Since the early 1960s benzodiazepines have become widely available, reaching prescription peaks in the 1970s (Reference LaderLader, 1991). Subsequently more and more data were reported indicating the disadvantages of long-term benzodiazepine use, such as the risk of dependence, a higher risk of accidents and falls, and cognitive disturbances (Reference Taylor, McCracken and WilsonTaylor et al, 1998). In the past few years the prevalence rate of benzodiazepine consumption in most European countries is estimated to be stable or slightly decreasing (Reference Stillwell and FountainStillwell & Fountain, 2002), but remains at levels varying between 2% and 3% of the general population (Reference Zandstra, Führer and Van de LisdonkZandstra et al, 2002). Although long-term therapeutic use of benzodiazepines is controversial, limited evidence suggests long-term efficacy in specific diagnostic groups such as panic disorder and social phobia (Reference Schweizer, Rickels and WeissSchweizer et al, 1993; Reference Otto, Pollack and GouldOtto et al, 2000). The prevalence of these disorders among people who are long-term benzodiazepine users, however, is relatively low (Reference Zandstra, Van Rijswijk and RijndersZandstra et al, 2004).
Problems experienced by patients stopping long-term benzodiazepine use initiated the development of treatment strategies for discontinuing these drugs. Russell & Lader (1993) proposed a stepped care approach to address the problem of long-term use. They advised starting with a minimal intervention and, if this failed, gradually intensifying treatment from supervised gradual withdrawal after patient assessment to specialised care including augmentation strategies. In order to summarise the evidence for the individual steps of such programmes, we carried out meta-analyses of the success rates of the different benzodiazepine discontinuation strategies.
METHOD
Identification of studies
An initial search was made of the databases PubMed and PsycINFO for the period 1966 to September 2004 and the Cochrane Library in December 2004, using the keywords BENZODIAZEPINE(S) in combination with WITHDRAWAL, DETOXIFICATION, DEPENDENCE, DISCONTINUATION or LONG-TERM. This search was extended by a manual search of the reference lists of all benzodiazepine discontinuation studies and benzodiazepine discontinuation augmentation studies (Fig. 1).

Fig. 1 Search strategy. Note: the study by Oude Voshaar et al (Reference Oude Voshaar, Gorgels and Mol2003a ) was included twice owing to its three-condition randomised controlled design.
Inclusion criteria
Papers were included in the review if they met the following criteria:
-
(a) the study had a randomised controlled design;
-
(b) the outcomes of discontinuation were presented separately for each treatment arm;
-
(c) long-term benzodiazepine use was defined as daily use for at least 3 months.
Excluded were case series, review papers, double publications, experimental research or clinical trials evaluating the efficacy of benzodiazepine treatment for a fixed period, and animal research. Authors R.C.O.V. and J.E.C. independently checked the inclusion and exclusion criteria of the identified studies.
Selection procedure, data extraction and quality assessment
Included studies were coded twice by R.C.O.V. and J.E.C. Discrepancies in the two coding forms were resolved by consensus after discussion or by referring to the data in the original article. This method yielded one coding form per article. The intervention type was added to the coding form by distinguishing between minimal interventions and systematic discontinuation programmes. Minimal interventions were defined as simple interventions applicable to large groups of people, for example, an advisory letter or a meeting in which patients who are long-term benzodiazepine users are advised to stop taking the drug. Systematic discontinuation programmes were defined as treatment programmes guided by a physician or psychologist. We sub-categorised these treatment programmes into systematic discontinuation alone or discontinuation with either psychotherapy or pharmacotherapy. The coding form consisted of the following items:
-
(a) inclusion criteria (minimum duration of benzodiazepine use 3.6 or 12 months) and diagnosed benzodiazepine dependence (yes/no);
-
(b) results at post-treatment outcome;
-
(c) year of publication;
-
(d) domain of use (i.e. psychiatric diagnosis or symptoms of included patients);
-
(e) steps of taper (abrupt, fixed or symptom-guided);
-
(f) tapered withdrawal after transfer to a long-acting benzodiazepine (yes/no);
-
(g) history of benzodiazepine use (dosage, type, duration of use);
-
(h) in-patient treatment (yes/no);
-
(i) setting (primary care, psychiatric clinic or addiction clinic).
Mean equivalent benzodiazepine dosages were obtained from the articles or calculated in diazepam equivalents (Reference Zitman and CouvéeZitman & Couvée, 2001). If no information was available to calculate the dosage in diazepam equivalents, we categorised the dosages as low (within the therapeutic range, or less than 15 mg), high (above the therapeutic range, or more than 30 mg) or medium (patients using benzodiazepines within and above the therapeutic range, or 15-30 mg).
The quality of the included articles was assessed twice by R.C.O.V., J.E.C. and/or A.J.L.M.v.B. using the Amsterdam-Maastricht consensus list, which covers the Chalmers criteria usually applied in the assessment of study quality (Reference Van Tulder, Assendelft and KoesVan Tulder et al, 1997; Reference Van Boeijen, Van Balkom and Van OppenVan Boeijen et al, 2005).
Statistical analysis
Since we were interested in the success rates of benzodiazepine discontinuation (binary outcome) and because in some studies data were sparse, we used stratified exact (conditional) methods with odds ratios as fixed-effects association measures. Exact P-values for testing significance and homogeneity of odds ratios across studies were calculated, and exact 95% confidence intervals were estimated. In cases in which homogeneity had to be rejected (P<0.05) we introduced a random effect in order to account for between-study variability of the odds ratios. In such cases the asymptotic direct pooling method was used for calculating significance levels and confidence limits.
RESULTS
The initial search yielded 5264 reference titles in PubMed, 1260 in PsychINFO and 666 in the Cochrane Library. Of these, 275 titles were identified by R.C.O.V. and J.E.C. as having possible relevance to discontinuation of long-term benzodiazepine use. (The full reference list is presented in data supplement 1 to the online version of this paper.) After screening of the abstracts and if necessary the full text, 246 papers were excluded (Fig. 1) and 29 papers met the inclusion criteria (Reference Tyrer, Rutherford and HuggettTyrer et al, 1981; Reference Lader and OlajideLader & Olajide, 1987; Reference Ashton, Rawlins and TyrerAshton et al, 1990; Reference Cantopher, Olivieri and CleaveCantopher et al, 1990; Reference JonesJones, 1990; Reference Udelman and UdelmanUdelman & Udelman, 1990; Reference Garcia-Borreguero, Bronisch and ApeltGarcia-Borreguero et al, 1991; Reference Schweizer, Rickels and CaseSchweizer et al, 1991; Reference Di Costanzo and RoveaDi Costanzo & Rovea, 1992; Reference Lader, Farr and MortonLader et al, 1993; Reference Otto, Pollack and SachsOtto et al, 1993; Reference Bashir, King and AshworthBashir et al, 1994; Reference Cormack, Sweeney and Hughes-JonesCormack et al, 1994; Reference Schweizer, Case and Garcia-EspanaSchweizer et al, 1995; Reference Tyrer, Ferguson and HallstromTyrer et al, 1996; Reference Lemoine, Touchon and BillardonLemoine et al, 1997; Reference Hantouche, Guelfi and CometHantouche et al, 1998; Reference Garfinkel, Zisapel and WainsteinGarfinkel et al, 1999; Reference Petrovic, Pevernagic and Van den NoortgatePetrovic et al, 1999; Rickels et al, Reference Rickels, Schweizer and Garcia1999, Reference Rickels, DeMartinis and Garcia-Espana2000; Reference Cialdella, Boissel and BelonCialdella et al, 2001; Reference Zitman and CouvéeZitman & Couvée 2001; Reference Gerra, Zaimovic and GiustiGerra et al, 2002; Reference Vorma, Naukkarinen and SarnaVorma et al, 2002; Reference Baillargeon, Landreville and VerreaultBaillargeon et al, 2003; Reference Oude Voshaar, Gorgels and MolOude Voshaar et al, 2003a ; Reference Rynn, Garcia-Espana and GreenblattRynn et al, 2003; Reference Morin, Bastien and GuayMorin et al, 2004).
Table 1 lists the scores for methodological quality of the included studies measured with the Amsterdam-Maastricht consensus list. The sumscore (range 0-18) can be considered to be a proxy of study quality. For studies evaluating psychotherapy augmentation strategies, however, the maximum score is 17. The quality of the included studies ranged from 8 to 17, corresponding with a moderate to excellent study quality. Recency of the study correlated moderately with better quality (Spearman's rank correlation coefficient 0.44, P=0.02). Patient numbers and demographic characteristics of the samples in the included papers are summarised in Table 2. The numbers of patients leaving the studies were relatively low, which can be explained by the fact that patient withdrawal was classified as discontinuation failure in the 14 studies reporting intention-to-treat analyses (Table 1). No difference was found in withdrawal rates between studies of different treatment modalities. Compared with those using benzodiazepine in the general population, minimal intervention studies included a higher proportion of women and the participants had a relatively higher age (Reference Zandstra, Führer and Van de LisdonkZandstra et al, 2002). Age and gender distribution of patients recruited in the only controlled study of systematic discontinuation alone was comparable with that of long-term benzodiazepine users in the population, as found by Zandstra et al (Reference Zandstra, Führer and Van de Lisdonk2002). Systematic discontinuation studies with augmentation strategies, on the contrary, included a lower proportion of women and a relatively lower age compared with the ‘average’ person using benzodiazepines in the population. The characteristics of the included studies according to the main items of the coding form are given in data supplement 2 to the online version of this paper.
Table 1 Validity scores of included studies assessed with the Amsterdam–Maastricht consensus list
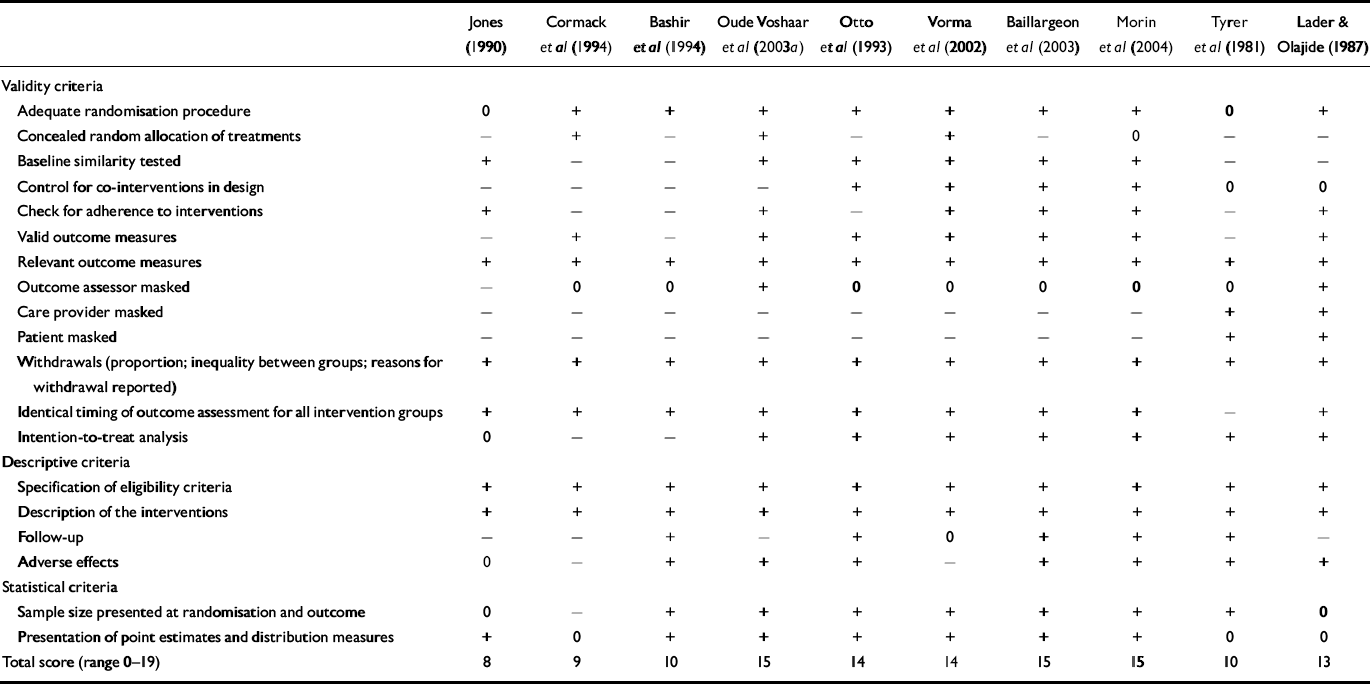
| Jones (Reference Jones1990) | Cormack et al (Reference Cormack, Sweeney and Hughes-Jones1994) | Bashir et al (Reference Bashir, King and Ashworth1994) | Oude Voshaar et al (Reference Oude Voshaar, Gorgels and Mol2003a ) | Otto et al (Reference Otto, Pollack and Sachs1993) | Vorma et al (Reference Vorma, Naukkarinen and Sarna2002) | Baillargeon et al (Reference Baillargeon, Landreville and Verreault2003) | Morin et al (Reference Morin, Bastien and Guay2004) | Tyrer et al (Reference Tyrer, Rutherford and Huggett1981) | Lader & Olajide (Reference Lader and Olajide1987) | Ashton et al (Reference Ashton, Rawlins and Tyrer1990) | Cantopher et al (Reference Cantopher, Olivieri and Cleave1990) | Udelman & Udelman (Reference Udelman and Udelman1990) | Garcia-Borreguero et al (Reference Garcia-Borreguero, Bronisch and Apelt1991) | Schweizer et al (Reference Schweizer, Rickels and Case1991) | Di Costanzo & Rovea (Reference Di Costanzo and Rovea1992) | Lader et al (Reference Lader, Farr and Morton1993) | Schweizer et al (Reference Schweizer, Case and Garcia-Espana1995) | Tyrer et al (Reference Tyrer, Ferguson and Hallstrom1996) | Lemoine et al (Reference Lemoine, Touchon and Billardon1997) | Garfinkel et al (Reference Garfinkel, Zisapel and Wainstein1999) | Hantouche et al (Reference Hantouche, Guelfi and Comet1998) | Petrovic et al (Reference Petrovic, Pevernagic and Van den Noortgate1999) | Rickels et al (Reference Rickels, Schweizer and Garcia1999) | Rickels et al (Reference Rickels, DeMartinis and Garcia-Espana2000) | Cialdella et al (Reference Cialdella, Boissel and Belon2001) | Zitman & Couvée (Reference Zitman and Couvée2001) | Gerra et al (Reference Gerra, Zaimovic and Giusti2002) | Rynn et al (Reference Rynn, Garcia-Espana and Greenblatt2003) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Validity criteria | |||||||||||||||||||||||||||||
| Adequate randomisation procedure | 0 | + | + | + | + | + | + | + | 0 | + | + | + | + | 0 | + | + | + | + | + | + | + | + | 0 | + | + | + | + | + | + |
| Concealed random allocation of treatments | – | + | – | + | – | + | – | 0 | – | – | – | – | 0 | – | – | + | – | – | – | – | – | 0 | – | – | – | – | + | – | – |
| Baseline similarity tested | + | – | – | + | + | + | + | + | – | – | + | + | + | – | + | + | – | – | – | + | + | + | – | + | + | + | + | + | + |
| Control for co-interventions in design | – | – | – | – | + | + | + | + | 0 | 0 | + | + | + | + | – | 0 | 0 | – | – | 0 | – | + | – | – | – | + | – | – | 0 |
| Check for adherence to interventions | + | – | – | + | – | + | + | + | – | + | + | + | + | – | + | + | + | + | – | + | + | + | – | + | + | + | + | + | + |
| Valid outcome measures | – | + | – | + | + | + | + | + | – | + | + | + | + | + | + | + | + | + | 0 | + | + | + | + | + | + | + | + | + | + |
| Relevant outcome measures | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Outcome assessor masked | – | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | + | + | 0 | 0 | – | 0 | + | 0 | 0 | 0 | 0 | + | + | 0 | + | + | + | 0 | – | + |
| Care provider masked | – | – | – | – | – | – | – | – | + | + | + | + | + | – | + | 0 | + | + | + | + | + | 0 | – | 0 | + | + | + | – | + |
| Patient masked | – | – | – | – | – | – | – | – | + | + | + | + | + | – | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Withdrawals (proportion; inequality between groups; reasons for withdrawal reported) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | – | + |
| Identical timing of outcome assessment for all intervention groups | + | + | + | + | + | + | + | + | – | + | + | + | 0 | 0 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Intention-to-treat analysis | 0 | – | – | + | + | + | + | + | + | + | 0 | – | + | + | – | + | 0 | 0 | 0 | + | + | 0 | + | 0 | – | 0 | + | 0 | – |
| Descriptive criteria | |||||||||||||||||||||||||||||
| Specification of eligibility criteria | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 0 | + | + | + | + | + | + | + | + |
| Description of the interventions | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Follow-up | – | – | + | – | + | 0 | + | + | + | – | + | 0 | – | – | – | – | – | + | – | + | + | – | – | – | + | – | + | + | + |
| Adverse effects | 0 | – | + | + | + | – | + | + | + | + | + | + | + | + | + | 0 | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Statistical criteria | |||||||||||||||||||||||||||||
| Sample size presented at randomisation and outcome | 0 | – | + | + | + | + | + | + | + | 0 | + | + | 0 | 0 | + | – | – | + | – | – | + | + | + | + | – | + | + | 0 | – |
| Presentation of point estimates and distribution measures | + | 0 | + | + | + | + | + | + | 0 | 0 | 0 | + | 0 | + | + | + | + | + | + | + | + | + | + | + | 0 | + | + | 0 | + |
| Total score (range 0–19) | 8 | 9 | 10 | 15 | 14 | 14 | 15 | 15 | 10 | 13 | 16 | 15 | 13 | 9 | 14 | 14 | 12 | 14 | 10 | 15 | 16 | 15 | 11 | 14 | 14 | 16 | 17 | 10 | 15 |
+, Present; –, absent; 0, not reported
Table 2 Demographic characteristics of the population in the selected studies
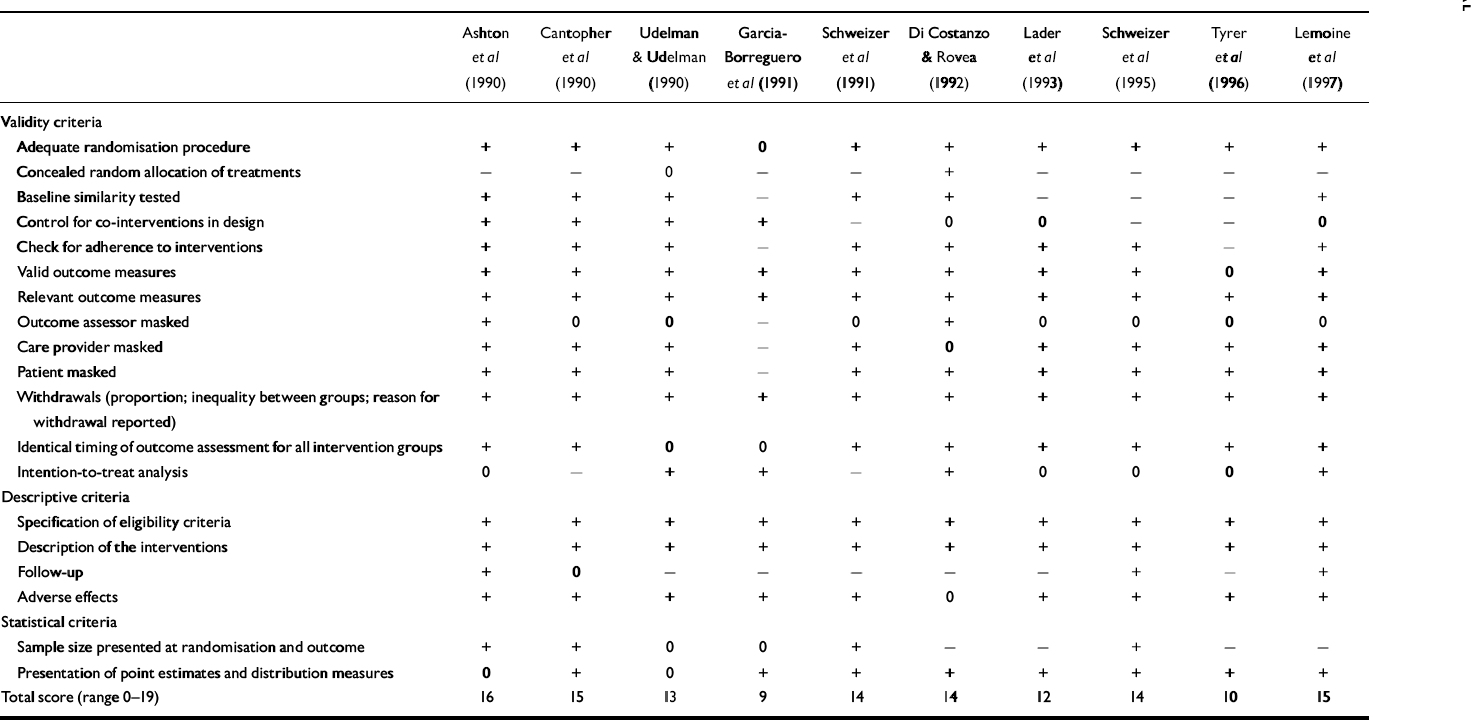
| Intervention | Studies n | Participants | Gender ratio M : F | Age, mean (years) | ||||
|---|---|---|---|---|---|---|---|---|
| Total n | Withdrew n | Completed n | ||||||
| Minimal intervention | 3 | 601 | 75 | 526 | 1 : 5 | 71 | ||
| Systematic discontinuation alone 1 | 1 | 107 | 23 | 84 | 1 : 2.6 | 62 | ||
| Systematic discontinuation with psychotherapy 1 | 5 | 357 | 40 | 317 | 1 : 1.4 | 56 | ||
| Systematic discontinuation with pharmacotherapy | 21 | 1333 | 130 | 1188 | 1 : 1.3 | 52 | ||
1. The study by Oude Voshaar et al (Reference Oude Voshaar, Gorgels and Mol2003a ) is included twice because it was a three-condition, controlled study
Findings of the meta-analysis
The three minimal intervention studies including 298 patients were homogeneous (P=0.76). The pooled odds ratio was 2.8 (95% CI 1.6-5.1). We found only one study that evaluated systematic discontinuation alone using a randomised controlled design (Reference Oude Voshaar, Gorgels and MolOude Voshaar et al, 2003a ) which showed an odds ratio of 6.1 (95% CI 2.0-18.6). (Further information is presented in data supplement 2 to the online version of this paper.)
All psychotherapy augmentation strategies evaluated the effect of cognitive-behavioural therapy. These studies appeared to be heterogeneous in outcome values (P<0.001), which could be explained by the cofactors setting, benzodiazepine dosage, group v. individual therapy and diagnosis (see Table 2). However, the studies of Baillargeon et al (Reference Baillargeon, Landreville and Verreault2003) and Morin et al (Reference Morin, Bastien and Guay2004) appeared to be comparable with respect to all variables evaluated with the coding form. Both studies evaluated group cognitive-behavioural therapy as an augmentation to systematic discontinuation alone using a fixed taper programme in a psychiatric out-patient setting among patients using low-dose benzodiazepines for insomnia. A post hoc heterogeneity analysis confirmed this finding (P=1.00) and a pooled odds ratio of 5.5 (95% CI 2.3-14.2) was found.
We found five pharmacological augmentation strategies with the compounds propranolol, buspirone, carbamazepine, trazodone and imipramine which were each evaluated at least twice. Statistical homogeneity was found for the studies evaluating carbamazepine (P=0.22), trazodone (P=0.35) and imipramine (P=0.051). The pooled analysis of studies evaluating the addition of imipramine found a significantly higher discontinuation success rate (P=0.03); augmentation with carbamazepine resulted in a higher success rate of borderline significance (P=0.06); whereas no significant effect was found for the addition of trazodone (P=0.12). The studies evaluating augmentation with propranolol and buspirone were heterogeneous in odds ratios (P=0.02 and P=0.004 respectively). The heterogeneity in odds ratios of the two studies evaluating propranolol was explained by differences in the steps of the tapering procedure, transfer to a long-acting benzodiazepine before dosage tapering, baseline benzodiazepine dosage, type of benzodiazepine and finally the diagnosis of included patients. The heterogeneity in odds ratios of the five studies evaluating buspirone was explained by the transfer to a long-acting agent, hospitalisation, baseline benzodiazepine dosage, type of benzodiazepine used before tapering, and diagnosis of included patients. Closer inspection did not reveal combinations of studies evaluating the addition of buspirone that might be homogeneous. Using a random-effects model we also did not find significant effects of the addition of propranolol (P=0.77) and buspirone (P=0.59).
DISCUSSION
The main finding of our meta-analysis was that minimal interventions are effective strategies for reducing benzodiazepine consumption, yielding an odds ratio of 2.8 in comparison with patients receiving usual care. More-intensive treatment in the form of systematic discontinuation with or without therapeutic augmentation was only once compared with usual care (Reference Oude Voshaar, Gorgels and MolOude Voshaar et al, 2003a ), with the finding of an odds ratio for patients receiving systematic discontinuation alone of 6.1. Although the clinical relevance was limited by the fact that systematic discontinuation alone was evaluated in one study only, the 62% success rate of systematic discontinuation alone in this study was comparable with the median success rate of 58% (range 25-100) in the control groups of studies evaluating systematic discontinuation augmentation strategies which consisted of systematic discontinuation alone or systematic discontinuation with placebo. Moreover, two large and well-designed (but uncontrolled) studies of benzodiazepine discontinuation also found discontinuation success rates of 62% (Reference Rickels, Schweizer and CaseRickels et al, 1990; Reference Schweizer, Rickels and CaseSchweizer et al, 1990). The three minimal intervention studies, as well as the study by Oude Voshaar et al (Reference Oude Voshaar, Gorgels and Mol2003a ), were conducted in general practice. Therefore, evidence for treatment of patients referred for help with benzodiazepine discontinuation is scarce.
A total of 17 different augmentation strategies were evaluated. Although these studies were conducted in a variety of settings, the age and gender distribution of patients in the samples suggests selective recruitment towards younger, male patients. Six augmentation strategies were evaluated in at least two studies each; for imipramine, carbamazepine and trazodone augmentation the studies were homogeneous. Of these three agents, only for imipramine was a significantly superior effect on benzodiazepine discontinuation success rate found (P=0.03); the effect of carbamazepine did not reach significance (P=0.06). A post hoc analysis showed that group cognitive-behavioural therapy had additive value for patients using low-dose benzodiazepines (<15 mg diazepam equivalent) for insomnia. Finally, the following strategies showed significantly higher benzodiazepine discontinuation success rates in single studies: group cognitive-behavioural therapy for patients with panic disorder, melatonin therapy for patients with insomnia, and for long-term benzodiazepine use generally also sodium valproate or flumazenil (Reference Otto, Pollack and SachsOtto et al, 1993; Reference Garfinkel, Zisapel and WainsteinGarfinkel et al, 1999; Reference Rickels, Schweizer and GarciaRickels et al, 1999; Reference Gerra, Zaimovic and GiustiGerra et al, 2002).
Limitations
Large generalisations from our meta-analysis are limited owing to heterogeneity of the included studies. We strove to explain heterogeneity with variables that have previously been suggested to be associated with discontinuation outcome (Reference Ashton, Rawlins and TyrerAshton et al, 1990; Rickels et al, Reference Rickels, Schweizer and Case1990, Reference Rickels, DeMartinis and Garcia-Espana2000; Schweizer et al, Reference Schweizer, Rickels and Case1990, Reference Schweizer, Rickels and De Martinis1998; Reference Murphy and TyrerMurphy & Tyrer, 1991; Reference Oude Voshaar, Mol and GorgelsOude Voshaar et al, 2003b ). However, the current state of knowledge precludes any firm conclusion as to the effects of these variables. In addition, more important variables might not have been identified or measured in the included studies, such as a clear DSM-IV Axis I diagnosis (American Psychiatric Assocation, 1994) or personality characteristics. For example, in a relatively large, uncontrolled study (n=165) personality factors were found to explain 24% of the variance in discontinuation outcome (Reference Schweizer, Rickels and De MartinisSchweizer et al, 1998).
Clinical implications
Although establishing the efficacy of individual treatment strategies is clinically relevant, stepped care approaches are even more important for treatment planning in the case of treatment-resistant benzodiazepine dependence. This meta-analysis was conducted in order to establish the clinical evidence for the individual steps in a stepped care approach in order to discontinue long-term benzodiazepine use. Following the stepped care approach proposed by Russell & Lader (1993), we now know that use of the first two steps - namely starting with a minimal intervention strategy, followed by systematic discontinuation alone for cases resistant to treatment in primary care - is supported by the results of randomised controlled trials. With respect to this statement, it has to be mentioned that the single study evaluating systematic discontinuation alone was conducted among people with long-term benzodiazepine use who did not respond to a minimal intervention strategy (Reference Oude Voshaar, Gorgels and MolOude Voshaar et al, 2003a ; Reference Gorgels, Oude Voshaar and MolGorgels et al, 2005). However, much research has still to be conducted in this field; for example, we do not know which variables and treatment characteristics are associated with a favourable outcome. The taper schedules described in published studies vary from abrupt discontinuation (Reference Rickels, Schweizer and CaseRickels et al, 1990), to 25% weekly reduction of dosage (Reference Schweizer, Rickels and CaseSchweizer et al, 1990; Reference Oude Voshaar, Gorgels and MolOude Voshaar et al, 2003a ), discontinuation in steps of about one-eighth of the daily dose every 2 weeks (Russell & Lader, 1993) to, finally, symptom-guided withdrawal with the time needed for withdrawal varying from about 4 weeks to a year or more (Reference AshtonAshton, 1987). However, different taper schedules have never been directly compared in a randomised controlled study. We also do not know which strategy should be followed if the first two steps fail. Although augmentation was not evaluated among patients who failed to discontinue their benzodiazepine use by systematic discontinuation alone, our meta-analysis found a higher discontinuation success rate after the addition of imipramine and carbamazepine in general, or group cognitive-behavioural therapy for patients with insomnia. Moreover, adding sodium valproate or flumazenil and adding melatonin or group cognitive-behavioural therapy in specific patient groups (e.g. those with panic disorder) can be an option. It should be noted that these suggestions are based on small, single studies (patient numbers n=27 to n=40). Future research should evaluate more rigorously stepped care programmes and promising augmentation strategies.






eLetters
No eLetters have been published for this article.