Autism spectrum disorder (ASD) is a chronic childhood-onset neurodevelopmental condition with detrimental effects on adaptive functions throughout life. Reference Billstedt, Gillberg and Gillberg1–Reference Pinborough-Zimmerman, Bakian, Fombonne, Bilder, Taylor and McMahon3 The number of people with ASD diagnoses has increased during the last decades although the underlying reasons for this increase are not fully understood. Reference Wazana, Bresnahan and Kline4 Both clinical Reference Cederlund, Hagberg, Billstedt, Gillberg and Gillberg5,Reference Howlin, Goode, Hutton and Rutter6 and population-based studies Reference Billstedt, Gillberg and Gillberg1,Reference Gillberg and Steffenburg7 have reported poor long-term outcomes Reference Bölte, de Schipper, Robison, Wong, Selb and Singhal8 regarding education, employment, independent living and peer relations. A poor long-term outcome has been observed in both low-functioning ASD (i.e. individuals with ASD and a co-existing intellectual disability) and high-functioning ASD (i.e. individuals with ASD and intellectual ability in the average or above range). Psychiatric comorbidity is common in individuals with ASD, Reference Caamano, Boada, Merchan-Naranjo, Moreno, Llorente and Moreno9–Reference Skokauskas and Gallagher13 and especially low-functioning ASD may be part of a known genetic syndrome (‘syndromic’ autism), such as fragile X syndrome, Down syndrome or tuberous sclerosis. Reference Persico and Napolioni14
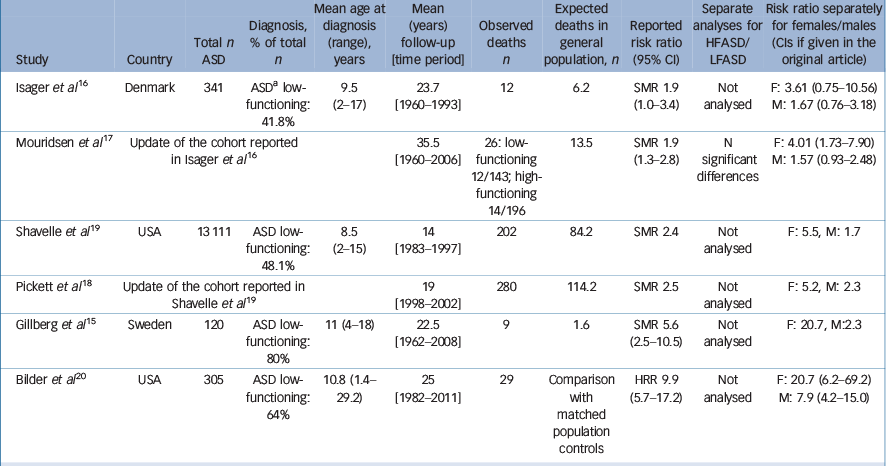
Risk of premature mortality has been reported to be elevated among individuals with ASD, compared with the general population, Reference Gillberg, Billstedt, Sundh and Gillberg15–Reference Shavelle, Strauss and Pickett19 as well as compared with healthy cousin or sibling controls. Reference Bilder, Botts, Smith, Pimentel, Farley and Viskochil20 To date, studies on mortality in ASD with long-term follow-ups comprise two clinical cohorts Reference Isager, Mouridsen and Rich16–Reference Shavelle, Strauss and Pickett19 and two population-based cohorts, Reference Gillberg, Billstedt, Sundh and Gillberg15,Reference Bilder, Botts, Smith, Pimentel, Farley and Viskochil20 diagnosed with ASD as children. Compared with mortality statistics from the general population or general population controls, the risk of premature mortality has been estimated to be twofold to 10-fold higher in the ASD population. Characteristics of the previous studies on the outcome of mortality in ASD are summarised in Table 1.
Table 1 A summary of study cohorts and main results from previous studies on mortality in ASD
| Study | Country | Total n
ASD |
Diagnosis, % of total n |
Mean age
at diagnosis (range), years |
Mean (years) follow-up [time period] |
Observed deaths n |
Expected deaths in general population, n |
Reported risk ratio (95% CI) |
Separate analyses for HFASD/ LFASD |
Risk ratio
separately for females/males (CIs if given in the original article) |
|---|---|---|---|---|---|---|---|---|---|---|
| Isager et al Reference Isager, Mouridsen and Rich16 | Denmark | 341 | ASD
a
low- functioning: 41.8% |
9.5 (2–17) |
23.7 [1960–1993] |
12 | 6.2 | SMR 1.9 (1.0–3.4) |
Not analysed |
F: 3.61 (0.75–10.56) M: 1.67 (0.76–3.18) |
| Mouridsen et al Reference Mouridsen, Bronnum-Hansen, Rich and Isager17 | Update of the cohort reported in Isager et al Reference Isager, Mouridsen and Rich16 |
35.5 [1960–2006] |
26: low- functioning 12/143; high- functioning 14/196 |
13.5 | SMR 1.9 (1.3–2.8) |
N significant differences |
F: 4.01 (1.73–7.90) M: 1.57 (0.93–2.48) |
|||
| Shavelle et al Reference Shavelle, Strauss and Pickett19 | USA | 13 111 | ASD
low- functioning: 48.1% |
8.5 (2–15) |
14 [1983–1997] |
202 | 84.2 | SMR 2.4 | Not analysed |
F: 5.5, M: 1.7 |
| Pickett et al Reference Pickett, Paculdo, Shavelle and Strauss18 | Update of the cohort reported
in Shavelle et al Reference Shavelle, Strauss and Pickett19 |
19 [1998–2002] |
280 | 114.2 | SMR 2.5 | Not analysed |
F: 5.2, M: 2.3 | |||
| Gillberg et al Reference Gillberg, Billstedt, Sundh and Gillberg15 | Sweden | 120 | ASD
low- functioning: 80% |
11 (4–18) | 22.5 [1962–2008] |
9 | 1.6 | SMR 5.6 (2.5–10.5) |
Not analysed |
F: 20.7, M:2.3 |
| Bilder et al Reference Bilder, Botts, Smith, Pimentel, Farley and Viskochil20 | USA | 305 | ASD
low- functioning: 64% |
10.8 (1.4– 29.2) |
25 [1982–2011] |
29 | Comparison with matched population controls |
HRR 9.9 (5.7–17.2) |
Not analysed |
F: 20.7 (6.2–69.2) M: 7.9 (4.2–15.0) |
ASD, autism spectrum disorder; SMR, standardised mortality ratio; HRR, hazard rate ratio; CI, confidence interval; F, female; M, male.
a. The previous Scandinavian diagnosis Borderline childhood psychosis was included as a proxy for Asperger syndrome.
IQ was tested in 60% of cases and in the rest of the cases was based on clinical assessments.
Based on the well-known association between ASD and medical conditions (e.g. epilepsy), Reference Woolfenden, Sarkozy, Ridley, Coory and Williams21 and especially in individuals with low-functioning ASD, it has been suggested that the excess mortality in ASD may be related to the presence of comorbid medical conditions and intellectual disability, rather than ASD per se. Reference Bilder, Botts, Smith, Pimentel, Farley and Viskochil20 However, the only study to date analysing differences in mortality between individuals with low-functioning ASD and high-functioning ASD did not identify significant between-group differences in overall mortality. Reference Mouridsen, Bronnum-Hansen, Rich and Isager17 In addition, in most previous studies sample sizes have been too small to compare mortalities in low-functioning and high-functioning ASD reliably. Thus, the potentially moderating effect of intellectual disability in mortality and causes of death in ASD remain unclear, and it has not been possible to determine whether ASD per se carries an increased mortality. Reference Gillberg, Billstedt, Sundh and Gillberg15
Gender is another possible moderator of excess mortality in ASD. Relative to males, females with ASD have been reported to have an elevated mortality risk. Reference Gillberg, Billstedt, Sundh and Gillberg15–Reference Bilder, Botts, Smith, Pimentel, Farley and Viskochil20 However, there has been considerable variation in the reported risk ratios ranging from 3.6 to 20.7 for females, whereas the risk ratios for males have ranged between 1.6 and 7.9. Reference Gillberg, Billstedt, Sundh and Gillberg15–Reference Bilder, Botts, Smith, Pimentel, Farley and Viskochil20 Large confidence intervals (CIs) in some of the studies indicate imprecise estimations.
Large-scale studies are needed to explore the predictive role of risk factors (such as comorbid intellectual disability or the potential role of gender) for mortality in ASD. Moreover, access to data from a large sample including a broad age range and a substantial follow-up period is needed to study different causes of death. On this point, most studies did not have adequate statistical power to examine less frequent causes of death.
The population-based studies conducted to date have identified such causes of death as associated medical conditions (including epilepsy), as well as cardiovascular and respiratory deaths. Reference Gillberg, Billstedt, Sundh and Gillberg15,Reference Bilder, Botts, Smith, Pimentel, Farley and Viskochil20 In a clinical cohort, a pattern of causes of death resembling that of the background population was observed, with exception for a very strong association for deaths associated with epilepsy. Reference Isager, Mouridsen and Rich16,Reference Mouridsen, Bronnum-Hansen, Rich and Isager17 The largest study on causes of death in ASD was based on a clinical cohort including ambulatory Californians with autism. Reference Pickett, Paculdo, Shavelle and Strauss18,Reference Shavelle, Strauss and Pickett19 Nevertheless, on excluding concomitant conditions such as cerebral palsy, tuberous sclerosis and Down syndrome, the results showed an elevated risk for several causes of death.
The aim of the current study was to analyse all-cause and cause-specific mortality in ASD using nationwide Swedish population-based registers. A further aim was to address the role of intellectual disability and gender as possible moderators of mortality and causes of death in ASD.
Method
Study design
We conducted a matched case cohort study.
Study setting
Two nationwide population-based Swedish registers were linked: the National Patient Register and the Cause of Death Register, both held by the National Board of Health and Welfare. The data were linked using the unique 10-digit personal identification number used in registers for all Swedish residents, including migrants with a residence permit. The National Patient Register includes diagnoses for all in-patient treatment episodes for psychiatric disorders in Sweden since 1973, as well as for out-patients (including diagnostic assessments with no further contact with psychiatric services) since 2001. The diagnoses are coded according to the Swedish versions of the ICD by the World Health Organization (WHO).
Study population
Individuals with ICD diagnosis codes for any ASD were identified from the National Patient Register. The validity or diagnostic accuracy of ASD diagnoses in Swedish health registries has been shown to be good. Reference Idring, Rai, Dal, Dalman, Sturm and Zander22 We first identified all individuals with an ASD diagnosis in the National Patient Register between 1987 and 2009. The ICD-9 23 ASD diagnoses (1987–1996; 299xx) were converted to corresponding ICD-10 24 diagnoses (1997 onwards) using a conversion instrument provided by the Swedish National Board of Health and Welfare. In the final cohort, the included diagnoses were autism (F84.0), Asperger syndrome (F84.5), atypical autism (F84.1) and pervasive developmental disorder – not otherwise specified (F84.9), other childhood disintegrative disorder (F84.3) and other pervasive developmental disorders (F84.8). Diagnoses of Rett syndrome (F84.2) and overactive disorder associated with mental retardation and stereotyped movements (F84.4) were excluded, as these are no longer considered core ASD diagnoses in current psychiatry (e.g. no longer classified in DSM-5). 25 In Swedish clinical practice, diagnostic assessment of ASD was rare before 1990 (less than 2% of the final study cohort) and a majority of the study cohort (88.2%) was diagnosed after 2001, i.e. after inclusion of out-patient data in the National Patient Register. The dichotomisation into low-functioning ASD and high-functioning ASD groups was based on the registered ICD codes for mental retardation. The ICD-9 codes 317–319 were converted to the corresponding ICD-10 diagnoses mild (F70), moderate (F71), severe (F72), profound (F73), other (F78) and unspecified (F79) intellectual disability. Thus, individuals with a co-existing intellectual disability were classified as low-functioning ASD regardless of which ASD diagnosis they had. The same type of classification strategy has been applied in previous studies Reference Idring, Magnusson, Lundberg, Ek, Rai and Svensson26,Reference Magnusson, Rai, Goodman, Lundberg, Idring and Svensson27 based on high-functioning ASD v. low-functioning ASD as two key categories for the specification of ASD in DSM-5.
For each proband identified with ASD from the National Patient Register, up to 100 controls were randomly selected from the Total Population Register. The controls were alive at the time-point of inclusion (when the case with ASD was registered the first time) and were not diagnosed with ASD during the study period. Controls were matched with cases by birth year, gender and county of residence in the year when the matched cases received their first ASD diagnosis.
Classification of the specific causes of death
ICD-9 codes for specific causes of death (for deaths during 1987–1996) were converted into corresponding ICD-10 diagnoses (1997–2009) using the conversion instrument provided by the National Board of Health and Welfare. The main causes of death were grouped into the following categories (ICD-10 chapters and codes are specified in the Appendix):
-
(a) Infections
-
(b) Neoplasms
-
(c) Endocrine
-
(d) Mental and behavioural disorders
-
(e) Diseases of the nervous system
-
(f) Diseases of the circulatory system
-
(g) Diseases of the respiratory system
-
(h) Diseases of the digestive system
-
(i) Diseases of the genitourinary system
-
(j) Congenital malformations
-
(k) Symptoms, signs and abnormal findings not elsewhere classified
-
(l) External causes of morbidity and mortality: intentional self-harm/suicide was analysed separately from other external causes of death. X60-X84 in ICD-10 was combined with undetermined suicide Y10-Y34 in ICD-10 and corresponding codes from ICD-9. These codes were combined to limit the temporal and geographic variation in the ascertainment. This practice is common in research and reporting concerning public health statistics. Reference Lager, Berlin, Heimerson and Danielsson28,Reference Mittendorfer-Rutz, Rasmussen and Wasserman29 A sensitivity analysis proved the comparability of the two diagnostic groups. The combined measure is referred to hereafter as suicide. In Other external causes we included remaining X and Y diagnoses and V diagnoses, as well as W diagnoses.
-
(m) Other causes of death: in this category we included chapters with only a few cases (in total, 247 cases; 15 of these with ASD) (for information on ICD chapters and codes, see Appendix).
Statistical analyses
Conditional logistic regression analyses were performed and odds ratios (ORs) with 95% CIs were calculated for all-cause and cause-specific mortality in cases of an ASD diagnosis in the National Patient Register (1987–2009). In the first step, the ORs were calculated for the total ASD group, as well as separately for the genders. In the second step, the ORs were calculated separately for the low- and the high-functioning ASD groups (compared with the matched controls). Analyses were performed stratified for gender if the difference between the entire ASD group and controls was significant (all other categories except Infections). For the analysis of interaction (ASD × gender), an interaction term was added as a covariate in the conditional logistic regression analyses and significant interaction was tested with the partial likelihood ratio test. If any cell included fewer than five cases at any level of the analysis, that level was dropped and the data were thus not shown. In the analyses of Mental and behavioural disorders, ASD diagnoses as primary causes of death (n = 2) were regarded as ill-defined causes of death and excluded. A partial likelihood ratio test was also used to compare the fit of the null model of diagnostic status (ASD or control) with the alternative model (low-functioning ASD, high-functioning ASD or control). Thus, if the data fitted the alternative model significantly better, an interaction effect between low-functioning ASD and high-functioning ASD was assumed and the null model was rejected. The alpha level was set at P<0.05. All statistical analyses were planned a priori.
Ethical approval
The Regional Ethics Committee in Stockholm approved the study (0229/939-31/5).
Results
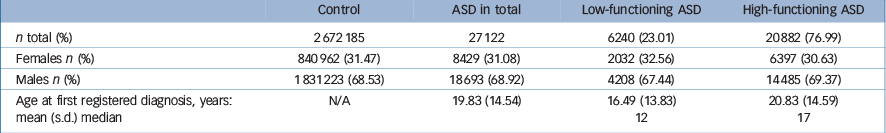
Characteristics of the study sample are described in Table 2.
Table 2 Characteristics of the study groups
| Control | ASD in total | Low-functioning ASD | High-functioning ASD | |
|---|---|---|---|---|
| n total (%) | 2 672 185 | 27 122 | 6240 (23.01) | 20 882 (76.99) |
| Females n (%) | 840 962 (31.47) | 8429 (31.08) | 2032 (32.56) | 6397 (30.63) |
| Males n (%) | 1 831 223 (68.53) | 18 693 (68.92) | 4208 (67.44) | 14 485 (69.37) |
| Age at first registered
diagnosis, years: mean (s.d.) median |
N/A | 19.83 (14.54) | 16.49 (13.83) | 20.83 (14.59) |
| 12 | 17 | |||
ASD, autism spectrum disorder.
All-cause mortality
At the time of the follow-up, 24 358 persons (0.91%) in the general population group had died, whereas the corresponding figure in the ASD group was 706 (2.60%; Table 3).
Table 3 Risk for all-cause mortality for the entire autism spectrum disorder (ASD) group, as well as separately for females and males, and low-functioning ASD and high-functioning ASD groups

| Controls | ASD OR (95% CI) | Low-functioning ASD OR (95% CI) | High-functioning ASD OR (95% CI) | |
|---|---|---|---|---|
| Number of deaths (%) | Number of deaths (%) | Number of deaths (%) | Number of deaths (%) | |
| Total | 2.56 (2.38–2.76) | 5.78** (4.94–6.75) | 2.18 (2.00–2.38) | |
| 24 358 (0.91) | 706 (2.60) | 169 (2.71) | 537 (2.57) | |
| Females | 2.24 (1.99–2.51) | 8.52 (6.55–11.08) | 1.88 (1.65–2.14) | |
| 11 693 (1.39) | 296 (3.51) | 61 (3.00) | 235 (3.67) | |
| Males | 2.87* (2.60–3.16) | 4.88 (4.02–5.93) | 2.49 (2.22–2.80) | |
| 12 665 (0.69) | 410 (2.19) | 108 (2.57) | 302 (2.08) | |
ASD, autism spectrum disorder; OR, odds ratio; CI, confidence interval.
* Partial likelihood ratio test for interaction effect ASD × gender, P=0.001.
** Partial likelihood ratio test for model selection (low-functioning ASD/high-functioning ASD), P<0.001.
Individuals with ASD had a 2.56-fold increased odds of mortality compared with matched general population controls (Table 3). Mortality was significantly elevated in both genders relative to the general population (males: OR = 2.87; females OR = 2.24), whereas the significant interaction effect indicated higher mortality among males (Table 3). Moreover, all-cause mortality was increased in both the low-functioning ASD (OR = 5.78) and the high-functioning ASD (OR = 2.18) groups, compared with the general population. The excess mortality was significantly higher in the low-functioning ASD group, as indicated by the significance of the partial likelihood ratio test (Table 3). Mortality was increased in both females (OR = 8.52) and males (OR = 4.88) with low-functioning ASD, as well as in both females (OR = 1.88) and males (OR = 2.49) with high-functioning ASD, compared with controls of the same gender.
Individuals in the control group died at a mean age of 70.20 years (s.d. = 24.16, median = 80), whereas the corresponding figure for the entire ASD group was 53.87 years (s.d. = 24.78, median = 55), for low-functioning ASD 39.50 years (s.d. = 21.55, median = 40) and high-functioning ASD 58.39 years (s.d. = 24.01, median = 63) respectively. The time period between registered ASD diagnosis and death (regardless of cause of death) was on average 5.30 years (s.d. = 4.85) for low-functioning ASD and 3.79 years (s.d. = 4.17) for the high-functioning ASD group.
Cause-specific mortality
Specific causes of death in the whole ASD group
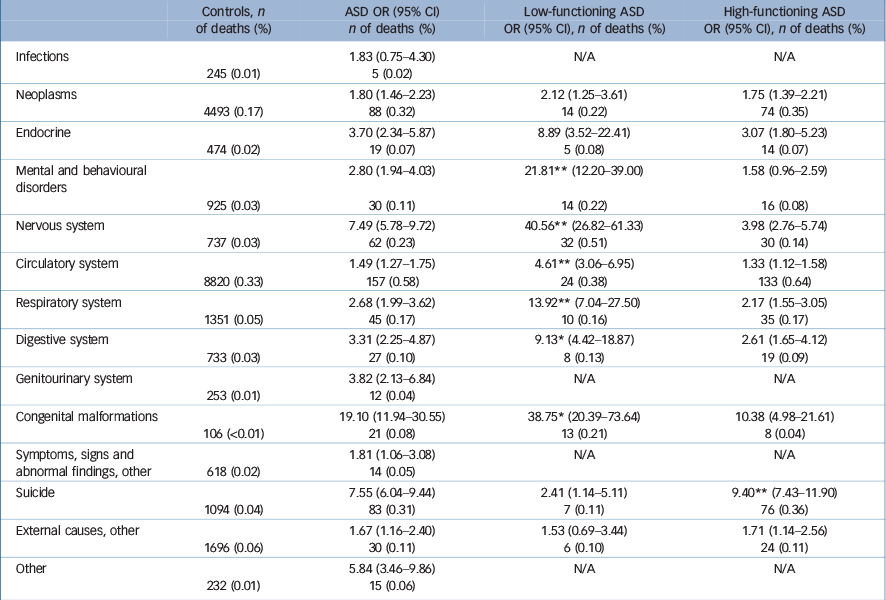
Significantly elevated mortality was noted among individuals with ASD in all analysed categories of specific causes of death except for infections (Table 4). The ICD diagnoses including only a few cases were combined and, also in this category, an excess mortality of the ASD group was observed. The mortality was 1.5-fold to eightfold increased compared with the general population (with exception for congenital malformations (OR = 19.10); however, the broad CIs indicate an imprecise estimation). ORs were highest in cases of mortality because of diseases of the nervous system (OR = 7.49) and because of suicide (OR = 7.55), in comparison with matched general population controls.
Table 4 Cause-specific mortality in relation to ASD and separately for low-functioning ASD and high-functioning ASD a
| Controls,
n
of deaths (%) |
ASD OR (95% CI) n of deaths (%) |
Low-functioning
ASD OR (95% CI), n of deaths (%) |
High-functioning
ASD OR (95% CI), n of deaths (%) |
|
|---|---|---|---|---|
| Infections | 245 (0.01) | 1.83 (0.75–4.30) 5 (0.02) |
N/A | N/A |
| Neoplasms | 4493 (0.17) | 1.80 (1.46–2.23) 88 (0.32) |
2.12 (1.25–3.61) 14 (0.22) |
1.75 (1.39–2.21) 74 (0.35) |
| Endocrine | 474 (0.02) | 3.70 (2.34–5.87) 19 (0.07) |
8.89 (3.52–22.41) 5 (0.08) |
3.07 (1.80–5.23) 14 (0.07) |
| Mental and
behavioural disorders |
925 (0.03) | 2.80 (1.94–4.03) 30 (0.11) |
21.81** (12.20–39.00) 14 (0.22) |
1.58 (0.96–2.59) 16 (0.08) |
| Nervous system | 737 (0.03) | 7.49 (5.78–9.72) 62 (0.23) |
40.56** (26.82–61.33) 32 (0.51) |
3.98 (2.76–5.74) 30 (0.14) |
| Circulatory system | 8820 (0.33) | 1.49 (1.27–1.75) 157 (0.58) |
4.61** (3.06–6.95) 24 (0.38) |
1.33 (1.12–1.58) 133 (0.64) |
| Respiratory system | 1351 (0.05) | 2.68 (1.99–3.62) 45 (0.17) |
13.92** (7.04–27.50) 10 (0.16) |
2.17 (1.55–3.05) 35 (0.17) |
| Digestive system | 733 (0.03) | 3.31 (2.25–4.87) 27 (0.10) |
9.13* (4.42–18.87) 8 (0.13) |
2.61 (1.65–4.12) 19 (0.09) |
| Genitourinary system | 253 (0.01) | 3.82 (2.13–6.84) 12 (0.04) |
N/A | N/A |
| Congenital malformations | 106 (<0.01) | 19.10 (11.94–30.55) 21 (0.08) |
38.75* (20.39–73.64) 13 (0.21) |
10.38 (4.98–21.61) 8 (0.04) |
| Symptoms, signs
and abnormal findings, other |
618 (0.02) | 1.81 (1.06–3.08) 14 (0.05) |
N/A | N/A |
| Suicide | 1094 (0.04) | 7.55 (6.04–9.44) 83 (0.31) |
2.41 (1.14–5.11) 7 (0.11) |
9.40** (7.43–11.90) 76 (0.36) |
| External causes, other | 1696 (0.06) | 1.67 (1.16–2.40) 30 (0.11) |
1.53 (0.69–3.44) 6 (0.10) |
1.71 (1.14–2.56) 24 (0.11) |
| Other | 232 (0.01) | 5.84 (3.46–9.86) 15 (0.06) |
N/A | N/A |
ASD, autism spectrum disorder; OR, odds ratio; CI, confidence interval.
a. Missing data on primary cause of death (n = 2677, <0.5% in both groups) are not included in the analyses; N/A analyses were not performed owing to the low number of cases in certain cells; partial likelihood ratio test for model selection (low-functioning ASD/high-functioning ASD).
* P<0.01 (Digestive P=0.009; Congenital malformations P = 0.007);
** P<0.001.
Specific causes of death in low-functioning v. high-functioning ASD
In most of the specific causes of death (Mental and behavioural disorders; Nervous system; Circulatory system; Respiratory system; Digestive system; and Congenital malformations), the low-functioning ASD group had higher mortality relative to the high-functioning ASD group, although both groups had significantly elevated mortality compared with the general population controls (Table 4). The most common cause of death in the low-functioning ASD group was epilepsy. In contrast, the high-functioning ASD group had a significantly more elevated suicide risk than the low-functioning ASD group, whereas, again, both groups had an increased risk compared with controls. The time period between registered ASD diagnosis and suicide was on average 2.86 years (s.d. = 2.41) in the low-functioning ASD group and 2.53 years (s.d. = 2.65) in the high-functioning ASD group. Online Table DS1 specifies the most common subcategories of the main causes of death for controls, the entire ASD group, and low-functioning and high-functioning ASD groups.
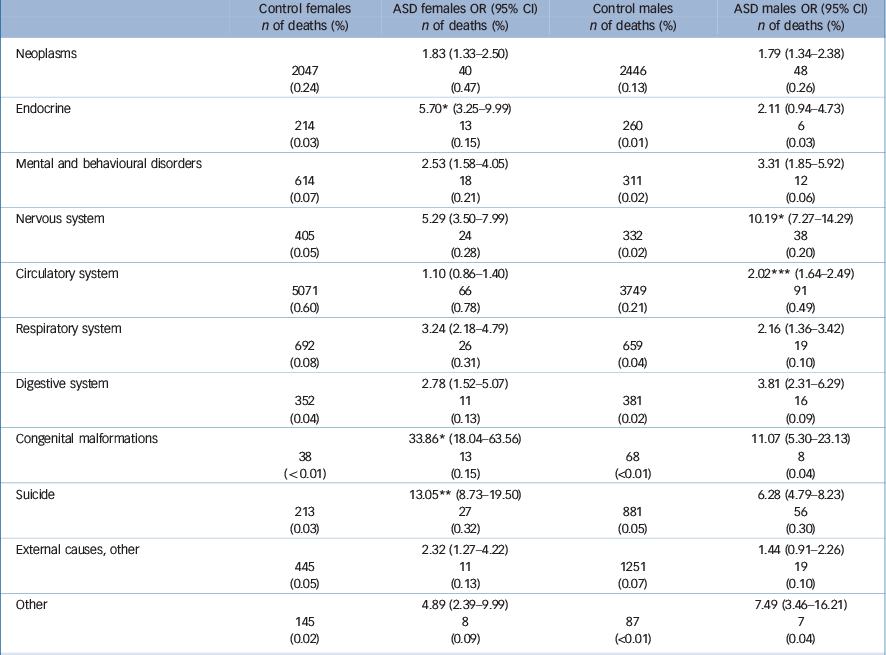
Gender differences in specific causes of death
For most diagnostic categories, the pattern of mortality risk was comparable in females and males with ASD. Nevertheless, males with ASD had a higher relative risk than females of mortality owing to diseases of the nervous and circulatory systems. On the other hand, females with ASD had higher relative mortality risk than males in diseases owing to endocrine diseases, congenital malformations and suicide (Table 5).
Table 5 Cause-specific mortality analysed separately for females and males a
| Control females n of deaths (%) |
ASD females OR (95% CI) n of deaths (%) |
Control males n of deaths (%) |
ASD males OR (95% CI) n of deaths (%) |
|
|---|---|---|---|---|
| Neoplasms | 1.83 (1.33–2.50) | 1.79 (1.34–2.38) | ||
| 2047 | 40 | 2446 | 48 | |
| (0.24) | (0.47) | (0.13) | (0.26) | |
| Endocrine | 5.70* (3.25–9.99) | 2.11 (0.94–4.73) | ||
| 214 | 13 | 260 | 6 | |
| (0.03) | (0.15) | (0.01) | (0.03) | |
| Mental and behavioural disorders | 2.53 (1.58–4.05) | 3.31 (1.85–5.92) | ||
| 614 | 18 | 311 | 12 | |
| (0.07) | (0.21) | (0.02) | (0.06) | |
| Nervous system | 5.29 (3.50–7.99) | 10.19* (7.27–14.29) | ||
| 405 | 24 | 332 | 38 | |
| (0.05) | (0.28) | (0.02) | (0.20) | |
| Circulatory system | 1.10 (0.86–1.40) | 2.02*** (1.64–2.49) | ||
| 5071 | 66 | 3749 | 91 | |
| (0.60) | (0.78) | (0.21) | (0.49) | |
| Respiratory system | 3.24 (2.18–4.79) | 2.16 (1.36–3.42) | ||
| 692 | 26 | 659 | 19 | |
| (0.08) | (0.31) | (0.04) | (0.10) | |
| Digestive system | 2.78 (1.52–5.07) | 3.81 (2.31–6.29) | ||
| 352 | 11 | 381 | 16 | |
| (0.04) | (0.13) | (0.02) | (0.09) | |
| Congenital malformations | 33.86* (18.04–63.56) | 11.07 (5.30–23.13) | ||
| 38 | 13 | 68 | 8 | |
| (<0.01) | (0.15) | (<0.01) | (0.04) | |
| Suicide | 13.05** (8.73–19.50) | 6.28 (4.79–8.23) | ||
| 213 | 27 | 881 | 56 | |
| (0.03) | (0.32) | (0.05) | (0.30) | |
| External causes, other | 2.32 (1.27–4.22) | 1.44 (0.91–2.26) | ||
| 445 | 11 | 1251 | 19 | |
| (0.05) | (0.13) | (0.07) | (0.10) | |
| Other | 4.89 (2.39–9.99) | 7.49 (3.46–16.21) | ||
| 145 | 8 | 87 | 7 | |
| (0.02) | (0.09) | (<0.01) | (0.04) | |
ASD, autism spectrum disorder; OR, odds ratio; CI, confidence interval.
a. Missing data on primary cause of (n = 2677, <0.5% in both groups) are not included in the analyses; partial likelihood ratio test for interaction effect (ASD gender).
* P<0.05 (Endocrine P = 0.039; Nervous system P = 0.014; Congenital malformations P=0.021);
** P<0.01 (Suicide P=0.004);
*** P< 0.001.
Discussion
In this large population-based study, we observed increased mortality in individuals with ASD. Mortality was increased in both low-functioning and high-functioning ASD, as well as in both genders. However, the risk was particularly high for females with low-functioning ASD. Patterns of specific causes of death were somewhat different for low-functioning ASD compared with high-functioning ASD.
The observed OR of 2.56 for ASD in relation to all-cause mortality is in line with most of the previous clinical and population-based mortality studies. Reference Gillberg, Billstedt, Sundh and Gillberg15–Reference Bilder, Botts, Smith, Pimentel, Farley and Viskochil20 We found that increased mortality in ASD was not limited to certain causes of death, such as diseases of nervous system, but was elevated for all analysed categories according to the ICD, apart from infectious diseases.
In most previous studies, the samples have been too small and/or selected to adequately analyse the role of intellectual disability regarding mortality in ASD. Therefore, we performed all analyses comparing low-functioning ASD and high-functioning ASD groups. Based on the well-known association of low-functioning ASD with several medical conditions, the higher mortality in low-functioning ASD than in high-functioning ASD was expected. Reference Woolfenden, Sarkozy, Ridley, Coory and Williams21 In approximately 10% of cases, ASD (predominantly low-functioning ASD) is part of a known genetic syndrome, Reference Persico and Napolioni14 which may be associated with both intellectual disability and many of the comorbid diseases. Opposing results from the only previous study focusing on differences in mortality between low-functioning ASD and high-functioning ASD, Reference Mouridsen, Bronnum-Hansen, Rich and Isager17 the current study showed increased mortality in mental and behavioural disorders, diseases of nervous, circulatory, respiratory and digestive systems, as well as congenital malformations in the low-functioning ASD group compared with the high-functioning ASD group. However, our results clearly indicated that mortality was elevated across a multiplicity of causes of death in ASD as a whole, including high-functioning ASD. Thus, our results add to the accumulating evidence indicating that ASD accounts for substantial health loss across the lifespan. Reference Baxter, Brugha, Erskine, Scheurer, Vos and Scott30
Suicide was the only specific cause of death showing a higher risk in high-functioning ASD compared with low-functioning ASD. High-functioning ASD often presents with co-existing psychiatric disorders. Reference Caamano, Boada, Merchan-Naranjo, Moreno, Llorente and Moreno9–Reference Skokauskas and Gallagher13 In a recent study, Reference Cassidy, Bradley, Robinson, Allison, McHugh and Baron-Cohen31 high prevalence of suicidal ideation and suicide attempts was reported among individuals with Asperger syndrome. Suicidality was increased in, but not limited to, individuals with Asperger syndrome and a history of depression. Reference Cassidy, Bradley, Robinson, Allison, McHugh and Baron-Cohen31 In addition to psychiatric comorbidity, individuals with high-functioning ASD may have psychological vulnerability, such as social disengagement, which may increase the risk of suicide. Reference Fowler32 Analysis of moderators and mediators of suicidal behaviours in individuals with ASD is an important area of future research, and should not only focus on risk factors but also resilience. Individuals with ASD may lack many of the protective factors that could decrease the risk of suicide, such as a supportive social network, Reference Fowler32 good coping skills Reference Oquendo, Dragatsi, Harkavy-Friedman, Dervic, Currier and Burke33 and overall life satisfaction. Reference Chioqueta and Stiles34 The risk of suicide may also be reduced by therapeutic and supportive contacts. Reference Fowler32 However, in individuals with ASD, difficulties in social interaction and communication (i.e. core symptoms of ASD) may seriously reduce the ability to seek and receive help and treatment. This may not only apply to help regarding psychological well-being but also somatic illness.
A large Swedish nationwide register-based study Reference Bjorkenstam, Ljung, Burstrom, Mittendorfer-Rutz, Hallqvist and Weitoft35 has indicated lower levels of somatic healthcare quality for psychiatric patients than for the general population. Thus, higher avoidable mortality Reference Rutstein, Berenberg, Chalmers, Child, Fishman and Perrin36 in psychiatric patients suggests that the medical care for physical disorders provided to psychiatric patients is less effective than in the general population. A recent systematic review Reference Baxter, Brugha, Erskine, Scheurer, Vos and Scott30 stressed the public health and policy implications of the substantial burden of ASD across the lifespan. Given that most individuals living with ASD today are adults, the support and interventions need to extend beyond paediatric and early education. Similarly, our findings may indicate a continuous need for improvement in public health and medical care for individuals with ASD. For instance, on average, age at initial diagnosis in the current sample was rather high in both low-functioning ASD and high-functioning ASD groups, and the time interval between ASD diagnosis and death was relatively short (3–5 years for overall mortality, but under 3 years regarding suicide as cause of death). However, the present study is unable to differentiate whether increased mortality in ASD is because of shortcomings in care provision, increased general biological vulnerability, or both.
Previous studies have reported higher mortality in females with ASD than males with ASD. Reference Gillberg, Billstedt, Sundh and Gillberg15–Reference Bilder, Botts, Smith, Pimentel, Farley and Viskochil20 Our results were in line with the previous studies in the low-functioning ASD group. However, we also observed opposite gender-specific mortality risk patterns among individuals with low-functioning ASD compared with high-functioning ASD. Among individuals with high-functioning ASD, females had a somewhat lower mortality risk than males. However, in the low-functioning ASD group, females had a higher risk than males. Thus, in the entire ASD group, females with low-functioning ASD seemed to be an especially vulnerable group in which the mortality risk was nine times higher than in the general population control group.
Strengths and limitations
The strengths of this population-based register study include the large study population with high statistical power and the good validity of Swedish registers. Reference Idring, Rai, Dal, Dalman, Sturm and Zander22 Consequently, we had the opportunity to analyse mortality even in less frequent causes of death for both low-functioning ASD and high-functioning ASD, as well as in both genders. Weaknesses of the present study include exclusive reliance on the National Patient Register for case ascertainment, leading to a selected and perhaps severely affected sample by only including individuals with ASD who had been in contact with clinical psychiatry services. This selection bias may be particularly relevant for individuals diagnosed before the year 2001 (i.e. before contact with out-patient psychiatric care services was included in the National Patient Register). However, after the year 2001 (88.2% in current data) all individuals having received an ASD diagnosis are registered in the National Patient Register because of the diagnostic assessment per se, i.e. also in cases with no further contact with psychiatric services. Another limitation is that this study did not in detail examine comorbidity for other ICD diagnoses than developmental intellectual disability. Future studies may highlight the likely possibility of psychiatric comorbidity moderating or mediating mortality in ASD. Finally, the generalisability of the present study is limited by the fact that healthcare is organised differently in different countries. Our results may not be fully generalisable for countries with very different healthcare systems.
Clinical implications
Our observation of excess cause-specific mortality in individuals with ASD may signify a generally increased biological vulnerability in ASD, as well as insufficient awareness, diagnoses and treatment of comorbid diseases within the healthcare system. As the mortality risk was increased for a number of different causes of death, a better knowledge of ASD appears to be desirable in all medical specialties. Health- and lifestyle-related issues may be a future focus for interventions directed at individuals with ASD and their significant others. Moreover, individuals with ASD may need support in communicating their symptoms and developing their skills in seeking help and treatment for problems involving psychological well-being or somatic health.
In summary, we observed markedly increased premature mortality in ASD owing to a multitude of medical conditions. The risk was particularly high for females with low-functioning ASD. However, individuals with high-functioning ASD had a high risk for suicide. Adequate and coordinated medical care for individuals with ASD and research into the phenomenon should be a target for a considerably broader audience of medical specialties than psychiatry and neurology.
Funding
The study was funded by the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet (grants no. 20120419 and 20130630, Tatja Hirvikoski), Karolinska Institutet in cooperation with the Stockholm County Council (KI/SLL, 2008:9, Sven Bölte), the Swedish Research Council (grant no. 523-2009-7054, Sven Bölte) and the Swedish Research Council in cooperation with the Swedish Research Council for Health, Working Life and Welfare, Vinnova and FORMAS (cross-disciplinary research programme concerning children's and youth's mental health, grant no. 259-2012-24, Sven Bölte).
Acknowledgements
We express our gratitude to psychiatrist Berit Lagerheim for providing us with an overview of Swedish clinical practice from 1980s onwards in diagnostic assessment of ASD and other neurodevelopmental disorders. We would also thank MD/Professor in psychiatry Jussi Jokinen for information on clinical aspects of ICD causes of death codes registration.
Appendix
ICD-10 chapters and codes used as a basis for categorisation of causes of death in current study
Chapter I: Infections (ICD codes A00-B99)
Chapter II: Neoplasms (C00-D48)
Chapter IV: Endocrine, nutritional and metabolic diseases (E00-E99)
Chapter V: Mental and behavioural disorders (F00-F99)
Chapter VI: Diseases of the nervous system (G00-G99)
Chapter IX: Diseases of the circulatory system (I00-I99)
Chapter X: Diseases of the respiratory system (J00-J99)
Chapter XI: Diseases of the digestive system (K00-K99)
Chapter XIV: Diseases of the genitourinary system (N00-N99)
Chapter XVII: Congenital malformations (Q00-Q99)
Chapter XVIII: Symptoms, signs, and abnormal findings not elsewhere classified (R00-R99)
Chapter XX: External causes of morbidity and mortality (V01-Y98)
Other causes of death: In this category, we included chapters with only a few cases chapter III (D50-D89, diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism); chapter VII (H00-H59, diseases of the eye and adnexa); chapter VIII (H60-H95, diseases of the ear and mastoid process); chapter XII (L00-L99, diseases of the skin and subcutaneous tissue); chapter XIII (M00-M99, diseases of the musculoskeletal system and connective tissue); chapter XV (O00-O99, pregnancy, childbirth and/or obstetric causes); chapter XVI (P00-P96, certain conditions originating in the perinatal period).








eLetters
No eLetters have been published for this article.