Through cross-sectional magnetic resonance imaging studies, it is now well established that brain volume reductions are present in schizophrenia (Reference Wright, Rabe-Hesketh and WoodruffWright et al, 2000). Were these reductions to reflect the disease process, one would expect them to mimic the clinical course and to be related to outcome. A cross-sectional magnetic resonance imaging study (Reference van Haren, Cahn and Hulshoff Polvan Haren et al, 2003) examined brain volume change as a predictor of outcome in recent-onset schizophrenia, but did not find any associations between the static baseline brain volume measurements and clinical and functional outcomes after 2 years. As brain volume reductions have been shown to be progressive (Reference Cahn, Hulshoff Pol and LemsCahn et al, 2002; Reference Lieberman, Tollefson and CharlesLieberman et al, 2005; Reference Woods, Ward and JohnsonWoods et al, 2005), dynamic brain volume changes, using two magnetic resonance measurements, might be more informative in relation to clinical and functional outcome. In a similar study, we examined 34 people with first-episode schizophrenia at baseline and after 1 year and compared them with 36 healthy people. An accelerated decline in total brain volume and in grey matter volume and an increase in lateral ventricle volume were found in patients compared with healthy individuals (Reference Cahn, Hulshoff Pol and LemsCahn et al, 2002). These progressive changes were not associated with symptoms, but did predict functional outcome 2 years after the initial assessment. Thus, in contrast to static brain volumes, dynamic brain measurements could be more useful in predicting outcome in schizophrenia. As the clinical course of schizophrenia reaches a plateau after about 5 years following the initial treatment (Reference Davidson and McGlashanDavidson & McGlashan, 1997), the present study clinically reexamined the same individuals 5 years after the initial evaluation, using various outcome measures. It was hypothesised that early brain volume changes in schizophrenia predict the longer-term outcome of the illness.
METHOD
Our initial study included 34 people with first-episode schizophrenia. After a mean follow-up period of 5.3 years (s.d.=0.8), three patients refused further participation. The present study included the remaining 31 participants (27 men and 4 women), with a mean age of 25.74 years (s.d.=4.88). Before inclusion, participants had used no or little antipsychotic medication: mean lifetime dose 163.85 mg (s.d.=72.26) haloperidol equivalents. All participants received a DSM–IV (American Psychiatric Association, 1994) diagnosis of schizophrenia (26) or schizoaffective disorder (5) and provided written informed consent.
Magnetic resonance images were acquired on a 1.5 Philips NT scanner and obtained at inclusion (T0) and after 1 year (T1). In-house developed software was used to measure mean (s.d.): total brain volume T0=1321.22 ml (108.57) and T1=1306.84 ml (113.66); grey matter T0=687.36 ml (50.29) and T1=668.91 ml (57.20); white matter T0=473.87 ml (64.06) and T1=476.06 ml (60.61); cerebellar T0=145.79 ml (11.99) and T1=147.04 ml (7.70); frontal lobe T0=291.86 ml (28.70) and T1=288.05 ml (23.11); lateral T0=14.84 ml (6.64) and T1=15.89 ml (7.70); and third ventricle T0=0.84 ml (0.39) and T1=0.88 ml (0.37). Images were checked and corrected manually if necessary. For a description of procedure and segmentation, see Hulshoff Pol et al (Reference Hulshoff Pol, Schnack and Bertens2002).
Patients were assessed with the Positive and Negative Syndrome Scale (PANSS; Reference Kay, Fiszbein and OplerKay et al, 1987), mean (s.d.) at: T0 positive symptoms 18.24 (5.77) and negative symptoms 18.90 (6.55); T1 positive symptoms 12.93 (4.61) and negative symptoms 15.40 (5.20); and T5 (5 years) positive symptoms 14.13 (5.71) and negative symptoms 15.38 (7.13). The Camberwell Assessment of Need (CAN; Reference Phelan, Slade and ThornicroftPhelan et al, 1995) mean (s.d.) was obtained after 2 years of follow up (T2): number of met and unmet needs 5.97 (3.77); and at T5: number of met and unmet needs 9.48 (6.61). In addition, Global Assessment of Functioning (GAF; American Psychiatric Association, 1994) mean (s.d.) 53.61 (21.57); and the assessment whether patients lived independently or not: (yes=17; no=14); were obtained at T5. Negative and positive PANSS symptom scores at T5 were used as measures of clinical outcome. Scores on the CAN, GAF and the assessment whether patients lived independently or not were used as measures of functional outcome.
To assess whether dynamic brain volume changes in the first year predict the 5-year clinical and functional outcome, linear regression analyses were performed with the unstandardised regression coefficient (β) representing the outcome score per millilitre volume change. Brain volume change (T0 to T1) of the various brain structures entered the analyses as predictor variables, with age and intracranial volume as covariates. As progressive brain volume change might have commenced before T0, baseline measures of the brain structures also entered the analyses as covariates. The various clinical and functional outcome scores obtained at T5 entered the analyses as dependent variables separately. Since clinical outcome might be variable initially but could stabilise over time, the same analyses were done with changes in PANSS scores (T1 to T5) as dependent variables. To assess whether clinical decline is progressive, paired-sample t-tests were performed with PANSS scores (T1 to T5) and CAN scores (T2 to T5) as paired variables.
RESULTS
A greater total brain volume decrease in the first year predicted a higher negative symptom score on PANSS (β=–0.1, t=–2.51, P=0.02) and a lesser likelihood of living independently (β=–0.01, t= –2.29, P=0.03) at 5-year follow up.
A greater grey matter volume decrease in the first year predicted a higher positive symptom score (β=–0.09, t=–2.29, P= –0.03), a higher negative symptom score (β=–0.16, t=–3.38, P=0.002) (Fig. 1a), a lower GAF score (β=0.40, t=2.79, P= 0.01) and a lesser likelihood of living independently (β=–0.01, t=–3.94, P=0.001) (Fig. 1b) at T5.
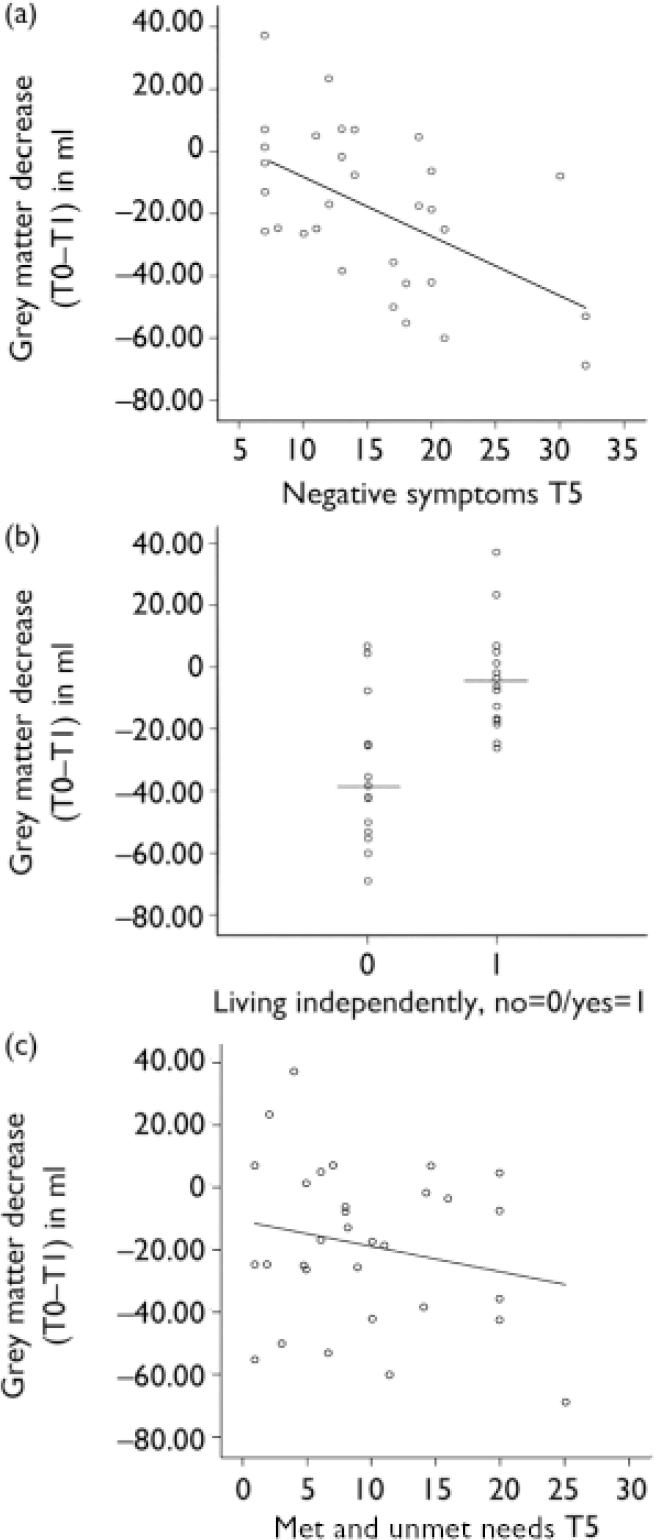
Fig. 1 Progressive brain volume changes in the first year predict 5-year outcome in schizophrenia. T0, baseline, T1, follow up at 1 year, T5, follow up 5 years. (a) Change in grey matter (T0–T1) and negative symptoms as measured with the Positive and Negative Syndrome Scale (T5). (b) Change in grey matter (T0–T1) and living independently (T5). (c) Change in lateral ventricle volume (T0–T1) and need of care measured with the Camberwell Assessment of Need (T5).
A greater white matter volume increase in the first year predicted higher positive symptom score (β=0.09, t=2.29, P=0.03) at T5.
A greater lateral ventricle volume increase in the first year predicted a greater number of met and unmet needs (β=1.47, t=2.82, P=0.009) (Fig. 1c) as measured with the CAN and a lesser likelihood to live independently (β=–1.0, t=–2.31, P=0.03) at T5. A greater cerebellar volume decrease in the first year predicted a higher negative symptom score (β=–0.79, t= –2.24, P=0.03) at T5.
Frontal lobe and third ventricle volume change did not predict outcome. A greater grey matter (β=–0.12, t=–2.8, P=0.009) and cerebellar (β–0.68, t=–2.43, P=0.02) volume decrease in the first year predicted a greater increase in the negative symptom score of the PANSS (T1 to T5). A greater white matter increase (β=0.19, t=3.12, P=0.004) predicted a greater increase in the positive symptom score of the PANSS. There was no significant increase in symptoms as measured with PANSS between T1 and T5, but total CAN scores significantly increased (t=3.24, d.f.=27, P=0.003) between T2 and T5.
DISCUSSION
This study found that early progressive brain volume changes in the first year can predict the 5-year outcome of schizophrenia. Those patients who had the largest decrease in grey matter in the first year had the highest negative symptom scores and were less likely to live independently 5 years after the first evaluation. Thus, it appears that early dynamic brain changes are not only associated with symptomatic outcome but also with functional outcome. Furthermore, these findings suggest that brain changes in the early stages of schizophrenia are related to the disease process and are clinically relevant in determining prognosis, and they emphasise the importance of early intervention in the treatment of schizophrenia, aiming to slow down or stop progressive brain volume loss.
This view is strengthened by another longitudinal magnetic resonance study that examined dynamic brain volume changes in people at very high risk of developing schizophrenia (Reference Pantelis, Velakoulis and McGorryPantelis et al, 2003). This study reported progressive grey matter decrease in very-high-risk individuals who developed a psychotic illness within 12 months. None the less, whether these early progressive brain volume changes in prodromal and first-episode schizophrenia are caused by the disease itself or are a consequence of the disease (and its treatment) still remains unanswered. The treatment of schizophrenia should focus on slowing this early progressive brain loss, with the goal of affecting the clinical course favourably.




eLetters
No eLetters have been published for this article.