People with autistic-spectrum disorder display pervasive abnormalities in socio-emotional communication and stereotyped and obsessional behaviour. Reference Wing1,Reference Gillberg2 These clinical symptoms have a profound impact on daily life as well as social and economic outcome, with estimated societal costs in the UK exceeding £1 billion per year. Reference Jarbrink and Knapp3 However, the neurobiological determinants of behavioural abnormalities in autistic-spectrum disorder are poorly understood.
Problems with self- and socially motivated behaviour and social interaction are thought to result from a lack of perceived reward feedback in people with autistic-spectrum disorder. Reference Garretson, Fein and Waterhouse4,Reference Dawson, Osterling, Rinaldi, Carver and McPartland5 However, activation of the brain's frontostriatal and frontolimbic reward system induced by reward feedback has not been investigated in autistic-spectrum disorder, and differences in social interaction have not been related to brain response with regard to reward. In vivo structural imaging studies have shown that the anatomy of the frontostriatal limbic system is abnormal in autistic-spectrum disorder. Reference Courchesne and Pierce6–Reference Piven, Berthier, Starkstein, Nehme, Pearlson and Folstein10 Generalised impairments in stimulus–reward associations have also been found in children Reference Garretson, Fein and Waterhouse4,Reference Dawson, Osterling, Rinaldi, Carver and McPartland5 and adults with autism. Reference Schultz, Gauthier, Klin, Fulbright, Anderson, Volkmar, Skudlarski, Lacadie, Cohen and Gore11 However, nobody has examined brain response to reward in autistic-spectrum disorder using functional imaging.
Thus, we investigated neural substrates of reward feedback in the context of a sustained attention task with monetary reward in adults with autistic-spectrum disorder and matched control individuals using rapid, mixed-trial event-related functional magnetic resonance imaging (fMRI). We hypothesised that compared with controls, individuals with autistic-spectrum disorder would show activation differences in frontostriatal limbic brain areas when achieving monetary reward, and that brain areas which were functionally different would be anatomically abnormal. Also, based on prior findings of Schultz et al, Reference Schultz, Gauthier, Klin, Fulbright, Anderson, Volkmar, Skudlarski, Lacadie, Cohen and Gore11 we hypothesised that differences in brain activation during reward feedback might be related to clinical symptoms as measured by the Autism Diagnostic Interview. Reference Lord, Rutter and Le Couteur12
Methods
Participants
We studied ten healthy men (controls) and ten right-handed adult men of normal IQ with autistic-spectrum disorder (seven with Asperger syndrome and three with high-functioning autism). Control participants were recruited by local advertisement. Individuals with autism were recruited through the Institute of Psychiatry at the Maudsley Hospital, London. All participants gave written informed consent as approved by the local research ethics committee (Institute of Psychiatry, South London and Maudsley Trust). Individuals were between 20 and 50 years of age at time of inclusion and did not differ significantly in age, socio-economic status or IQ (see online Table DS1). Asperger syndrome and high-functioning autism were diagnosed by a consultant psychiatrist (D.M.), using ICD–10 criteria. 13 In addition, where parental informants were available, the Autism Diagnostic Interview Reference Lord, Rutter and Le Couteur12 was carried out (this was possible in eight out of ten individuals with autistic-spectrum disorder).
All participants underwent a structured clinical examination, including eyesight, neurological examination for handedness (questionnaire) and routine blood tests, to exclude comorbid medical and psychiatric disorders (e.g. epilepsy, tuberous sclerosis and/or psychosis) and biochemical, haematological or chromosomal abnormalities (e.g. fragile-X syndrome) possibly affecting brain function. None of the participants had a history of major medical illness or psychiatric disorder other than autistic-spectrum disorder. The revised short form Wechsler Adult Intelligence Scale 13 was used to measure IQ. None of the participants was taking medication at the time of testing.
Neuroimaging
All participants were familiarised with the task and scanning procedure before MRI scanning. Scanning took place at the Neuroimaging Unit of the Institute of Psychiatry, London, using a 1.5 T GE Signa System (General Electric, Milwaukee, Wisconsin, USA). All anatomical and functional images were acquired in the same session. During the 6 min fMRI, 208 event-related functional images with a repetition time (TR) of 1.8 s were acquired using a T2* weighted gradient echo, echo planar imaging sequence, sensitive to blood oxygen level dependent (BOLD) contrast (TR=1.8 ms, echo time TE=40 ms, flip angle 90°, matrix: 64 × 64, field of view FOV=240 mm, 12 slices, slice thickness 7 mm (0.7 mm gap), 3.15 mm in-plane resolution). To allow equilibrium to reach steady state, four echo planar imaging volumes corresponding to 8 s were introduced before each sequence and discarded from analysis. In the same scanning session, structural volumetric images (using axial spoiled gradient recall acquisition in steady state) were acquired with full head coverage, 124 contiguous slices (1.5 mm thick with 0.89 × 0.89 mm in-plane resolution), a 256 × 256 × 124 matrix and a TR/TE time ratio of 24/5 ms (flip angle 45°, FOV=24 cm) and a quadrature (birdcage) head coil for radiofrequency transmission and reception. Consistent image quality was ensured by a semi-automated quality control procedure.
During event-related fMRI acquisition, visual images of the experimental paradigm continuous performance test (CPT) were projected into the bore of the magnet using an active matrix video projector (model LC-XIP1999 EGA-mode, 70 Hz refresh rate, Eiki, Japan) and presented on a screen viewed via an integrated periscope assembly. For response, the right button on a button box connected to an Intel 3 PC running Visual-Basic software for stimulus presentation was used by the participants. All responses were recorded in real time.
A rapid, mixed-trial, randomised presentation design was used. Inter-trial intervals were randomly jittered between 800 ms and 1000 ms, and the appearance of target events was randomised to optimise statistical efficacy. It has been shown that both jittering of the inter-trial intervals and randomisation of stimulus type reduces the response overlap distortions and therefore improves the efficiency of fast event-related fMRI designs. Reference Crawford, Mychalkiw, Johnson and Moore14,Reference Burock, Buckner, Woldorff, Rosen and Dale15
Experimental paradigm
The computerised fMRI-compatible CPT with monetary incentive, taken from the Maudsley Attention and Response Suppression task battery was used. Reference Dale16 All participants received standardised instructions for the task.
The CPT task consisted of a letter stream of 418 stimuli, each of 300 ms presentation time, with a gap of 400 ms (total intra-trial interval time 900 ms). The letter stream included presentation of eaach of the target stimuli (the letters O and X) 24 times. One of these target letters (X or O) would be linked to monetary reward. Participants were told before the experiment whether the letter X or O was linked to the reward (this was varied randomly). Money could be earned by pressing the right button on a button box as response to the target stimuli. Two rising score bars – one yellow, the other blue – were displayed on the right-hand side of the screen. The amount of money earned during the task would be continuously shown by the yellow bar. The blue bar would rise when the other target letter was correctly identified, but this would not receive monetary reward as a feedback (see online Fig. DS1). The participants were asked to respond always to both target stimuli (X and O). The monetary reward was about 30 p for each correct response. For 100% correct responses £8 could be earned (at the time £1 was equivalent to $1.85). All participants were shown the amount of £8 (in £1 coins) prior to scanning. The target stimuli X and O were interspersed with at least six non-target stimuli randomly selected from the letters A–N. At least 5.4 s and at the most 9 s separated the target stimuli. Reaction time to target stimuli was recorded via a computerised response file linked to the response keypad. For each individual mean reaction time, omission errors to target stimuli, and commission errors (responses to non-target stimuli) were recorded. Each person received the total of £8 after completion of the task, regardless of their performance.
Imaging analysis
All event-related fMRI data were processed using SPM2 (Wellcome Department of Imaging Neuroscience, London, http://www.fil.ion.ucl.ac.uk/spm) modified for event-related designs. The functional scans were corrected for participants' head motion by realignment and co-registration using a rigid body transformation and sinc-interpolation (mean intra-participant head motion was below 3 mm translation and 2° rotation). They were then normalised using the same transformation matrix as the anatomical images, and smoothed with a 10 mm full-width half-maximum Gaussian-kernel. Statistical parametric maps were calculated for all data using a general linear model, with separate haemodynamic response functions, modelling the events of the functional task (estimated model: target events contrasted with non-target events, adjusted for target stimuli position in paradigm). The estimated model, a within-participant design implemented in the general linear model, resulted in an SPM(f) map per person. The significantly activated brain regions were obtained for each person, reflecting brain activation during response to the monetary reward-related target stimuli (e.g. X v. O) by using a linear contrast of regression coefficient at an individual (within participant) level. To test for regionally specific task effects, group activation maps (for the group of individuals with autistic-spectrum disorder and the control group, separately) were created using a threshold of P<0.001, uncorrected, SPM(t).
To test for group differences per task, group by task interactions were calculated, using one-way analysis of variance (ANOVA) against the null hypothesis of zero event-related activation differences between the two groups. SPM2 contrasts between −1 and 1 were calculated to estimate voxel values per group/task. The set of voxel values for each group comparison was thresholded and corrected for multiple comparisons at P<0.05 and using family-wise error correction. Furthermore, only those voxels were accepted as significant that belonged to a cluster of at least 10 significantly activated neighbouring voxels (minimum cluster size 10 voxels, extended height threshold of P<0.0001, surviving correction for multiple comparisons at P<0.05). Voxels and clusters were localised using the Montreal Neurological Institute coordinates and transformed into Talairach and Tournoux coordinates: Reference Talairach and Tournoux18 where possible, Brodmann areas were classified. Reference Brodmann19,Reference Brett, Johnsrude and Owen20
Signal intensity values of anterior cingulate cortex cluster (voxels (n=92)) surviving the correction of multiple comparisons (P<0.05, corrected) of the group by task interactions were extracted from Matlab (Matlab, The MathWorks, Inc., Natick, Massachusetts, USA) and transferred into SPSS (SPSS 11.1 for Windows, SPSS, Inc., Chicago, Illinois, USA). Using nonparametric statistics (Spearman's rho), significantly different voxel values and ADI scores were correlated.
Structural MRI analysis
Optimised voxel-based morphometry analysis
We used optimised voxel-based morphometry implemented in SPM to identify regional differences in white and grey matter concentration (density) of individuals with autistic-spectrum disorder compared with control individuals. Optimised voxel-based morphometry techniques, including template creation, spatial normalisation, tissue segmentation and smoothing, Reference Ashburner and Friston21 were employed. For statistical comparison, grey and white matter segments were smoothed with a 10 mm full-width half-maximum isotropic Gaussian kernel. Regional grey and white matter differences between participants with autistic-spectrum disorder and controls were assessed using t-statistics. Student t-tests were carried out, investigating group differences on a voxel-by-voxel basis for grey and white matter segments of individuals with autistic-spectrum disorder compared with control individuals (n=20). T-tests for group comparisons were thresholded at P<0.05, corrected for multiple comparisons, with a minimal cluster size (cluster extend threshold at P<0.0001) of 50 voxels.
Results
Behavioural data
During the rewarded CPT task, there was no within-group effect on reaction time differences between rewarded and non-rewarded stimuli. The amount of omission or commission errors did not differ significantly between groups.
Imaging data
For reward achievement (contrast of successful rewarded–successful non-rewarded stimuli), control individuals significantly activated (P<0.001, uncorrected) the right insula and the anterior cingulate cortex and middle frontal gyrus, bilaterally (Table 1). Individuals with autistic-spectrum disorder significantly activated (P<0.001, uncorrected) the left anterior cingulate cortex and left middle and superior frontal gyrus and the right superior parietal lobe (Table 1).
Table 1 Functional activation during correct responses to target stimuli
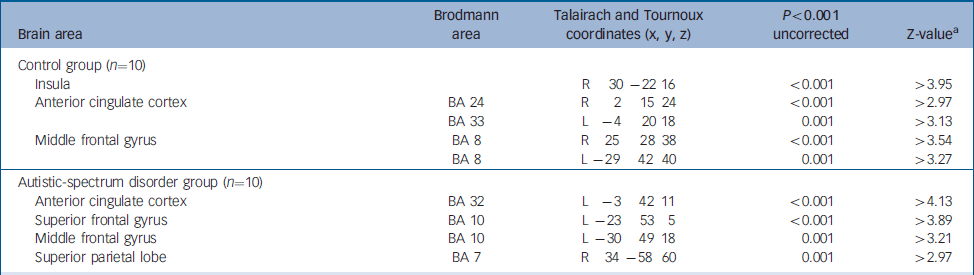
| Brain area | Brodmann area | Talairach and Tournoux coordinates (x, y, z) | P<0.001 uncorrected | Z-valuea |
|---|---|---|---|---|
| Control group (n=10) | ||||
| Insula | R 30 -22 16 | <0.001 | >3.95 | |
| Anterior cingulate cortex | BA 24 | R 2 15 24 | <0.001 | >2.97 |
| BA 33 | L -4 20 18 | 0.001 | >3.13 | |
| Middle frontal gyrus | BA 8 | R 25 28 38 | <0.001 | >3.54 |
| BA 8 | L -29 42 40 | 0.001 | >3.27 | |
| Autistic-spectrum disorder group (n=10) | ||||
| Anterior cingulate cortex | BA 32 | L -3 42 11 | <0.001 | >4.13 |
| Superior frontal gyrus | BA 10 | L -23 53 5 | <0.001 | >3.89 |
| Middle frontal gyrus | BA 10 | L -30 49 18 | 0.001 | >3.21 |
| Superior parietal lobe | BA 7 | R 34 -58 60 | 0.001 | >2.97 |
For reward achievement in a group comparison (contrast of rewarded–non-rewarded stimuli, and individuals with autistic-spectrum disorder compared with control individuals), participants with autism showed significantly increased brain activation in the left anterior cingulate gyrus (Fig. 1). Increased activation was also found in the left middle frontal gyrus.
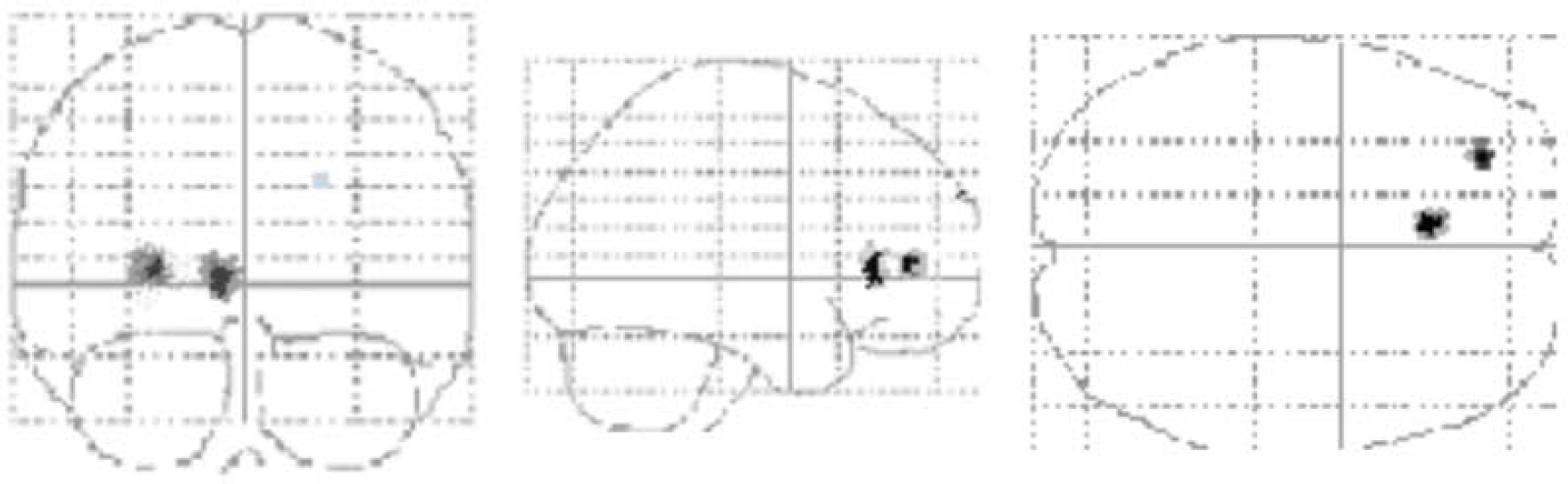
Fig. 1 Functional activation differences in individuals with autistic-spectrum disorder (n 10) compared with control individuals (n 10). Significantly (P<0.05, corrected for multiple comparisons) increased functional activation to target stimuli in individuals with autistic-spectrum disorder, compared with control individuals in the left anterior cingulate gyrus (Brodmann area 32; Talairach & Tournoux coordinates −6, 32, 26; z=4.65). Significantly (uncorrected P<0.001) increased functional activation of the same comparison also in the left middle frontal gyrus (Brodmann area 10; Talarach & Tournoux coordinates −23, 44, −10).
Group by task effect correlation with Autism Diagnostic Interview scores
The relative voxel values of significantly (P<0.05 corrected) increased activation of the left anterior cingulate gyrus correlated (P<0.002, two-tailed, correlation coefficient 0.780, Spearman's rho, using Holms-Bonferroni correction) with individual scores on ADI domain A (qualitative abnormalities in reciprocal social interaction) in individuals with autistic spectrum disorder (Fig. 2). Domain B (qualitative abnormalities in communication) and domain C (repetitive behaviour) did not correlate significantly with measures of brain activation.
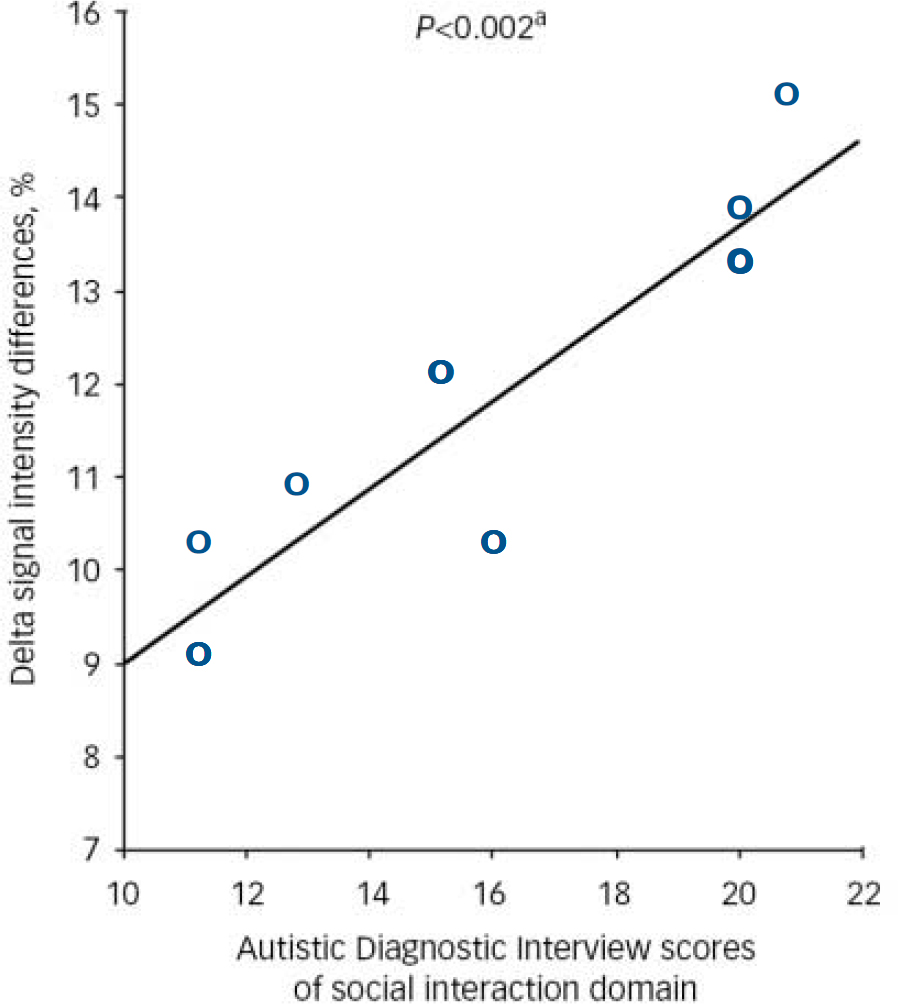
Fig. 2 Significant (P<0.002) correlation of social interaction with signal intensity increases in the anterior cingulate gyrus in individuals with autistic spectrum disorder. a. Significant at P<0.001; two-tailed (Spearman's ρ=0.879, Pearson's correlation=0.902).
Structural volumetric data
Compared with control participants, individuals with autistic-spectrum disorder had significantly decreased peri-ventricular white matter volume (P<0.05, corrected) of the left frontal lobe (Talairach and Tournoux coordinates [x, y, z]: −8, 31, 3). No significantly increased white matter density and no significant differences in grey matter density were found between participant groups (see online Fig. DS2).
Discussion
We investigated functional brain activation during reward achievement in people with autistic-spectrum disorder and matched control individuals. Reward achievement (correct responses to target stimuli accompanied by a monetary reward feedback compared with responses to non-rewarded target stimuli) elicited task-relevant brain activation in both groups of participants, mainly in the middle and superior frontal cortices, anterior cingulate gyrus, insula and superior parietal lobes. Control individuals activated a network of brain areas encompassing bilateral anterior cingulate and frontal cortices, and the right insula. Individuals with autism activated a left hemispheric network encompassing the left anterior cingulate, middle and superior frontal gyrus, and the right parietal lobe. In a direct comparison with control participants, individuals with autism showed significantly greater activation of the left anterior cingulate gyrus during reward achievement. Increased brain activation correlated with clinical abnormalities in social interaction in autistic-spectrum disorder (assessed using the Autism Diagnostic Interview Reference Lord, Rutter and Le Couteur12 ). Also, people with autistic-spectrum disorder had a significantly reduced peri-ventricular white matter density in the left frontal lobe.
Reward feedback in the context of cognitive tasks is mediated by frontostriatal and frontolimbic connections and in particular by paralimbic brain regions that lie at the interface between emotion and cognition, such as the anterior cingulate gyrus. Reference Pochon, Levy, Fossati, Lehericy, Poline, Pillon, Le Bihan and Dubois22 The anterior cingulate cortex is thought to act as central executer and coordinator for predominantly right-hemispheric neural networks, maintaining arousal and alertness, while receiving input from prefrontal and parietal brain areas via the corpus callosum. Reference Mottaghy, Willmes, Horwitz, Mueller, Krause and Sturm23 The anterior cingulate cortex is a brain region strategically placed at the interface between motivation and cognition, and therefore thought to play an important role in higher cognitive functions, such as selective and executive attention and conflict detection, as well as being involved in motivation and arousal. Reference Bush, Luu and Posner24
During reward achievement, people with autistic-spectrum disorder show increased activation of the rostral part of the anterior cingulate cortex. This area is thought to be important for performance monitoring based on reward-feedback. Reference Bloom and Hynd25,Reference Rogers, Ramnani, Mackay, Wilson, Jezzard, Carter and Smith26 Meta-analyses suggest that based on reward feedback, rostral areas of the anterior cingulate cortex are responsible for more cognitive aspects of error detection and risk assessment, while caudal, subgenual regions are mediating emotional functions. Reference Bush, Luu and Posner24
Using fMRI in healthy adults, Kirsch et al demonstrated that the anterior cingulate cortex facilitates selective attention towards highly motivational stimuli. Reference Kirsch, Schienle, Stark, Sammer, Blecker, Walter, Ott, Burkart and Vaitl27 Increased pregenual anterior cingulate cortex activation has been reported for the anticipation of monetary gains, whereas subcallosal anterior cingulate cortex activation was increased during winning compared with losing. Reference Rogers, Ramnani, Mackay, Wilson, Jezzard, Carter and Smith26 Clinical studies provide evidence that increased anterior cingulate cortex activation is associated with obsessive–compulsive symptoms and abnormal social behaviour, whereas reduced anterior cingulate cortex activation is correlated with social bluntness, lack of self-initiated behaviour and depression. Reference Ohnishi, Matsuda, Hashimoto, Kunihiro, Nishikawa, Uema and Sasaki28
In autistic-spectrum disorder, increased anterior cingulate cortex activation is a novel finding. However, reduced anterior cingulate cortex activation has been observed in tasks of social emotion (‘theory of mind’) Reference Happe, Ehlers, Fletcher, Frith, Johansson, Gillberg, Dolan, Frackowiak and Frith29 and spatial working memory. Reference Luna, Minshew, Garver, Lazar, Thulborn, Eddy and Sweeney30 Ohnishi et al Reference Ohnishi, Matsuda, Hashimoto, Kunihiro, Nishikawa, Uema and Sasaki28 reported a positive correlation between reduced anterior cingulate cortex cerebral blood flow and deficits in theory of mind tasks. In addition, increased anterior cingulate cortex grey matter volume has been reported in people with autistic-spectrum disorder. Reference Waiter, Williams, Murray, Gilchrist, Perrett and Whiten31 Increased grey matter volume could be an indicator for differences in apoptosis and neuronal overgrowth, possibly influencing cognitive or motivational performance. Furthermore, the anterior cingulate cortex is involved in the intuitive assessment of complex situations (as mediated by Von Economo neurons), Reference Allman, Watson, Tetreault and Hakeem32 an ability highly deficient in autistic-spectrum disorder. Murphy et al Reference Murphy, Daly, Schmitz, Toal, Murphy, Curran, Erlandsson, Eersels, Kerwin, Ell and Travis33 reported that reduced 5-HT2A receptor binding in the anterior cingulate cortex (and posterior cingulate cortex) correlated with the degree of abnormal social behaviour in people with autistic-spectrum disorder. Further, Haznedar et al Reference Haznedar, Buchsbaum, Hazlett, LiCalzi, Cartwright and Hollander34,Reference Haznedar, Buchsbaum, Metzger, Solimando, Spiegel-Cohen and Hollander35 reported a significant correlation between reduced glucose metabolism of the anterior and posterior cingulate gyrus, and qualitative social interaction in autistic-spectrum disorder. The authors suggested that abnormalities in the metabolism of the anterior cingulate cortex (and frontotemporal regions) underpin deficits in social interaction (and social learning). Our finding of a positive correlation between increased anterior cingulate cortex activation and abnormalities in social interaction are in line with these previous findings. In addition, our findings of increased functional activation of the anterior cingulate cortex during reward achievement in autistic-spectrum disorder are in line with the evidence for anatomical abnormalities in this brain region. Increased activation of the anterior cingulate cortex when performing a task well may reflect an increased need for feedback-related performance monitoring in autistic-spectrum disorder. Alternative interpretations might also suggest increased arousal or enhanced attention to rewarded stimuli. Since the more cognitive part of the rostral anterior cingulate cortex showed increased activation during reward achievements in our sample of individuals with autistic-spectrum disorder, it might reflect increased effort to achieve a desired outcome by actively choosing a goal-directed behaviour with immediate return.
Another explanation for the increased anterior cingulate cortex activation could be that monetary reward is intrinsically a greater incentive for individuals with autistic-spectrum disorder than for people without autism because, although money can be seen as a social reward, through operant learning it has also been strongly associated with primary reinforcers such as food. Reference Delgado, Labouliere and Phelps36
We did not find that areas which differed functionally in participants with autistic-spectrum disorder were also anatomically abnormal. Nevertheless, a left hemispheric reduction of frontal white matter density in autistic-spectrum disorder could be an indicator for disrupted inter- and intra-hemispheric transfer – possibly demanding increased neuronal recruitment of frontal areas. The left hemispheric functional anterior cingulate cortex abnormalities that we observed and reduced frontal white matter density in our autistic-spectrum disorder group, are in line with emerging evidence for left hemispheric functional and structural abnormalities in the disorder. Reference McAlonan, Cheung, Cheung, Suckling, Lam, Tai, Yip, Murphy and Chua8,Reference Waiter, Williams, Murray, Gilchrist, Perrett and Whiten31,Reference Chung, Dalton, Alexander and Davidson37 We also earlier observed functional differences in left prefrontal and paralimbic brain regions in adults with autistic-spectrum disorder during cognitive tasks and corresponding grey matter abnormalities in homologue frontal cortex areas. Reference Schmitz, Rubia, Daly, Smith, Williams and Murphy38
Reward achievement, social interaction and frontal lobe maturation
Our combined findings of increased left anterior cingulate cortex activation during reward achievement and reduced left frontal white matter density in individuals with autistic-spectrum disorder compared with control individuals suggest that left frontal lobe white matter mal-development may affect reward-related brain activation. Increased anterior cingulate cortex activation could be a compensatory mechanism for dysfunctional communication and abnormal frontal white matter connectivity in individuals with autistic-spectrum disorder. Reference Courchesne and Pierce6 The left hemisphere normally develops later than the right, and frontostriatal connections are only established relatively late in adolescence. Reference Sowell, Thompson, Holmes, Jernigan and Toga39,Reference Paus, Koski, Caramanos and Westbury40 Thus, neurodevelopmental delay in autistic-spectrum disorder may have an impact on the left hemisphere in particular and consequently explain some of the developmental abnormalities, including social interaction deficits, found in the disorder. We demonstrated a link between social interaction deficits and reward-related left hemispheric brain activation. Reward achievements, like other cognitive behavioural abnormalities such as socio-emotional intelligence and theory of mind, Reference Happe, Ehlers, Fletcher, Frith, Johansson, Gillberg, Dolan, Frackowiak and Frith29,Reference Baron-Cohen, Ring, Wheelwright, Bullmore, Brammer, Simmons and Williams41 seem predominantly mediated by frontal left hemispheric structures, and require more frontal lobe brain activation in individuals with less social interaction abilities. Neurodevelopmental delay of the left hemisphere in autism could, therefore, influence brain activation patterns and behavioural outcome.
Study limitations
Our sample is small and only high-functioning adults with autistic-spectrum disorder were included. Our findings cannot, therefore, be applied to the wider spectrum of people with the disorder, for example, children or adults with ‘typical’ autism (i.e. those with intellectual disability and developmental language delay). The relationship between increased brain function during monetary gain and clinical symptoms, and the biological basis of this hyper-function, need to be clarified in future studies using larger sample. Furthermore, reward motivation in its entirety needs to be investigated further to pinpoint the exact motivational incentives which drive reward-related behaviour in individuals with and without autistic-spectrum disorder.
Acknowledgements
This study was supported by the Medical Research Council (MRC UK AIMS network).






eLetters
No eLetters have been published for this article.