Dementia with Lewy bodies (DLB) is a common form of dementia in old age, accounting for 15-20% of cases in hospital autopsy series (Reference WeinerWeiner, 1999), a prevalence confirmed in a UK community-based dementia case register study (Reference Holmes, Cairns and LantosHolmes et al, 1999). The disorder shares clinical and pathological features with both Alzheimer's disease and Parkinson's disease. The differentiation of DLB from these other disorders therefore poses diagnostic difficulties — where do the boundaries begin and end?
PATHOLOGY
Lewy bodies are neuronal inclusions composed of abnormally phosphorylated, neurofilament proteins aggregated with ubiquitin and α-synuclein. In Parkinson's disease, Lewy body formation and neuron loss in brain-stem nuclei, particularly the substantia nigra, lead to movement disorder. In DLB, significant Lewy body formation also occurs in paralimbic and neocortical structures, and extensive depletion of acetylcholine neurotransmission in neocortical areas occurs as a result of degeneration in the brain-stem and basal forebrain cholinergic projection neurons. A distinctive pattern of ubiquitin and α-synuclein immunoreactive neuritic degeneration has additionally been identified in DLB. ‘Lewy neurites’ are seen in the substantia nigra, hippocampal region CA2/3, dorsal vagal nucleus, basal nucleus of Meynert and transentorhinal cortex. These extensive neuritic changes are probably more relevant for neuropsychiatric symptom formation than the relatively sparsely distributed cortical Lewy bodies (Reference Gomez-Tortosa, Newell and IrizarryGómez-Tortosaet al, 1999).
Some Alzheimer-type changes are also present in the majority of patients with DLB. Senile plaques, diffuse and neuritic, are present in similar density and distribution as in Alzheimer's disease, but the other hallmark lesions of Alzheimer's disease, neurofibrillary tangles, are relatively few (Reference WeinerWeiner, 1999), as are biochemical measures of tau pathology. Stringent neuropathological criteria for Alzheimer's disease require above-threshold numbers of neocortical neurofibrillary tangles. Therefore, most DLB cases should probably be regarded as distinct from Alzheimer's disease and not as a ‘variant’ of it. Minor vascular disease also occurs in about 30%, but its clinical significance is unknown. Mixed pathological changes are therefore usually seen in DLB (Reference Holmes, Cairns and LantosHolmes et al, 1999), cases of pure DLB without any plaques, tangles or vascular lesions being uncommon.
CLINICAL FEATURES
Dementia is usually, but not always, the presenting feature — a minority of patients present with parkinsonism alone, some with psychiatric disorder in the absence of dementia, and others with orthostatic hypotension, falls or transient disturbances of consciousness (Reference Byrne, Lennox and LoweByrne et al, 1989; Reference McKeith, Perry and FairbairnMcKeith et al, 1992) (Table 1).
Table 1 A comparison of clinical symptoms in Alzheimer's disease and dementia with Lewy bodies
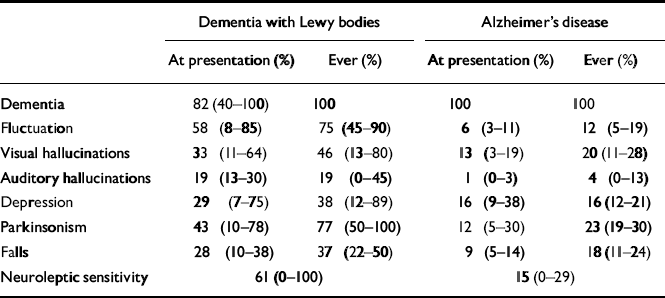
| Dementia with Lewy bodies | Alzheimer's disease | |||
|---|---|---|---|---|
| At presentation (%) | Ever (%) | At presentation (%) | Ever (%) | |
| Dementia | 82 (40-100) | 100 | 100 | 100 |
| Fluctuation | 58 (8-85) | 75 (45-90) | 6 (3-11) | 12 (5-19) |
| Visual hallucinations | 33 (11-64) | 46 (13-80) | 13 (3-19) | 20 (11-28) |
| Auditory hallucinations | 19 (13-30) | 19 (0-45) | 1 (0-3) | 4 (0-13) |
| Depression | 29 (7-75) | 38 (12-89) | 16 (9-38) | 16 (12-21) |
| Parkinsonism | 43 (10-78) | 77 (50-100) | 12 (5-30) | 23 (19-30) |
| Falls | 28 (10-38) | 37 (22-50) | 9 (5-14) | 18 (11-24) |
| Neuroleptic sensitivity | 61 (0-100) | 15 (0-29) | ||
Age at onset ranges from 50 to 83 years and at death from 68 to 92 years, with a slight excess of males. Mean survival time is similar to that for Alzheimer's disease, although some DLB patients show rapid symptom progression and death within 1-2 years of onset. Cognitive function appears to worsen at the same rate as in Alzheimer's disease, and parkinsonism at a rate similar to that in Parkinson's disease: approximately 10% decline per year for each.
Fluctuation in cognitive performance and level of consciousness is the most characteristic feature of DLB. It is usually evident on a day-to-day basis, and often apparent within much shorter periods. The marked amplitude between best and worst performance distinguishes it from the minor day-to-day variations that commonly occur in dementia of any cause. About two-thirds of patients report visual hallucinations, repeatedly seeing people and animals that appear to be real but make no noise. Emotional responses to these experiences vary from intense fear to indifference or even amusement. Depressive symptoms are common and 40% of patients have a major depressive episode, similar to the rate in Parkinson's disease and significantly greater than in Alzheimer's disease.
Up to 70% of patients have parkinsonism; bradykinesia, limb rigidity and gait disorder are the most common manifestations. When present, these symptoms may be as severe in DLB as in Parkinson's disease. Recurrent falls and syncope occur in up to a third, presumably reflecting autonomic nervous system involvement. Transient disturbances of consciousness in which patients are found mute and unresponsive for periods of several minutes may represent the extreme of fluctuation in attention and arousal, but are often mistaken for transient ischaemic attacks, despite a lack of focal neurological signs.
DIAGNOSIS AND DIFFERENTIAL DIAGNOSIS
There are four main categories of disorder that should be considered in the differential diagnosis of DLB. These are other dementia syndromes; other causes of delirium; other neurological syndromes such as Parkinson's disease, progressive supranuclear palsy, multi-system atrophy or Creutzfeldt—Jakob disease; and other psychiatric disorders such as late-onset delusional disorder, depressive psychosis and mania. The most frequent clinical misdiagnosis of DLB is as Alzheimer's disease. Accurate diagnosis rests upon careful history-taking and physical and mental state examinations. Although no specific biological test or marker is available, clinical investigations may be helpful (Table 2).
Table 2 The role of clinical investigations in discriminating Alzheimer's disease and dementia with Lewy bodies
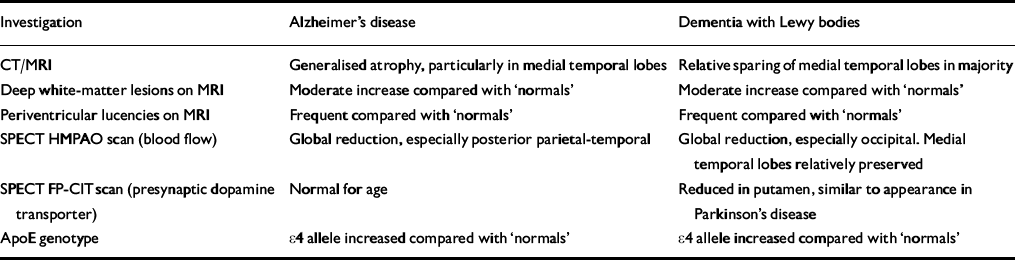
| Investigation | Alzheimer's disease | Dementia with Lewy bodies |
|---|---|---|
| CT/MRI | Generalised atrophy, particularly in medial temporal lobes | Relative sparing of medial temporal lobes in majority |
| Deep white-matter lesions on MRI | Moderate increase compared with ‘normals’ | Moderate increase compared with ‘normals’ |
| Periventricular lucencies on MRI | Frequent compared with ‘normals’ | Frequent compared with ‘normals’ |
| SPECT HMPAO scan (blood flow) | Global reduction, especially posterior parietal-temporal | Global reduction, especially occipital. Medial temporal lobes relatively preserved |
| SPECT FP-CIT scan (presynaptic dopamine transporter) | Normal for age | Reduced in putamen, similar to appearance in Parkinson's disease |
| ApoE genotype | ϵ 4 allele increased compared with ‘normals’ | ϵ 4 allele increased compared with ‘normals’ |
Structural brain imaging in DLB reveals generalised atrophy, although 40% of patients show preservation of medial temporal lobe structures, unlike Alzheimer's disease. There is no difference from Alzheimer's disease in terms of degree of ventricular enlargement, frontal lobe atrophy or presence of white-matter changes on magnetic resonance imaging (Reference Barber, Scheltens and GholkarBarber et al, 1999). Using single-photon emission computed tomography (SPECT), significant reduction in striatal uptake of a ligand for the presynaptic dopamine transporter site (FP-CIT) is seen in DLB but not in Alzheimer's disease, and this may prove to be a highly specific and widely applicable diagnostic test (Reference Walker, Costa and IncaWalker et al, 1999).
The consensus criteria for the clinical diagnosis of DLB are given in the Appendix (Reference McKeith, Galasko and KosakaMcKeith et al, 1996). Particular emphasis needs to be given to recognising the characteristic pattern of cognitive dysfunction with prominent attentional deficits, visuospatial impairment and frontosubcortical dysfunction. In contrast to Alzheimer's disease there is often a relative preservation of short-term memory. Probable DLB is diagnosed if any two of the three core symptoms (fluctuating cognition, visual hallucinations, spontaneous motor features of parkinsonism) are additionally present.
Several autopsy validation studies have examined the diagnostic accuracy of the consensus criteria and find uniformly high specificity, usually 0.9-1.0, but sensitivity of case detection is more variable and generally lower (0.22-0.83). Identifying cognitive fluctuation poses clinicians the greatest diagnostic difficulty. Standardised methods for quantifying fluctuation in dementia have now been published. The Clinician Assessment of Fluctuation is a clinician-administered severity scale, and the One Day Fluctuation Assessment Scale is based on a caregiver questionnaire. If independently confirmed, these methods have the potential to significantly increase the reliability — and therefore the validity — of a diagnosis of DLB (Reference Walker, Ballard and AyreWalker et al, 2000).
MANAGEMENT
Antipsychotic pharmacotherapy
Managing psychosis is one of the most difficult challenges in the care of patients with DLB. It is a major source of distress to patients, exacerbates the carer's burden, and is a determinant of earlier transfer to institutional care and an increased mortality. Neuroleptic agents are usually the drugs of choice for psychosis in dementia. In DLB, however, they are preferably avoided or used only with great caution, since severe neuroleptic sensitivity reactions can precipitate irreversible parkinsonism, further impair consciousness level, and induce autonomic disturbances reminiscent of neuroleptic malignant syndrome. They occur in 40-50% of neuroleptic-treated patients with DLB and are associated with a two— to threefold increased mortality. Acute D2 receptor blockade is thought to mediate these effects, and despite some promising initial reports, atypical and novel antipsychotic agents such as risperidone and olanzapine seem to be about as likely to cause adverse reactions as older, ‘typical’ drugs. Sedation, increased confusion and worsening of parkinsonism are the most common side-effects.
Cholinesterase inhibitors
There have been several reports that patients who respond well to cholinesterase inhibitor (ChEI) treatments are more likely to have DLB than Alzheimer's disease at autopsy. This is consistent with the neurochemical profile of DLB and the fact that post-synaptic cortical muscarinic receptors are functionally intact. Case reports suggest that apathy, somnolence, hallucinations and delirium are symptoms of DLB that improve substantially with ChEI therapy (e.g. Reference Samuel, Caliguri and GalaskoSamuel et al, 2000). Eleven patients with DLB, mean age 78.5 years, treated with rivastigmine in an open-label study over 12 weeks had reductions in delusions (73%), apathy (61%), agitation (52%) and hallucinations (31%) compared with baseline. Forty-five per cent (5) of the patients experienced significant clinical improvements, defined by a reduction of more than 50% in a composite score comprising hallucinations, delusions, apathy and agitation. Five of seven patients lost all of their delusions, three of eight stopped hallucinating and three had significantly fewer or less troublesome hallucinations. Carers and patients regarded these improvements, which had not previously been observed with other treatments, including low-dose neuroleptics, as substantial. No patient became more Parkinsonian (Reference McKeith, Grace and WalkerMcKeith et al, 2000a ).
A multi-centre, randomised controlled trial of rivastigmine supports these early case reports. One hundred and twenty patients from the UK, Spain and Italy, fulfilling consensus criteria for probable DLB, were given rivastigmine at daily doses up to 12 mg (mean end-dose 9.4 mg) or placebo for 20 weeks. A 3-week drug-free period followed. Patients on rivastigmine showed significantly reduced core psychiatric symptoms of DLB, i.e. they were less apathetic and had less anxiety, delusions and hallucinations while receiving treatment. Approximately twice as many patients on rivastigmine (63%) as on placebo (30%) showed at least a 30% improvement from baseline with regard to psychiatric symptoms typical of DLB. Active-treatment patients were also able to perform significantly faster and better on a computerised battery of neuropsychological tests, particularly on tasks with a substantial attentional component. After drug discontinuation the differences between rivastigmine and placebo tended to disappear. Some of the known adverse events of ChEI (nausea, vomiting, anorexia) were seen more frequently on rivastigmine than on placebo, but safety and tolerability of the drug were judged acceptable (Reference McKeith, Spano and Del SerMcKeith et al, 2000b ).
Although further, confirmatory studies are required, there is substantial evidence accumulating that ChEIs are effective in some (although not all) DLB patients, and the class should be considered as first-line pharmacological treatments for cognitive dysfunction, apathy, psychosis and agitation in this disorder.
Other medications
There is no available information about the effects of antidepressants, anticonvulsants or benzodiazepines on psychiatric and behavioural symptoms. It has been suggested that clonazepam may be helpful in controlling the characteristic sleep disturbance of DLB with its vivid dreams and a loss of normal muscle atonia in rapid eye movement sleep, which lead to excessive jerking or complex vigorous movements. Although limited levodopa responsiveness has generally been reported in DLB, this may reflect a failure to treat, or underdosage, because of concerns about exacerbating psychotic symptoms. A cautious trial of levodopa is recommended in DLB patients with clinically significant motor disability.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
• Dementia with Lewy bodies (DLB) accounts for 15-20% of all dementia in old age that can now be accurately diagnosed using operationalised criteria. The most frequent clinical misdiagnosis of DLB is as Alzheimer's disease.
-
• Fifty per cent of patients with DLB show neuroleptic sensitivity reactions with a two— to threefold increase in mortality.
-
• Cholinesterase inhibitors improve cognition, psychosis and other neuropsychiatric features in DLB. They may soon become established as first-line treatment.
LIMITATIONS
-
• Diagnostic accuracy for DLB has not yet been established outside specialised research settings.
-
• The boundaries between DLB and Parkinson's disease with dementia/psychosis remain indistinct.
-
• The National Institute for Clinical Excellence does not yet recommend the use of cholinesterase inhibitors in DLB. Further placebo-controlled trials are urgently needed.
APPENDIX
Consensus criteria for the clinical diagnosis of probable and possible dementia with Lewy bodies
-
(a) The central feature required for a diagnosis of dementia with Lewy bodies (DLB) is progressive cognitive decline of sufficient magnitude to interfere with normal social or occupational function. Prominent or persistent memory impairment may not necessarily occur in the early stages but is usually evident with progression. Deficits on tests of attention and of frontal-subcortical skills and visuospatial ability may be especially prominent.
-
(b) Two of the following core features are essential for a diagnosis of probable DLB, one is essential for possible DLB.
-
(i) Fluctuating cognition with pronounced variations in attention and alertness.
-
(ii) Recurrent visual hallucinations which are typically well formed and detailed.
-
(iii) Spontaneous motor features of parkinsonism.
-
-
(c) Features supportive of the diagnosis are:
-
(i) Repeated falls
-
(ii) Syncope
-
(iii) Transient loss of consciousness
-
(iv) Neuroleptic sensitivity
-
(v) Systematised delusions
-
(vi) Hallucinations in other modalities.
-
-
(d) A diagnosis of DLB is less likely in the presence of:
-
(i) Stroke disease, evident as focal neurological signs or on brain imaging.
-
(ii) Evidence on physical examination and investigation of any physical illness, or other brain disorder, sufficient to account for the clinical picture.
-
See McKeith et al (Reference McKeith, Galasko and Kosaka1996).





eLetters
No eLetters have been published for this article.