Research on the association between diet and depression has focused primarily on nutrients such as fatty acids, Reference Lin and Su1–Reference Tiemeier, van Tuijl, Hofman, Kiliaan and Breteler4 and nutrients involved in the homocysteine pathway such as vitamins B6,B9 and B12, Reference Murakami, Mizoue, Sasaki, Ohta, Sato and Matsushita2,Reference Gilbody, Lightfoot and Sheldon5–Reference Tolmunen, Hintikka, Ruusunen, Voutilainen, Tanskanen and Valkonen7 with inconclusive results. Recent years have seen a move away from analysing associations between isolated nutrients and health to consideration of the effects of dietary patterns. Reference Hu8 For example, a meta-analysis published in 2008 showed that greater adherence to a Mediterranean dietary pattern (high intake of fruits, vegetables and fish, and low intake of meat and dairy products) was associated with a lower incidence of Parkinson's and Alzheimer's diseases. Reference Sofi, Cesari, Abbate, Gensini and Casini9 However, the health outcomes of that meta-analysis did not include depression and, to the best of our knowledge, no previous prospective study has investigated the association between dietary patterns and the occurrence of depressive symptoms. Thus, the objective of this study was to examine the association between dietary patterns, derived from a food frequency questionnaire using factor analysis, and depression in a large British middle-aged population, the Whitehall II study participants. We were able to control for a large range of sociodemographic variables, health behaviours and health parameters including chronic diseases and cognitive functioning.
Method
The target population for the Whitehall II study was all London-based office staff, aged 35–55 years, working in 20 civil service departments. Reference Marmot and Brunner10 Baseline screening (phase 1) took place during 1985–8 (n = 10 308), and involved a clinical examination and a self-administered questionnaire containing sections on demographic characteristics, health, lifestyle factors, work characteristics, social support and life events. The clinical examination included measures of blood pressure, anthropometric and biochemical factors, neuroendocrine function and subclinical markers of cardiovascular disease. Subsequent phases of data collection alternated between postal questionnaire alone – phases 2 (1989–90), 4 (1995–6), 6 (2001) and 8 (2006) – and postal questionnaire accompanied by a clinical examination – phases 3 (1991–3), 5 (1997–9) and 7 (2002–4). Analyses reported in this study were restricted to the 3486 White European participants with data on dietary patterns and all covariates at phase 5 and depression at phase 7. Black (n = 175) and Asian (n = 331) participants were excluded owing to differences in eating patterns.
After complete description of the study to the participants, written informed consent was obtained; the University College London ethics committee approved the study.
Dietary assessment at phase 5 and determination of dietary pattern
A machine-readable Food Frequency Questionnaire (FFQ), Reference Brunner, Stallone, Juneja, Bingham and Marmot11 based on the one used in the US Nurses Health Study, Reference Willett, Sampson, Stampfer, Rosner, Bain and Witschi12 was sent to the participants. The food list (127 items) from the original questionnaire was anglicised, and foods commonly eaten in the UK were added. Reference Bingham, Gill, Welch, Cassidy, Runswick and Oakes13 A common unit or portion size for each food was specified, and participants were asked how often, on average, they had consumed that amount of the item during the previous year. Response to all items was on a nine-point scale, ranging from ‘never, or less than once per month’ to ‘six or more times per day’. The selected frequency category for each food item was converted to a daily intake.
According to nutrient profile and culinary use of food items, the 127 items of the FFQ were grouped into 37 predefined food groups by adding food items within each group (online Table DS1). Reference Akbaraly, Singh-Manoux, Marmot and Brunner14 Dietary patterns were identified using principal component analysis of these 37 groups. The factors were rotated by an orthogonal transformation (varimax rotation function in SAS software to achieve a simple structure, allowing greater interpretability. Two dietary patterns were identified using multiple criteria: the diagram of eigenvalues, the scree plot, the interpretability of the factors and the percentage of variance explained by the factors (online Table DS2). The factor score for each pattern was calculated by summing intakes of all food groups weighted by their factor loadings. Factor loadings represent correlation coefficients between the food groups and the dietary pattern. The first pattern was heavily loaded by high intake of vegetables, fruits and fish, labelled the ‘whole food’ pattern. The second pattern, labelled ‘processed food’, was heavily loaded by high consumption of sweetened desserts, chocolates, fried food, processed meat, pies, refined grains, high-fat dairy products and condiments. Each participant received a factor score for each identified pattern. Factor analysis does not group individuals into clusters; instead, all individuals contribute to both factors and it is the homogeneity between food items that defines the factors. The validity and the reliability of this version of the FFQ in terms of nutrient and food consumption have been documented in detail both in our cohort and in another UK cohort. Reference Brunner, Stallone, Juneja, Bingham and Marmot11,Reference Bingham, Gill, Welch, Cassidy, Runswick and Oakes13 To assess the validity of the dietary patterns resulting from this a posteriori food grouping, we reran the principal component analyses using the 127 individual food items, with similar results.
Depression measurement at phase 7
The Center for Epidemiologic Studies – Depression scale (CES–D) is a short self-report scale designed to measure depressive symptoms in the general population. Reference Radloff15 The 20 items of the scale measure symptoms associated with depression and have been validated against longer scales. Reference Radloff15 Participants were asked to score the frequency of occurrence of specific symptoms during the previous week on a four-point scale (0, ‘less than 1 day’; 1, ‘1–2 days’; 2, ‘3–4 days’; and 3, ‘5–7 days’). These were summed to yield a total score between 0 and 60. Participants scoring more than 15 were categorised as cases of CES–D depression. Reference Radloff15
Covariates at phase 5
Sociodemographic variables
Sociodemographic variables consisted of age, gender, marital status, employment grade and education. The British civil service employment grade, defined on the basis of salary, social status and level of responsibility, consisted of three levels, with grade 1 representing the highest level and grade 3 the lowest. Highest educational attainment was grouped into five levels (no academic qualification, lower secondary education, higher secondary education, university degree, higher university degree).
Health behaviours
Health behaviours measured were smoking (non-smoker, former smoker, current smoker) and physical activity, converted to metabolic equivalent (MET) scores, Reference Singh-Manoux, Hillsdon, Brunner and Marmot16 categorised as ‘mildly energetic’ (MET values below 3), ‘moderately energetic’ (MET values ranging from 3 to 6) and ‘vigorous’ (MET values of 6 or above) physical activity.
Health status
Health status was ascertained using a number of measures: prevalence of coronary heart disease, based on clinically verified events, including non-fatal myocardial infarction and definite angina; self-reported stroke or transient ischaemic attack; diabetes (defined by a fasting glucose ≥7.0 mmol/l or a 2-hour post-load glucose ≥11.1 mmol/l or reported doctor diagnosed diabetes or use of diabetes medication); 17 hypertension (systolic or diastolic blood pressure ≥140 mmHg or ≥90 mmHg respectively, or use of hypertensive drugs); use of antidepressants; and cognitive functioning assessed by the Alice Heim reasoning test 4–I, Reference Heim18 a series of 65 verbal and mathematical reasoning items of increasing difficulty: low cognitive score was defined as a performance in the lowest quintile. For sensitivity analyses we used the General Health Questionnaire (GHQ), Reference Goldberg19 assessed at both phase 3 and phase 5 of the study, which captured common mental disorders and included the four-item depression subscale. All items were scored from 0 to 3 and then summed; cut-off points of 4 out of 12 were used to identify depression cases.
Statistical analysis
Neither natural thresholds nor clinically based thresholds are defined for the factor score measures of the two dietary patterns. We divided both scores into thirds based on their distribution in order to allow a robust estimation of self-reported depression across levels of dietary patterns that was not driven by extreme values. Logistic regression was used to model the association between the tertiles of the two dietary patterns and CES–D depression. In the first model the analyses were adjusted for age, gender and energy intake; in the second model they were also adjusted for employment grade, educational level, marital status, smoking and physical activity; and in the final model the analyses were further adjusted for health measures. Interactions between each dietary pattern and the covariates were tested and were found not to be statistically significant. To examine whether the association between dietary pattern and CES–D depression was robust, we ran two sensitivity analyses, the first adjusting for additional covariates such as dyslipidaemia (low-density lipoprotein cholesterol ≥4.1 mmol/l or use of lipid-lowering drugs) and body mass index (BMI, in kg/m2) in a subsample for whom these data were available, and the second excluding individuals receiving antidepressive treatment or who had GHQ-defined depression at phase 5. All analyses were conducted using SAS software (version 9.1 for Windows).
Results
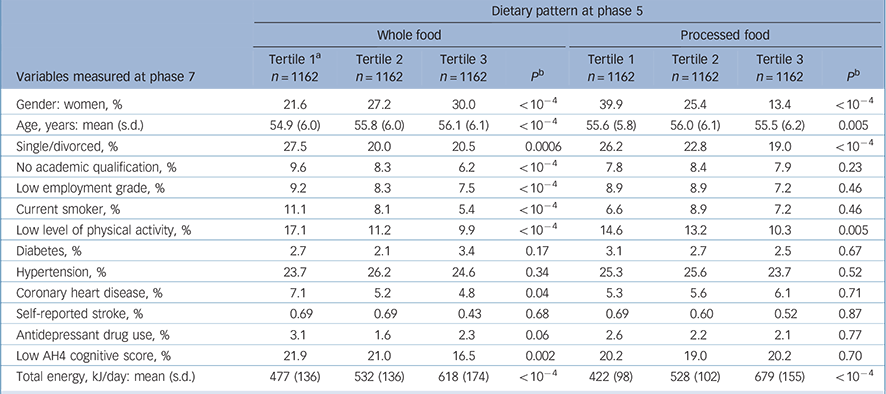
Compared with the 6943 individuals still alive at phase 7, the 3486 participants included in the analyses were more likely to be men (73.8% v. 66.7%), and less likely to be in the low occupational grade (8.3% v. 18.0%) or have no academic qualification (8.0% v. 10.0%). A total of 5990 individuals had a CES–D assessment at phase 7; compared with those excluded from the analyses, the prevalence of CES–D depression was lower in our study sample (11.9% v. 19.2%). Concerning dietary habits, participants included in analyses were more likely to be in the highest tertile of both whole food and processed food dietary patterns. At phase 7, a total of 416 participants were categorised as CES–D cases (score >15). Characteristics of the participants as a function of the presence of CES–D depression are presented in Table 1. Factors associated with tertiles of the two dietary patterns (whole food and processed food) at phase 5 are shown in Table 2.
Table 1 Characteristics of participants at phase 5 according to the presence of CES–D depression at phase 7

| CES—D depression at phase 7 a | ||||
|---|---|---|---|---|
| No n = 3070 | Yes n = 416 | P b | ||
| Gender: women, % | 25.3 | 33.2 | 0.0006 | |
| Age, years: mean (s.d.) | 55.7(6.0) | 54.5 (6.2) | 0.0002 | |
| Single or divorced, % | 18.4 | 31.7 | < 10-4 | |
| No academic qualification, % | 7.6 | 11.5 | 0.09 | |
| Low employment grade, % | 7.8 | 12.0 | < 10-4 | |
| Current smoker, % | 7.3 | 14.7 | < 10-4 | |
| Low level of physical activity, % | 12.0 | 18.3 | < 10-4 | |
| Diabetes, % | 2.5 | 5.0 | 0.003 | |
| Hypertension, % | 25.1 | 22.6 | 0.26 | |
| Coronary heart disease, % | 5.4 | 7.7 | 0.06 | |
| Self-reported stroke, % | 0.59 | 0.72 | 0.74 | |
| Antidepressant drug use, % | 1.9 | 5.5 | < 10-4 | |
| Low AH4 cognitive score, c % | 11.9 | 14.4 | 0.14 | |
| Total energy intake, kJ/day: mean (s.d.) | 542 (158) | 544 (181) | 0.85 | |
AH4, Alice Heim test 4-I; CES—D, Center for Epidemiologic Studies — Depression scale.
a. Depression assessed with CES—D questionnaire; participants scoring more than 15 were classified as having ‘CES—D depression’.
b. Value for heterogeneity.
c. Below first quintile.
Table 2 Associations between dietary pattern at phase 5 and covariates at phase 5
| Dietary pattern at phase 5 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Whole food | Processed food | ||||||||||||||
| Variables measured at phase 7 | Tertile 1 a n = 1162 | Tertile 2 n = 1162 | Tertile 3 n = 1162 | P b | Tertile 1 n = 1162 | Tertile 2 n = 1162 | Tertile 3 n = 1162 | P b | |||||||
| Gender: women, % | 21.6 | 27.2 | 30.0 | < 10-4 | 39.9 | 25.4 | 13.4 | < 10-4 | |||||||
| Age, years: mean (s.d.) | 54.9 (6.0) | 55.8 (6.0) | 56.1 (6.1) | < 10-4 | 55.6 (5.8) | 56.0 (6.1) | 55.5 (6.2) | 0.005 | |||||||
| Single/divorced, % | 27.5 | 20.0 | 20.5 | 0.0006 | 26.2 | 22.8 | 19.0 | < 10-4 | |||||||
| No academic qualification, % | 9.6 | 8.3 | 6.2 | < 10-4 | 7.8 | 8.4 | 7.9 | 0.23 | |||||||
| Low employment grade, % | 9.2 | 8.3 | 7.5 | < 10-4 | 8.9 | 8.9 | 7.2 | 0.46 | |||||||
| Current smoker, % | 11.1 | 8.1 | 5.4 | < 10-4 | 6.6 | 8.9 | 7.2 | 0.46 | |||||||
| Low level of physical activity, % | 17.1 | 11.2 | 9.9 | < 10-4 | 14.6 | 13.2 | 10.3 | 0.005 | |||||||
| Diabetes, % | 2.7 | 2.1 | 3.4 | 0.17 | 3.1 | 2.7 | 2.5 | 0.67 | |||||||
| Hypertension, % | 23.7 | 26.2 | 24.6 | 0.34 | 25.3 | 25.6 | 23.7 | 0.52 | |||||||
| Coronary heart disease, % | 7.1 | 5.2 | 4.8 | 0.04 | 5.3 | 5.6 | 6.1 | 0.71 | |||||||
| Self-reported stroke, % | 0.69 | 0.69 | 0.43 | 0.68 | 0.69 | 0.60 | 0.52 | 0.87 | |||||||
| Antidepressant drug use, % | 3.1 | 1.6 | 2.3 | 0.06 | 2.6 | 2.2 | 2.1 | 0.77 | |||||||
| Low AH4 cognitive score, % | 21.9 | 21.0 | 16.5 | 0.002 | 20.2 | 19.0 | 20.2 | 0.70 | |||||||
| Total energy, kJ/day: mean (s.d.) | 477 (136) | 532 (136) | 618 (174) | < 10-4 | 422 (98) | 528 (102) | 679 (155) | < 10-4 | |||||||
AH4, Alice Heim test 4-I.
a. Tertiles 1, 2 and 3 represent individuals in the lowest, intermediate and highest thirds of the dietary factor score.
b. Value for trend.
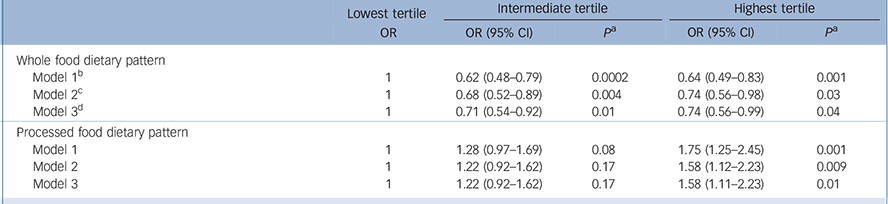
Table 3 shows the association between the two dietary pattern scores categorised in tertiles at phase 5 and CES–D depression at phase 7. Participants with the highest intake of whole food were less likely to report CES–D depression: OR = 0.64 (95% CI 0.49–0.83) after adjusting for age, gender and energy intake (model 1). This association was not much attenuated after adjustment for all covariates (model 3): OR = 0.74 (95% CI 0.56–0.99). In contrast, in the fully adjusted analyses participants with a high intake of processed food had higher odds of CES–D depression compared with those with the lowest intake: OR = 1.58 (95% CI 1.11–2.23).
Table 3 Associations between dietary pattern scores at phase 5 and CES–D depression at phase 7 (n = 3486)
| Intermediate tertile | Highest tertile | ||||||
|---|---|---|---|---|---|---|---|
| Lowest tertile OR | OR (95% CI) | P a | OR (95% CI) | P a | |||
| Whole food dietary pattern | |||||||
| Model 1 b | 1 | 0.62 (0.48-0.79) | 0.0002 | 0.64 (0.49-0.83) | 0.001 | ||
| Model 2 c | 1 | 0.68 (0.52-0.89) | 0.004 | 0.74 (0.56-0.98) | 0.03 | ||
| Model 3 d | 1 | 0.71 (0.54-0.92) | 0.01 | 0.74 (0.56-0.99) | 0.04 | ||
| Processed food dietary pattern | |||||||
| Model 1 | 1 | 1.28 (0.97-1.69) | 0.08 | 1.75 (1.25-2.45) | 0.001 | ||
| Model 2 | 1 | 1.22 (0.92-1.62) | 0.17 | 1.58 (1.12-2.23) | 0.009 | ||
| Model 3 | 1 | 1.22 (0.92-1.62) | 0.17 | 1.58 (1.11-2.23) | 0.01 | ||
CES—D, Center for Epidemiologic Studies — Depression scale.
a. Value for trend.
b. Model 1: adjusted for gender, age and energy intake.
c. Model 2: model 1 plus adjustment for marital status, employment grade, education, physical activity and smoking habits.
d. Model 3: model 2 plus adjustment for hypertension, diabetes, cardiovascular disease, self-reported stroke, use of antidepressive drugs and cognitive functioning.
Sensitivity analyses
Additional analyses were undertaken on a subsample with data on BMI and dyslipidaemia (n = 2702) at phase 5. Among them, 323 participants had CES–D depression at phase 7. High whole food intake at phase 5 remained associated with lower odds of subsequent CES–D depression at phase 7 (OR = 0.75, 95% CI 0.54–1.03), whereas participants with high processed food scores had higher odds of CES–D depression (OR = 1.76, 95% CI 1.19–2.62) after adjustment for all potential confounders, including BMI and dyslipidaemia.
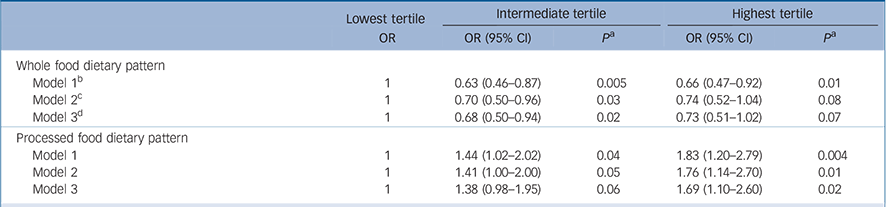
In an attempt to elucidate whether the association shown in Table 3 was due to an effect of diet on depression and not the reverse, the analysis was repeated after excluding the 427 participants who identified themselves as having depression at phase 5. As CES–D scores were not available at phase 5, self-reported depression was identified on the basis of a score of 4 or more on the GHQ depression subscale (n = 374) or reported antidepressant treatment (n = 81). As can be seen in Table 4, among the remaining 3059 individuals of whom 265 had CES–D depression at phase 7, the results are comparable to those reported in Table 3, reinforcing our original observation that poor diet is a risk factor for self-reported depression.
Table 4 Associations between dietary pattern scores at phase 5 and CES–D depression at phase 7 after excluding participants identified as having depression at phase 5 (total n = 3059) a
| Intermediate tertile | Highest tertile | ||||||
|---|---|---|---|---|---|---|---|
| Lowest tertile OR | OR (95% CI) | P a | OR (95% CI) | P a | |||
| Whole food dietary pattern | |||||||
| Model 1 b | 1 | 0.63 (0.46-0.87) | 0.005 | 0.66 (0.47-0.92) | 0.01 | ||
| Model 2 c | 1 | 0.70 (0.50-0.96) | 0.03 | 0.74 (0.52-1.04) | 0.08 | ||
| Model 3 d | 1 | 0.68 (0.50-0.94) | 0.02 | 0.73 (0.51-1.02) | 0.07 | ||
| Processed food dietary pattern | |||||||
| Model 1 | 1 | 1.44 (1.02-2.02) | 0.04 | 1.83 (1.20-2.79) | 0.004 | ||
| Model 2 | 1 | 1.41 (1.00-2.00) | 0.05 | 1.76 (1.14-2.70) | 0.01 | ||
| Model 3 | 1 | 1.38 (0.98-1.95) | 0.06 | 1.69 (1.10-2.60) | 0.02 | ||
CES—D, Center for Epidemiologic Studies — Depression scale.
a. Participants defined as having depression using the General Health Questionnaire depression subscale (n = 374) or those taking antidepressant drugs (n = 81).
b. Model 1: adjusted for gender, age and energy intake.
c. Model 2: model 1 plus adjustment for marital status, employment grade, level of education, physical activity and smoking habits.
d. Model 3: model 2 plus adjustment for hypertension, diabetes, cardiovascular disease, self-reported stroke, use of antidepressive drugs and cognitive functioning.
A further test of the reverse causality hypothesis examined GHQ depression at phase 3 (n = 397) as a predictor of dietary pattern at phase 5. We found no evidence (P = 0.24 for the whole food pattern and P = 0.92 for the processed food pattern) to suggest that dietary patterns at phase 5 were worse among participants who met criteria for GHQ depression at phase 3.
Discussion
We examined associations between two distinct dietary patterns, whole food (rich in fruit, vegetables and fish) and processed food (rich in processed meat, chocolates, sweet desserts, fried food, refined cereals and high-fat dairy products), and CES–D depression 5 years later in a middle-aged population. The whole food pattern was associated with lower odds of subsequent CES–D depression and the processed food pattern with higher odds of CES–D depression. These associations were robust to adjustments for a range of health parameters and behavioural factors. Dietary patterns have been investigated in relation to many health outcomes, but the research on depression has mostly focused on the risk of depression associated with single nutrients. Our results suggest a protective effect of an overall diet rich in fruits, vegetables and fish, whereas an overall diet rich in processed meat, chocolates, sweetened desserts, fried food, refined cereals and high-fat dairy products seems to be deleterious for depression. These findings are in line with a recent meta-analysis showing that adherence to a diet characterised by a high intake of fruits, vegetables and fish and a low intake of meat and dairy products (‘Mediterranean’ diet) was associated with lower overall mortality, lower mortality from cancer and cardiovascular disease, and lower incidence of neurodegenerative diseases. Reference Sofi, Cesari, Abbate, Gensini and Casini9
Plausible mechanisms
There are several plausible mechanisms underlying the association we observed between the whole food pattern and self-reported depression. The high content of antioxidants in fruits and vegetables could be protective, 20 as some studies have shown higher antioxidant levels to be associated with lower depression risk. Reference Sarandol, Sarandol, Eker, Erdinc, Vatansever and Kirli21 The potential protective effect of the whole food diet could also come from folate found in large amounts in some cruciferous vegetables (broccoli, cabbage, Brussels sprouts), leafy vegetables (spinach), other green vegetables (asparagus, avocado) and dried legumes (lentil, chickpea). 22 It has been suggested that low levels of folate might increase the risk of depression and result in reduced availability of S-adenosylmethionine, a universal methyl donor, which can result in impaired formation of myelin, neurotransmitters and membrane phospholipids. Reference Selhub, Bagley, Miller and Rosenberg23 In line with this, a large study of Finnish middle-aged men found an increased risk of depression in participants with lower dietary intake of folate. Reference Tolmunen, Hintikka, Ruusunen, Voutilainen, Tanskanen and Valkonen7 However, some studies have found no association between folate levels and depression in elderly populations. Reference Kamphuis, Geerlings, Grobbee and Kromhout6 A further plausible mechanism involves fish consumption. The whole food dietary pattern includes a high intake of fish and there is evidence suggesting an association between high levels of fish consumption and low incidence of depression. Reference Hibbeln24 This protective effect of fish consumption has been traditionally attributed to its high content of long-chain ω-3 polyunsaturated fatty acids. Reference Astorg, Arnault, Czernichow, Noisette, Galan and Hercberg25 These are a major component of neuron membranes and have vascular and anti-inflammatory properties. Evidence of this association has come from observational studies that have shown an inverse association between ω-3 fatty acid levels measured in blood or estimated from intake and depression. Reference Sanchez-Villegas, Henriquez, Figueiras, Ortuno, Lahortiga and Martinez-Gonzalez3,Reference Tiemeier, van Tuijl, Hofman, Kiliaan and Breteler4 Finally, it is also possible that the protective effect of diet on depression comes from the cumulative and synergic effect of nutrients from different sources of foods rather than from the effect of one isolated nutrient.
The deleterious effect of processed food on self-reported depression is a novel finding. A previous cross-sectional study has shown a correlation between sugar consumption and the annual rate of depression in six countries. Reference Westover and Marangell26 Furthermore, the processed food diet is very close to the ‘Western’ pattern defined in the American population, Reference Hu8 which has been shown to be associated with higher risk of coronary heart disease and inflammation. Reference Hu8,Reference Lopez-Garcia, Schulze, Fung, Meigs, Rifai and Manson27 Several lines of investigation have suggested that coronary heart disease and inflammation are involved in the pathogenesis of depression. Reference Kamphuis28,Reference Tiemeier, Hofman, van Tuijl, Kiliaan, Meijer and Breteler29 However, further studies are needed to improve our understanding of the association between processed food intake, the inflammation process and depression.
Limitations of the study
There are several limitations to our study. First, reverse causation – with depression affecting the dietary pattern rather than the other way around – remains an alternative interpretation of the observed associations. To test this issue, we undertook sensitivity analyses and found no significant association between previous reports of depression (phase 3, 1991–3) using the GHQ depression subscale and dietary patterns assessed 6 years later. This suggests that depression did not predict dietary behaviour in our study. Furthermore, we also showed that our main finding – the association between dietary patterns (phase 5, 1997–9) and CES–D depression at phase 7 (2002–4) – remains significant after excluding participants who reported depression at phase 5, assessed using the GHQ depression subscale and report of antidepressant treatment. We were not able to use the CES–D to exclude prior depression as it was only introduced at phase 7 of the study. Even if the use of different tools to assess self-reported depression may decrease sensitivity of these analyses, results using the GHQ to exclude participants with prior depression show that the estimates of the association between dietary patterns and subsequent CES–D depression were similar to those reported in this paper. Thus, reverse causation seems an unlikely explanation for our findings.
Second, some bias owing to selective retention of participants is possible as we found socioeconomic position, depression and dietary patterns to be associated with the likelihood of being included in the analyses. If anything, this could contribute to an overestimation of the association between a whole food pattern and self-reported depression on account of the overrepresentation of individuals without depression who follow a health-conscious diet.
Third, is the use of a semi-quantitative food questionnaire that only covered specific foods and is recognised to be less precise than dietary assessment by diary questionnaire. However, we have shown previously in this study population that nutrient intake estimated by the FFQ method is well correlated with biomarker levels and with intake estimates from the generally more accurate 7-day diary. Reference Brunner, Stallone, Juneja, Bingham and Marmot11 The factor analyses approach used to identify these patterns involves several arbitrary decisions, such as the consolidation of food items into food groups, the number of factors extracted, the methods of rotation and the labelling of the factors. However, the two major eating patterns identified – the whole food and processed food diets – were similar to the ‘prudent’ and Western patterns determined in large American prospective cohorts. Reference Hu8
Fourth, the study participants were mainly office-based civil servants, not fully representative of the British population, and analyses were restricted to White participants, which may limit the generalisability of the findings. Finally, we cannot exclude the possibility of residual confounding in the analysis due to unmeasured or imprecisely measured factors. It is possible, for example, that a healthy diet is only one component of an overall healthy lifestyle which affords protection against depression. However, the effects of dietary patterns on depressive symptoms did not substantially attenuate after adjustment for other indicators of a healthy lifestyle, such as smoking, physical activity and body mass, and a range of other potential confounders. This provides evidence against the interpretation that we have found a spurious association that is simply a by-product of an overall healthy lifestyle.
Diet pattern and depression
Despite these limitations, our study is unique in expanding the focus in this field of research from single nutrients and single foods to overall diet patterns. Our study provides evidence of a robust association between two dietary patterns, the whole food and the processed food patterns, and depressive symptoms in a large prospective cohort of White, middle-aged British participants. The results suggest that consumption of fruits, vegetables and fish affords protection against the onset of depressive symptoms 5 years later, whereas a diet rich in processed meat, chocolates, sweet desserts, fried food, refined cereals and high-fat dairy products increases vulnerability. These findings suggest that existing healthy eating policies will generate additional benefits to health and well-being, and that diet should be considered as a potential target for the prevention of depressive disorders.
Funding
T.N.A. is sponsored by the Academy of Finland (projects 117604, 124322). A.S.-M. is supported by a European Young Investigator Award from the European Science Foundation. M.G.M. is supported by a Medical Research Council (MRC) research professorship. J.E.F. is supported by the MRC (grant G8802774) and M.K. is supported by the Academy of Finland. The Whitehall II study has been supported by grants from the MRC, the British Heart Foundation, the UK Health and Safety Executive, the UK Department of Health, the US National Heart, Lung and Blood Institute (grant HL36310), the US National Institute on Aging (grant AG13196), the US Agency for Health Care Policy and Research (grant HS06516) and the John D. and Catherine T. MacArthur Foundation Research Networks on Successful Midlife Development and Socio-economic Status and Health.
Acknowledgements
We thank all of the participating civil service departments and their welfare, personnel and establishment officers; the British Occupational Health and Safety Agency; the Council of Civil Service Unions; all participating civil servants in the Whitehall II study; and all members of the Whitehall II study team.







eLetters
No eLetters have been published for this article.