Memory encoding has been implicated as the key failure point in schizophrenia, Reference Cirillo and Seidman1 with differential patterns of functional magnetic resonance imaging (fMRI) activation identified in the prefrontal cortex and medial temporal lobe in addition to the posterior cingulate and other midline areas. Reference Ragland, Laird, Ranganath, Blumenfeld, Gonzales and Glahn2,Reference Achim and Lepage3 Moreover, the memory deficits observed in schizophrenia have been consistently related to a poorer functional Reference Green4 and clinical Reference Bodnar, Harvey, Malla, Joober and Lepage5-Reference Toulopoulou and Murray8 outcome. Yet, no previous study has explicitly examined the functional relationship between memory encoding and outcome. A better understanding of this relationship could help improve our understanding of the pathophysiology of schizophrenia Reference McGuire, Howes, Stone and Fusar-Poli9,Reference Saykin, Gur, Gur, Mozley, Mozley and Resnick10 and aid in the development of newer, target-specific treatments. Reference Toulopoulou and Murray8,Reference McGuire, Howes, Stone and Fusar-Poli9,Reference Hyman and Fenton11,Reference Ranganath, Minzenberg and Ragland12
Using fMRI, we explored for activation differences among individuals with remitted and non-remitted first-episode schizophrenia (the remitted and non-remitted groups respectively) and healthy controls (the control group); remission was defined following the proposed 2005 consensus criteria. Reference Andreasen, Carpenter, Kane, Lasser, Marder and Weinberger13 The fMRI paradigm used is sensitive to detecting activity in the prefrontal cortex, medial temporal lobe and midline posterior regions using three different contrasts: (a) associative v. item-oriented encoding strategy, (b) encoding semantically unrelated v. related image pairs, and (c) successful v. unsuccessful memory encoding. Reference Achim, Bertrand, Montoya, Malla and Lepage14,Reference Achim, Bertrand, Sutton, Montoya, Czechowska and Malla15 Previous work from our laboratory identified verbal memory deficits Reference Bodnar, Harvey, Malla, Joober and Lepage5,Reference Bodnar, Malla, Joober and Lepage6 and reduced grey matter in the medial temporal lobe bilaterally (parahippocampal gyrus Reference Bodnar, Harvey, Malla, Joober and Lepage5 and hippocampus tail Reference Bodnar, Malla, Czechowska, Benoit, Fathalli and Joober16 ) as markers of a poor clinical outcome. Based on these results, together with our previous fMRI result that identified reduced medial temporal lobe activation in participants with a first-episode of psychosis using the semantic relatedness contrast, Reference Achim, Bertrand, Sutton, Montoya, Czechowska and Malla15 we hypothesised that the non-remitted group, compared with the remitted and control groups, would show a selective deactivation in the medial temporal lobe for this particular contrast. Furthermore, regarding the fMRI behavioural data, we hypothesised that the non-remitted group would display a poorer recognition memory compared with both the remitted and the control groups.
Method
Participants and treatment protocol
All the participants with schizophrenia were treated at the Douglas Mental Health University Institute in Montreal, Canada, at the Prevention and Early Intervention Program for Psychoses (PEPP) - a specialised service providing treatment to individuals aged 14-30 years from a local catchment area with either affective or non-affective psychosis. Individuals with an IQ higher than 70 who had not taken antipsychotic medication for more than 1 month were consecutively admitted as in- or out-patients. See Malla et al Reference Malla, Norman, McLean, Scholten and Townsend17 (or visit www.douglas.qc.ca/pages/view?section_id=165) for more details.
For the neuroimaging study, only individuals aged 18-30 years with no previous history of neurological disease or head trauma causing loss of consciousness were eligible. In all, 45 individuals with first-episode schizophrenia (treated January 2004 to December 2008) had usable fMRI data. Three individuals were subsequently removed because of: excessive movement during scanning (n = 1) and failure to follow encoding instructions (n = 2; fMRI behavioural results below chance level). The remaining 42 participants were separated into two groups, the 15 who achieved remission (35.7%) and the 27 who did not. Remission was defined as mild (3) or less on eight key symptoms of the Positive and Negative Syndrome Scale Reference Kay, Fiszbein and Opler18 (PANSS; delusions, conceptual disorganisation, hallucinatory behaviour, blunted affect, social withdrawal, lack of spontaneity and flow of conversation, mannerisms and posturing, and unusual thought content) maintained for 6 consecutive months (in our case, from month 6 to 12 after admission). Reference Andreasen, Carpenter, Kane, Lasser, Marder and Weinberger13
Diagnoses included schizophrenia (remitted group n = 12, non-remitted group n = 21), schizoaffective disorder (remitted group n = 2, non-remitted group n = 6) and schizophreniform disorder (remitted group n = 1) according to the Structured Clinical Interview for DSM-IV Reference First, Spitzer, Gibbon and Williams19 confirmed between two senior research psychiatrists (A.K.M. and R.J.). Thirty-one healthy controls were recruited through advertisements in local newspapers and were included only if they had no current or previous history of (a) any Axis I disorders, (b) any neurological diseases, (c) head trauma causing loss of consciousness, and (d) a first-degree family member with schizophrenia or related schizophrenia-spectrum psychosis. See the online supplement for a description of the sociodemographic and clinical data collected.
After a comprehensive description of the study, written informed consent was obtained from all participants. Research protocols were approved by the McGill University Faculty of Medicine review board.
fMRI task
Stimuli consisted of pairs of clipart images (representing common objects, food or animals) arranged side by side on a screen presented through a projector and mirror system. Each trial consisted of an encoding cue presented for 3 s, then a pair of images for 3 s, followed by a fixation cross lasting 1 to 4 s (times randomly mixed between trials). The cues oriented the participant to perform one of two different encoding tasks: half promoted an associative strategy (compare images and select the one that was bigger in real-life size) and half promoted a deep item-oriented strategy (examine images and answer if at least one image represented a living entity); the encoding strategy contrast. Each of the two fMRI encoding sessions comprised of 56 different pairs of images: 28 semantically related (e.g. band-aid and medical kit) and 28 semantically unrelated (e.g. t-shirt and lizard); the semantic relatedness contrast. Semantic relatedness (unrelated and related) and encoding strategy (associative and item-oriented) were mixed randomly. See online Fig. DS1 for a pictorial representation of the task.
Following encoding, and while still in the scanner, recognition memory was assessed in two sessions by presenting all of the previously encoded pairs (intact and not rearranged) along with an equal number of distracter, never-seen-before pairs (half semantically related and half unrelated), for a total of 112 pairs per session. Participants were asked to respond to each pair as either old or new. The answers to the old pairs were used to distinguish successful encoding (old pairs recognised as such) from unsuccessful encoding (old pairs recognised as new); the subsequent memory effect contrast. Participants were told prior to encoding that recognition memory would be subsequently tested. Full details are published elsewhere. Reference Achim, Bertrand, Montoya, Malla and Lepage14,Reference Achim, Bertrand, Sutton, Montoya, Czechowska and Malla15
fMRI data acquisition, processing and statistical analyses
Scanning was carried out at the Montreal Neurological Institute on a 1.5T Siemens whole-body MRI system. Functional T 2*-weighted echo-planar images were acquired with blood oxygenation level-dependent contrast (repetition time (TR) = 2130 ms, echo time (TE) = 50 ms, flip angle = 90°) covering the entire brain (25 interleaved slices parallel to the anterior-posterior commissural plane; voxel size 4×4×5 mm); 214 volumes were acquired in each session. See online supplement for information about the participants' medication at the time of the scan.
Functional data were analysed using SPM2 on a Windows-based workstation. For preprocessing, images were time corrected to account for differences in sampling times for different slices, motion corrected, spatially normalised (final voxel size 2×2×2 mm) and smoothed with an 8 mm full-width at half maximum (FWHM) Gaussian kernel. Low-frequency temporal drifts were removed by applying a high-pass filter. The movement correction logs (obtained from the realignment procedure) were examined to ensure none of the participants had movements greater than 5 mm or 5°; one participant in the non-remitted group met that threshold and was removed. Data were analysed by the general linear model, in which individual events were modelled by a canonical haemodynamic response function.
Two design matrices were created for each participant: one for the encoding strategy and semantic relatedness contrasts and one for the subsequent memory effect contrast. The design matrices were modelled using correct and incorrect trials from both encoding sessions (see below for exceptions) differing in event types modelled. For the first matrix, four event types were modelled: (a) associative encoding of related pairs, (b) associative encoding of unrelated pairs, (c) item-oriented encoding of related pairs, and (d) item-oriented encoding of unrelated pairs; the six movement parameters were included as covariates. Only one of the two encoding sessions was included for two individuals (one each in the remitted and non-remitted groups) because only one session was completed; one participant in the non-remitted group was removed because of technical problems in acquiring responses. This amounted to 26 individuals in the non-remitted group, 15 in the remitted group and 31 in the control group.
For the second matrix, two event types were modelled: (a) successful encoding and (b) unsuccessful encoding; the six movement parameters were included as covariates. Only one of the two encoding sessions was included for one person in the remitted group because only one session was completed; two participants (one each in the remitted and non-remitted groups) were removed as the recognition task was not completed. This amounted to 26 individuals in the non-remitted group, 14 in the remitted group and 31 in the control group.
In all, three fMRI contrasts - encoding strategy (associative > item-oriented), semantic relatedness (unrelated > related), and subsequent memory effect (successful > unsuccessful) - were compared between (a) remitted and non-remitted groups, (b) non-remitted and control groups, and (c) remitted and control groups. To correct for multiple comparisons, we applied a cluster extent threshold determined by Monte Carlo simulation using software written by Slotnick Reference Slotnick, Moo, Segal and Hart20,Reference Slotnick and Schacter21 employing the following parameters: matrix 64×64, slices 25, original voxel dimension 4×4×5, resampled voxel resolution 2×2×2, smoothing 8 mm, P-corrected = 0.01, P-voxel = 0.001. After 10 000 iterations, an extent threshold of 100 contiguous voxels was determined; this procedure prevented a false-positive rate above 1% due to multiple testing. Cluster-based thresholding is an alternative to the voxel-based correction and is often more sensitive to activation when one can reasonably expect multiple contiguous activated voxels. Reference Forman, Cohen, Fitzgerald, Eddy, Mintun and Noll22,Reference Petersson, Nichols, Poline and Holmes23 For significant clusters, the parameter estimate of each voxel was extracted using EasyROI, a SPM2 utility created by Pernet (www.sbirc.ed.ac.uk/cyril/cp_download.html), and averaged to provide an overall measure of activation for that cluster. Peak voxel coordinates were converted from Montreal Neurological Institute (MNI) to Talairach using a non-liner transformation (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach), with structures identified using Talairach Client (www.talairach.org/client.html).
For the behavioural data, performance means and response times (encoding and recognition separately) were explored using a MANOVA, with group membership (non-remitted, remitted, control) as the between-group factor and encoding strategy (associative, item-oriented) and semantic relatedness (unrelated pairs, related pairs) as the within-group factors. Encoding performance was calculated as the percentage of correct answers to the encoding questions. One individual in the healthy control group performed at chance level for item-oriented encoding and was excluded. During debriefing, this participant reported giving a positive response only when both items were alive (only one needed to be alive). Since the distinction between associative and deep item-oriented encoding was not compromised by this response pattern, this participant was included in the fMRI analyses.
Recognition performance was calculated as: hit rate (HR) = (hits + 0.5)/(number of items + 1) to evaluate the effect of encoding strategy on subsequent retrieval. Furthermore, to account for false alarms (FA), a discrimination index (Pr = HR - FA) and response bias (Br = (FA/(1 - Pr)) - 0.5) (subtraction of 0.5 allowed an absence of bias to correspond to a value of 0) were calculated according to the two-high threshold model. Reference Snodgrass and Corwin24 The discrimination index provides an unbiased estimate of memory accuracy; Br is an index of the overall tendency to respond ‘old’ or ‘new’ regardless of accuracy - positive values indicate a familiarity bias (i.e. a tendency to say old) and negative values indicate a novelty bias (i.e. a propensity to say new). For discrimination index and response bias, only semantic relatedness and group membership were included in the analyses, as encoding strategy (associative v. item) was only part of the encoding phase. All performance data were log transformed to achieve normality; response time data were normally distributed. All analyses were two-tailed with a critical P-value of 0.05 and performed using SPSS version 12 on a Windows-based workstation.
TABLE 1 Sociodemographic characteristics for the non-remitted and remitted first-episode schizophrenia groups and the control group

| Non-remitted group (n = 27) | Remitted group (n = 15) | Control group (n = 31) | Analysis | ||||
|---|---|---|---|---|---|---|---|
| F | χ 2 | d.f. | P | ||||
| Age at scan, years: mean (s.d.) | 23.7 (4.2) | 24.2 (3.0) | 24.7 (3.3) | 0.59 | 2,70 | 0.558 | |
| Parental socioeconomic status, a mean (s.d.) | 3.4 (1.2) | 3.3 (1.3) | 3.1 (1.0) | 1.51 | 2 | 0.470 | |
| Education level, b mean (s.d.) | 11.4 (2.2) | 12.6 (3.1) | 14.8 (2.1) | 14.94 | 2,70 | <0.001 | |
| Full-scale IQ, c mean (s.d.) | 96.9 (14.3) | 98.7 (15.7) | 109.0 (12.6) | 6.67 | 2,68 | 0.005 | |
| Male, n (%) | 21 (77.8) | 10 (66.7) | 17 (54.8) | 3.38 | 2 | 0.185 | |
| Right handed, n (%) | 20 (74.1) | 12 (80.0) | 29 (93.5) | 4.16 | 2 | 0.125 | |
a Hollingshead socioeconomic status, in which one is highest and five is lowest.
b Number of years completed. Tukey's honestly significant difference tests revealed: non-remitted group = remitted group (P = 0.284); non-remitted group < control group (P < 0.001); remitted group < control group (P = 0.012).
c Measured with the Wechsler Adult Intelligence Scale (WAIS-III); Reference Wechsler25 data were available for only 28 individuals in the control group. Tukey's honestly significant difference tests revealed: non-remitted group = remitted (P = 0.917); non-remitted group < control group (P = 0.006); remitted group = control group (P = 0.060).
Results
Sociodemographic and clinical
The three groups did not significantly differ in age, parental socioeconomic status, gender or handedness. The remitted and non-remitted groups had significantly fewer years of education and a lower full-scale IQ compared with the control group; outcome groups did not differ (Table 1). Outcome groups did not differ in positive or negative symptoms at first assessment but the remitted group showed significantly lower totals at month 6 and 12, as expected. Total antipsychotic dosage (in chlorpromazine equivalents) was similar at first assessment but was higher (non-significant) for the non-remitted group at months 6 and 12. At the time of the scan, the non-remitted group had significantly higher positive and negative symptom totals and were receiving a higher (non-significant) dosage of antipsychotic medication. Medication adherence did not differ at any time point (online Table DS1).
Behavioural fMRI results
There were significant (group semantic relatedness) interactions for encoding accuracy and for recognition hit-rate and response times; there were no other significant group-related interactions. Of note, for encoding accuracy, the remitted and control groups responded more accurately to related pairs than to unrelated pairs, whereas the non-remitted group displayed no such difference. Other analyses revealed that the remitted and non-remitted groups did not significantly differ in any instance but various between-group differences with the control group were present (Table 2). For discrimination index, the non-remitted group displayed poorer overall recognition memory compared with both the remitted and control groups. For response bias, it was revealed that all participants showed a stronger novelty bias (propensity to say ‘new’) when recognising related pairs of images over unrelated pairs of images (Table 3). See online supplement for a complete description of results.
fMRI results
For the semantic relatedness contrast (unrelated > related) (Table 4 and Fig. 1), the ‘remitted > non-remitted’ comparison identified reduced activation in the left posterior cingulate in the non-remitted group. However, the parameter estimates from this cluster revealed that the non-remitted group actually had positive activation when encoding related pairs over unrelated pairs (t 25 = 3.33, P = 0.003); in contrast, the remitted group had negative activation when encoding related pairs over unrelated pairs (t 14 = –3.32, P = 0.005).
TABLE 2 Performance and response times for memory encoding and recognition for non-remitted, remitted and control groups

| Associative strategy | Item-oriented strategy | |||
|---|---|---|---|---|
| Unrelated, mean (s.d.) | Related, mean (s.d.) | Unrelated, mean (s.d.) | Related, mean (s.d.) | |
| Encoding | ||||
| Accuracy a | ||||
| Non-remitted group b | 0.91 (0.07) | 0.91 (0.09) | 0.84 (0.12) | 0.88 (0.11) |
| Remitted group b | 0.86 (0.09) | 0.94 (0.07) | 0.79 (0.22) | 0.84 (0.22) |
| Control group b | 0.93 (0.07) | 0.96 (0.05) | 0.91 (0.08) | 0.96 (0.08) |
| Response time, ms | ||||
| Non-remitted group | 1508 (190) | 1510 (166) | 1504 (252) | 1387 (177) |
| Remitted group | 1545 (182) | 1599 (160) | 1485 (220) | 1435 (243) |
| Control group | 1423 (205) | 1436 (193) | 1351 (207) | 1248 (199) |
| Recognition | ||||
| Hit rate a | ||||
| Non-remitted group b | 0.73 (0.15) | 0.78 (0.15) | 0.65 (0.18) | 0.70 (0.19) |
| Remitted group b | 0.80 (0.11) | 0.85 (0.09) | 0.69 (0.15) | 0.74 (0.15) |
| Control group b | 0.85 (0.07) | 0.87 (0.06) | 0.79 (0.10) | 0.78 (0.09) |
| Response time, ms | ||||
| Non-remitted group | 1395 (267) | 1267 (271) | 1360 (265) | 1313 (286) |
| Remitted group | 1359 (318) | 1198 (237) | 1448 (255) | 1223 (301) |
| Control group | 1158 (191) | 1071 (157) | 1209 (194) | 1102 (184) |
a Data are percentages of correct answers, presented as a proportion of the total possible.
b The means and standard deviations represent the raw data (i.e. before correction for the non-normal distribution) and should be interpreted with caution.
TABLE 3 Discrimination index and response bias for non-remitted, remitted and control groups

| Discrimination index | Response bias | |||
|---|---|---|---|---|
| Performance a | Unrelated, mean (s.d.) | Related, mean (s.d.) | Unrelated, mean (s.d.) | Related, mean (s.d.) |
| Non-remitted group b | 0.56 (0.23) | 0.63 (0.26) | –0.23 (0.18) | –0.29 (0.25) |
| Remitted group b | 0.68 (0.13) | 0.76 (0.13) | –0.28 (0.20) | –0.38 (0.17) |
| Control group b | 0.75 (0.10) | 0.78 (0.08) | –0.21 (0.18) | –0.32 (0.16) |
a Data are percentages of correct answers, presented as a proportion of the total possible.
b The means and standard deviations represent the raw data (i.e. before correction for the non-normal distribution) and should be interpreted with caution.
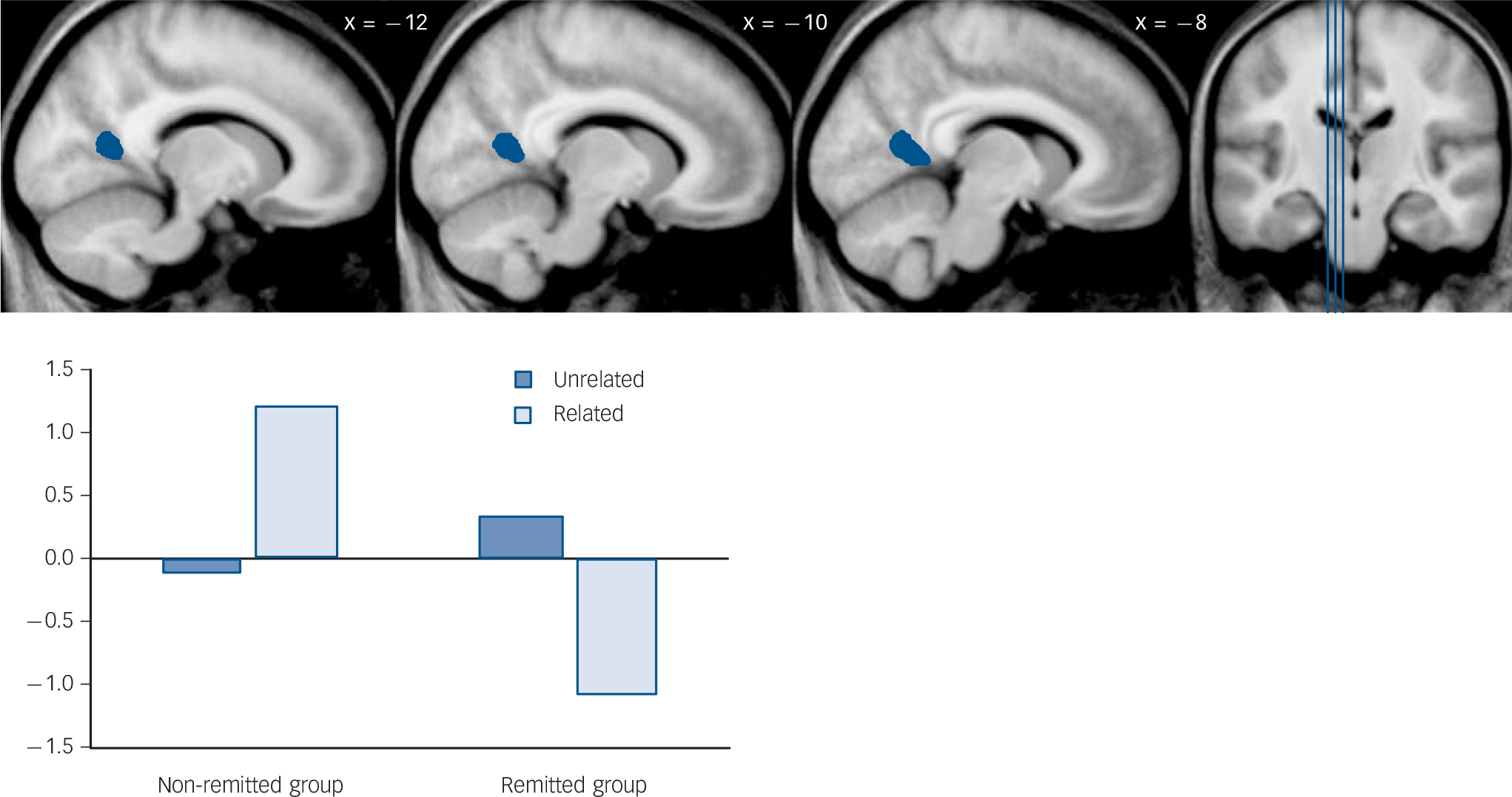
Fig. 1 Result of ‘remitted > non-remitted’ group comparison for semantic relatedness contrast (‘unrelated pairs > related pairs’).
TABLE 4 Results for contrast semantically unrelated > semantically related pairs a

| Coordinates | Parameter estimates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cluster Size | t | x | y | z | Side | Region (Brodmann area) | Unrelated | Related | ||
| Remitted > non-remitted group | Remitted group | Non-remitted group | Remitted group | Non-remitted group | ||||||
| 130 | 4.91 | –10 | –57 | 12 | Left | Posterior cingulate (30) b | 0.34 | –0.12 | –1.07 | 1.21 |
| Non-remitted > remitted group | ||||||||||
| No significant differences in activation | – | – | – | – | ||||||
| Control > non-remitted group | Control group | Non-remitted group | Control group | Non-remitted group | ||||||
| 310 c | 5.09 | –38 | –37 | –12 | Left | Parahippocampus (37) | 2.86 | 1.79 | 1.99 | 2.81 |
| 310 c | 4.16 | –28 | –14 | –16 | Left | Hippocampus (–) | ||||
| 310 c | 4.15 | –26 | –28 | –14 | Left | Parahippocampus (37) | ||||
| 101 d | 4.00 | 48 | –60 | 9 | Right | Middle temporal (39) | 9.27 | 6.19 | 7.11 | 5.85 |
| 101 d | 3.65 | 50 | –68 | 0 | Right | Inferior temporal (37) | ||||
| Non-remitted > control group | ||||||||||
| No significant differences in activation | – | – | – | – | ||||||
| Control > remitted group | Control group | Remitted group | Control group | Remitted group | ||||||
| No significant differences in activation | – | – | – | – | ||||||
| Remitted > control group | ||||||||||
| No significant differences in activation | – | – | – | – | ||||||
a The cluster size represents the number of voxels. The x, y, and z coordinates of local maxima are listed according to the Talairach coordinate system. The parameter estimates reported in the last columns were extracted from within each group for each comparison.
b Peak voxel identified as posterior cingulate but cluster overlaps calcarine fissure.
c These peaks belong to the same cluster of activation.
d These peaks belong to the same cluster of activation.
The ‘non-remitted > remitted’ comparison identified no significant differences. The ‘control > non-remitted’ comparison identified reduced activation in the non-remitted group in the left parahippocampus/hippocampus and right inferior/middle temporal gyrus. Parameter estimates from the parahippocampus/hippocampus cluster revealed that the non-remitted group had a larger positive activation when encoding related pairs over unrelated pairs in the parahippocampus (t 25 = 4.24, P<0.001). In contrast, the control group had a larger positive activation when encoding unrelated pairs over related pairs (t 30 = –3.27, P = 0.003). For the inferior/middle temporal cluster, the control group had a larger positive activation when encoding unrelated pairs over related pairs (t 30 = –6.45, P<0.001), whereas the non-remitted group did not differ in the same pattern of activation (t 25 = –1.23, P = 0.231). The ‘non-remitted > control’ comparison as well as the ‘control > remitted’ and ‘remitted > control’ comparisons identified no significant differences.
For the encoding strategy contrast (associative > item-oriented) (Table DS2), significant differences were identified for only the ‘control > remitted’ comparison, which identified reduced activation in the right precuneus and bilateral middle cingulate in the remitted group. Parameter estimates from the precuneus revealed that the remitted group had positive activation for item-oriented encoding over associative encoding (t 14 = –6.90, P<0.001); the control group did not differ in larger positive activation for item-oriented over associative encoding (t 30 = –1.74, P = 0.092). The remitted group had a larger negative activation for associative encoding over item-oriented encoding in the middle cingulate (t 14 = –4.49, P = 0.001), whereas the control group did not significantly differ in negative activation for associative or item-oriented encoding (t 30 = 0.15, P = 0.884).
For the subsequent memory effect contrast (successful > unsuccessful) (online Table DS3), significant differences were observed for only the ‘non-remitted > control’ comparison, which identified reduced activation in the left superior temporal gyrus/insula in the control group. Parameter estimates from this cluster revealed that the control group had a larger negative activation for successful encoding over unsuccessful encoding (t 30 = –5.12, P<0.001), whereas the non-remitted group did not differ in the larger negative activation for unsuccessful encoding over successful encoding (t 25 = 1.64, P = 0.114).
Supplementary analyses
The ‘non-remitted > remitted’ comparison for the semantic relatedness contrast was re-analysed including covariates with only minimal changes to the posterior cingulate result: handedness, smaller cluster size (122 voxels) and t-value (4.88); antipsychotic dosage at scan, smaller cluster size (124 voxels) but larger t-value (4.93); IQ, larger cluster size (137 voxels) and t-value (5.30); and the three covariates together, smaller cluster size (123 voxels) but larger t-value (5.07).
The parameter estimates (related and unrelated) from the left posterior cingulate were correlated with the eight symptoms from the remission definition using Spearman's correlations (critical P-value set at 0.006 (0.05/8); Bonferroni corrected). There were no notable correlations with symptom ratings at time of scan. Activation values were also correlated with encoding accuracy (critical P<0.025, (0.05/2)) and discrimination index (critical P<0.017, (0.05/3)) using Pearson's correlation, but no significant relationships were found (online Table DS4).
Discussion
Using three fMRI contrasts: encoding strategy (associative>item-oriented), semantic relatedness (unrelated>related), and subsequent memory effect (successful>unsuccessful), we explored for functional differences during memory encoding among participants with first-episode schizophrenia who later achieved remission v. those who did not and healthy controls.
Between outcome groups, the non-remitted group had a positive activation in the left posterior cingulate when encoding semantically related images over unrelated images, whereas those in the remitted group had a negative activation. No significant differences were identified using the encoding strategy or subsequent memory effect contrasts. For comparisons with the control group, for semantic relatedness, the non-remitted group had a larger positive activation when encoding related images over unrelated images in the left medial temporal lobe, whereas the control group had a larger positive activation when encoding unrelated images over related images. Both the non-remitted and control groups had larger positive activation for unrelated images over related images activation in the right temporal gyrus (non-significant in the non-remitted group). For encoding strategy, the remitted group had a larger negative activation in the middle cingulate bilaterally for associative encoding over item-oriented encoding, whereas the control group had no significant activation differences between encoding strategies. The remitted group also had a negative activation in the right precuneus for associative encoding over item-oriented encoding, whereas the control group had a positive activation. For the subsequent memory effect, controls had a larger negative activation in the left superior temporal gyrus for successful encoding over unsuccessful encoding, whereas the non-remitted group had a larger negative activation (non-significant) for unsuccessful encoding over successful encoding.
For the fMRI behavioural data, results were limited to semantic relatedness. During encoding, the remitted and control groups responded more accurately to related images over unrelated images, whereas the non-remitted group showed no difference suggesting an impaired use of semantic encoding. For recognition, the non-remitted group performed significantly worse in overall recognition memory when accounting for false alarms compared with both the remitted and the control groups. This supported the growing literature relating worse memory performance to a poor clinical outcome in schizophrenia. Reference Bodnar, Harvey, Malla, Joober and Lepage5-Reference Toulopoulou and Murray8
Semantic processing, positive activity and clinical outcome
There is little doubt semantic processing is impaired in schizophrenia, with impairments ranging from a lack of knowledge about people or objects to inabilities to form relationships between objects (or images). Reference Wang, Cheung, Gong and Chan26-Reference Binder, Desai, Graves and Conant28 More specifically regarding the latter, a recent meta-analysis of studies involving event-related potentials found that people with schizophrenia are more impaired when processing related conditions (words or images) compared with unrelated conditions. Reference Wang, Cheung, Gong and Chan26 This failure to process related images properly was supported by our results but was more particular to those participants who did not achieve remission. Behaviourally, the non-remitted group showed no advantage when encoding related images compared with the remitted and control groups.
Functionally, the non-remitted group had a positive activation in the left posterior cingulate cortex when encoding semantically related images over unrelated images; the remitted group had a negative activation. In addition, the non-remitted group had a larger positive activation in the left medial temporal lobe when processing related images over unrelated images, whereas the results were the opposite in the control group. Although a positive activation in the posterior cingulate cortex or medial temporal lobe seemed counterintuitive at first and was at variance with our initial hypothesis, several studies have associated positive (or increased) activity in the posterior cingulate cortex and medial temporal lobe with memory dysfunction in schizophrenia.
In schizophrenia, the posterior cingulate cortex has been shown to remain hyperactive when mentally engaged Reference Whitfield-Gabrieli, Thermenos, Milanovic, Tsuang, Faraone and McCarley29-Reference Garrity, Pearlson, McKiernan, Lloyd, Kiehl and Calhoun31 - the opposite of what is known to occur as per the default-mode network Reference Whitfield-Gabrieli, Thermenos, Milanovic, Tsuang, Faraone and McCarley29,Reference Broyd, Demanuele, Debener, Helps, James and Sonuga-Barke32 - with this hyperactivity associated with worse memory performance. Reference Whitfield-Gabrieli, Thermenos, Milanovic, Tsuang, Faraone and McCarley29,Reference Meda, Stevens, Folley, Calhoun and Pearlson30 With support from our main findings, we suggest that the posterior cingulate cortex remains hyperactive in the non-remitted group during an attention-oriented task (encoding related images) resulting in improper encoding and poorer memory. However, we found posterior cingulate cortex activity did not correlate with encoding accuracy or recognition memory (discrimination index). Nevertheless, the posterior cingulate cortex is believed to be crucial to memory encoding Reference Binder, Desai, Graves and Conant28,Reference Wang, Laviolette, O'Keefe, Putcha, Bakkour and Van Dijk33 and semantic processing Reference Binder, Desai, Graves and Conant28 but how it may be functionally related to clinical outcome requires further exploration. Regarding the medial temporal lobe, a recent fMRI meta-analysis of memory function in schizophrenia showed increased activity in the left parahippocampal gyrus during encoding. Reference Ragland, Laird, Ranganath, Blumenfeld, Gonzales and Glahn2 The authors suggested that in schizophrenia, the increased activity during encoding may result from inefficient, compensatory brain activity until a suitable encoding strategy is reached. Reference Ramsey, Jansma, Jager, Van Raalten and Kahn34 So, during encoding, it appears the non-remitted group may not be fully engaging the correct encoding mechanisms (left parahippocampal gyrus) when trying to encode related images. However, since parahippocampal activity only differed with the control group and not the remitted group, functional activity of this structure, as per memory encoding, may not be related to clinical outcome directly. More studies are required to explore this potential relationship.
Our results support that functional impairments exist in people with schizophrenia when processing related images but further demonstrate that these impairments may be specific to or driven by individuals with a poorer outcome. It would be interesting to explore if semantically related N400 differences Reference Wang, Cheung, Gong and Chan26 exist between the remitted and non-remitted groups.
Other functional differences with healthy controls
Compared with the control group, the non-remitted group had increased activity in the left superior temporal gyrus/insula for the subsequent memory effect contrast but nothing identified for the encoding strategy contrast. In contrast, the remitted group showed decreased activity in the right precuneus and bilateral middle cingulate for the encoding strategy contrast but nothing identified for the subsequent memory effect contrast. As with our previous study, we found limited results for both of these particular contrasts. Reference Achim, Bertrand, Sutton, Montoya, Czechowska and Malla15 However, we did not use an associative memory recognition task (which typically requires the participant to distinguish between intact and rearranged pairs of items) and therefore could not examine associative v. item memory during retrieval. Perhaps a real associative v. item task would have shown more significant group differences both at the behavioural and neuronal levels for these two contrasts.
Implications
We identified increased activity in the posterior cingulate cortex in the non-remitted group compared with the remitted group specific to semantic processing during encoding. This finding suggested that the functional activity of semantic processing may be sensitive to detecting differences within a patient sample (i.e. good v. poor outcome). As a region of interest, the posterior cingulate cortex should be explored further both structurally and functionally in relation to psychopathology for a better understanding of any underlying relationships. It could also be targeted using pharmaco-fMRI techniques Reference Honey and Bullmore35,Reference Stein36 in relation to the numerous receptor abnormalities (glutamate, gamma-aminobutyric acid and muscarnic) identified in schizophrenia Reference Newell, Zavitsanou and Huang37,Reference Newell, Zavitsanou, Jew and Huang38 to expand the research into developing newer, target-specific treatments for people with schizophrenia in the hope of achieving a better outcome.
Limitations
First, ethical approval allowed for a first-assessment scan and follow-up scans at 1 year and 2 years thereafter, with fMRI data collected only during the first-assessment scan. Thus, scanning could not take place when remission status was determined after 1 year of treatment. At the time of the fMRI scan, 1 of the 15 participants in the remitted group was in a non-remitted state, whereas 10 of the 27 participants in the non-remitted group were in a remitted state. Re-analysing the data with participants separated based on clinical data near the time of scan showed similar results. Of interest, the posterior cingulate cortex (cluster: 68 voxels, t-value = 4.27) was still hyperactive in the non-remitted group when encoding related images. Since the majority of the participants had obtained their 1-year remission status by the time of the fMRI scan, we feel our results would be similar had scanning taken place at month 12.
Second, not finding increased activity in the posterior cingulate cortex in the non-remitted v. control group or increased activity in the medial temporal lobe in the non-remitted v. remitted group may have limited the interpretation of our results. However, these activations were in fact present but were not detected due to our conservative threshold. Upon lowering the threshold (P<0.005, uncorrected), we found that the non-remitted group had positive activity in the left posterior cingulate cortex (x = –6, y = -48, z = 6, cluster: 222 voxels) compared with the control group and positive activity in the left parahippocampal gyrus (x = –28, y = –28, z = –19, cluster: 51 voxels) compared with the remitted group when encoding related pairs over unrelated pairs. However, these results may be a false-positive because of the low threshold used. Nevertheless, the non-remitted group do show altered activity compared with the remitted and control groups in both the posterior cingulate cortex and medial temporal lobe, supporting our initial interpretations.
Third, although the inclusion of medicated and relatively stable participants could imply that medication may have affected the cognitive processes being studied and limited the generalisability of our findings to unmedicated individuals with first-episode schizophrenia, including people who are stable enough to comply with the imaging procedures is likely to produce more reliable results for fMRI studies of cognitive functions than by including unmedicated and symptomatically unstable individuals who are not able to follow the task instructions. Although antipsychotic medications have been shown to affect fMRI results, Reference Abbott, Juarez, White, Gollub, Pearlson and Bustillo39,Reference Ettinger, Williams, Fannon, Premkumar, Kuipers and Moller40 we demonstrated our main result was unaffected when including antipsychotic dosage as a covariate. Nevertheless, caution is warned when interpreting our results.
A final limitation was not incorporating the two-design matrices into one matrix to analyse the fMRI data for the subsequent memory effect. This would have been advantageous since we had an interaction between recognition memory and semantic relatedness for the behavioural data. However, we could not employ a single matrix including both ‘successful v. unsuccessful memory encoding‘ and ‘unrelated v. related image pairs’ as there were not an equal number of events that could be modelled for all of the participants. Of those that could be modelled, our final sample size would have been reduced to 18 people in the control group, 10 in the remitted group and 18 in the non-remitted group. As such, we decided to explore only ‘successful v. unsuccessful memory encoding’ for the subsequent memory effect analysis.
Funding
This work was funded by operating grants from the Canadian Institutes of Health Research (CIHR; ) and the Sackler Foundation to M.L. and A.K.M. M.L. is supported by a salary award from the Fonds de la recherche en santé du Québec (FRSQ). A.K.M. is supported by the Canada Research Chairs programme.
Acknowledgements
We thank the PEPP-Montreal research staff for their help with recruitment and clinical assessments of all participants involved.








eLetters
No eLetters have been published for this article.