In the USA, nearly a quarter of all pregnancies are complicated by pre-pregnancy obesity and the prevalence is increasing(Reference Kim, Dietz and England1). Pregnant women who are obese are at higher risk of pre-eclampsia(Reference Cedergren2, Reference Hauger, Gibbons and Vik3), gestational diabetes mellitus(Reference Chu, Callaghan and Kim4), birth trauma, large-for-gestational-age birth(Reference Cedergren2) and stillbirth(Reference Cedergren2, Reference Cnattingius, Bergstrom and Lipworth5) compared with their lean counterparts. Nevertheless, the mechanism by which obesity contributes to poor outcomes remains uncertain.
Nutritional status during pregnancy may partially mediate the relationship between pre-pregnancy obesity and adverse pregnancy and birth outcomes. In non-pregnant populations, obesity has been associated with insufficiencies of micronutrients, including vitamin E, vitamin C, vitamin D, folate, vitamin A and carotenoids(Reference Kimmons, Blanck and Tohill6, Reference Andersen, Jacobs and Gross7). Obese individuals may also have lower levels of essential fatty acids (EFA)(Reference Kishino, Watanabe and Urata8, Reference Karlsson, Marild and Brandberg9) than lean patients. But the relationship between obesity and nutritional biomarkers has not been thoroughly researched in pregnancy. Micronutrients and EFA play critical roles in the healthy physical and neurological development of the fetus and prevent conditions such as anaemia and neural tube defects(10, Reference Uauy, Hoffman and Peirano11). Therefore, it is critical to explore these associations in pregnancy.
The objective of our study was to evaluate the association between pre-pregnancy BMI and patterns of nutritional biomarkers at ≤20 weeks’ gestation.
Methods and procedures
We conducted a secondary data analysis from the Antidepressant Use During Pregnancy (ADUP) Study, a prospective cohort study of pregnancy in Pittsburgh, PA, USA. The details of this study have been described previously(Reference Wisner, Sit and Hanusa12, Reference Bodnar, Wisner and Luther13). Pregnant women were recruited at ≤20 weeks’ gestation. Eligible women had singleton gestations and women were excluded if they had psychosis, bipolar disorder, active substance use disorder, gestational exposure to benzodiazepines or prescription drugs in the category of D or X (other than selective serotonin reuptake inhibitors) defined by the US Food and Drug Administration, or pre-existing chronic diseases. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving patients were approved by the University of Pittsburgh Institutional Review Board. Written informed consent was obtained from all patients.
The ADUP Study recruited women from 2000 to 2007. In 2004, the study protocol was modified to include nutrition measures, including biomarker assessment in maternal blood. Of the 197 eligible women interviewed from 2004 to 2007, 130 (66 %) provided a non-fasting blood sample at ≤20 weeks that was processed for a full panel of nutritional biomarkers. We excluded one woman with missing data for pre-pregnancy weight. Our final analytic sample was 129 women. Women included in the analysis were less likely to be nulliparous than women who were excluded for missing weight measurements or blood samples (33 % v. 51 %, P = 0·01). Other maternal characteristics such as pre-pregnancy BMI, age, education, race, marital status, smoking status, employment status and diagnosed major depressive disorder did not differ significantly (P > 0·05) between the two groups.
Our exposure of interest was general maternal adiposity before conception, measured using pre-pregnancy BMI (weight (kg)/height (m)2). Pre-pregnancy BMI was based on pre-pregnancy weight, self-reported at enrolment, and measured height. Because only four women in the sample were underweight (BMI < 18·5 kg/m2), we categorized women into three groups: lean (BMI < 25·0 kg/m2), overweight (BMI = 25·0–29·9 kg/m2) and obese (BMI ≥ 30·0 kg/m2)(14). Our outcome of interest was maternal nutritional status, as measured by patterns of nutritional biomarkers. At enrolment, women provided a non-fasting blood sample. Blood samples were assayed for red cell EFA, plasma folate, plasma homocysteine, plasma ascorbic acid, serum retinol, serum 25-hydroxyvitamin D, serum α-tocopherol, serum ferritin, serum soluble transferrin receptors and serum carotenoids using methods described previously(Reference Bodnar, Wisner and Luther13, Reference Tomedi, Bogen and Hanusa15).
Women identified their race/ethnicity as non-Hispanic white, non-Hispanic African American and other. Because only four women self-identified as ‘other’, we combined the African American and the ‘other’ groups into a non-white category for analysis. Women were categorized as nulliparous or as having a previous live birth. A diagnosis of major depressive disorder was made using the Structured Clinical Interview for DSM-IV(Reference First, Gibbon and Spitzer16). Educational status was defined as having less than a high school education, some college, college degree and post-graduate education. Women were classified as unemployed or employed (which included women who worked full time, part time or occasionally). For marital status, ‘married’ included married or living as married and ‘unmarried’ included single, separated, widowed and divorced. Women were classified as current smokers if they smoked at all during pregnancy or non-smokers if they did not.
We used Pearson χ 2 tests and Student t tests to determine differences in maternal characteristics by pre-pregnancy BMI categories. To describe skewed biomarkers, we calculated geometric means and log-transformed biomarkers before statistical testing. We conducted a factor analysis on the fifteen untransformed maternal dietary biomarkers as described previously(Reference Bodnar, Wisner and Luther13). Three patterns were generated and assigned names based on the biomarkers that loaded heavily on the pattern. Each represents biologically meaningful correlations between biomarkers: pattern 1 ‘Essential Fatty Acids (EFA)’, pattern 2 ‘Micronutrients’ and pattern 3 ‘Carotenoids’. Pattern scores were categorized based on tertiles of the distribution.
Logistic regression was used to assess the independent associations between pre-pregnancy BMI and the likelihood of being in the lowest tertile of each nutritional pattern. Potential confounders were maternal age, race/ethnicity, parity, education, marital status, smoking status, employment and depression (diagnosed with the Structured Clinical Interview for DSM-IV(Reference First, Gibbon and Spitzer16)). Only parity, race/ethnicity and age met our a priori definition of confounding (≥10 % change in the odds ratio after excluding the covariate from the full model). Analyses were conducted using statistical software package Stata version 11·0.
Results
The women in the cohort were primarily well-educated, non-Hispanic white, married, non-smokers and employed (Table 1). The mean pre-pregnancy BMI was 26·6 (sd 6·0) kg/m2. About half (49·6 %) of the women were lean, 22·5 % were overweight and 27·9 % were obese. Obese women were less likely to have a college degree (P < 0·01) and more likely to be nulliparous (P < 0·01) and depressed (P = 0·03) than lean women. Maternal age, race/ethnicity, marital status, smoking and employment did not differ significantly by pre-pregnancy BMI category.
Table 1 Maternal characteristics of the study population, stratified by pre-pregnancy BMI; Antidepressant Use During Pregnancy Study, Pittsburgh, PA, USA

*Based on Student's t test for maternal age and χ 2 test for the other covariates.
†As measured by the Structured Clinical Interview for DSM-IV(Reference First, Gibbon and Spitzer16).
In unadjusted analysis, women who were overweight before pregnancy had lower mean concentrations of plasma folate (P = 0·04), plasma ascorbic acid (P = 0·01), serum β-carotene (P = 0·01) and serum β-cryptoxanthin (P = 0·02) than lean women (Table 2). Compared with lean women, obese pregnant women had lower mean concentrations of red cell DHA (P < 0·01), red cell arachidonic acid (P = 0·01), plasma ascorbic acid (P = 0·03), serum 25-hydroxyvitamin D (P = 0·01), serum β-carotene (P < 0·01), serum lutein + zeaxanthin (P < 0·01) and serum β-cryptoxanthin (P < 0·01) and higher mean serum soluble transferrin receptors concentration (P = 0·03).
Table 2 MeanFootnote * maternal nutritional biomarkers at 20 weeks’ gestation, stratified by pre-pregnancy BMI; Antidepressant Use During Pregnancy Study, Pittsburgh, PA, USA
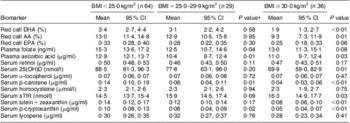
AA, arachidonic acid; 25(OH)D, 25-hydroxyvitamin D; sTfR, soluble transferrin receptors.
* Geometric means, all values are presented as mean and 95 % confidence interval.
† Student's t test, biomarkers log-transformed before tests were performed, BMI<25·0 kg/m2 is the reference group.
In unadjusted analysis, obese women had significantly greater odds of being in the lowest tertile of all three nutritional patterns. After adjustment for confounders, obese pregnant women were three and five times more likely to be in the lowest tertile of the EFA and Carotenoid pattern, respectively, than lean women (Table 3). After adjustment, there was no association between obesity and Micronutrient pattern score. There was no relationship between pre-pregnancy overweight and any pattern before or after adjustment.
Table 3 Association between pre-pregnancy BMI and the lowest tertileFootnote * of each nutritional pattern; Antidepressant Use During Pregnancy Study, Pittsburgh, PA, USA

Ref, referent category.
* Lowest tertile compared to the combined middle and highest tertiles of nutrient components.
† Adjusted for parity, race/ethnicity and age. Further adjustment for other covariates had no meaningful impact on the findings.
Discussion
Using factor analysis of an array of nutritional biomarkers, we found that a larger percentage of obese women were in the lowest tertile of the EFA, Micronutrient and Carotenoid patterns than lean women at ≤20 weeks’ gestation. After adjustment for maternal characteristics, pre-pregnancy obesity remained associated with poorer EFA and Carotenoid patterns.
To our knowledge, the present study is the first one to examine pre-pregnancy BMI relative to a wide range of maternal biomarkers. Our EFA and carotenoid conclusions are in agreement with previous studies of individual biomarkers in a variety of non-pregnant populations(Reference Andersen, Jacobs and Gross7, Reference Galan, Viteri and Bertrais17–Reference Micallef, Munro and Phang20). For example, in a cross-sectional analysis of 4512 non-pregnant women, both obese and overweight women were significantly more likely to be in the lowest 20th percentile of a sum of carotenoid concentrations than lean women(Reference Kimmons, Blanck and Tohill6). Although we observed that obesity was associated with lower Micronutrient pattern scores in unadjusted analysis, adjustment for parity, race/ethnicity and age eliminated this effect. In contrast, other researchers have reported poorer micronutrient concentrations among obese women in non-pregnant populations(Reference Kimmons, Blanck and Tohill6, Reference Aasheim, Hofso and Hjelmesaeth21). One study of non-pregnant women found that obese women were more likely to have low levels of vitamin E, vitamin C, vitamin D and serum folate than non-obese women(Reference Kimmons, Blanck and Tohill6). Studies in pregnancy report that maternal obesity increases the likelihood of poor maternal vitamin D(Reference Bodnar, Catov and Roberts22) and folate(Reference Han, Ha and Park23) status in pregnancy. The differences in our conclusions may be due in part to prenatal vitamin use. While prenatal vitamins always contain micronutrients, they do not uniformly contain EFA or many carotenoids. Given the high socio-economic status of our population, the use of prenatal vitamins and other dietary supplements may have been widespread, leading to a predominance of elevated micronutrient concentrations. If obese women in our cohort were taking prenatal supplements but consumed diets poor in fish, the major source of DHA and EPA, or fruits and vegetables, which contribute to carotenoids(Reference Laraia, Bodnar and Siega-Riz24), this may account for our results. Unfortunately, complete data on dietary intake and supplementation were not available at 20 weeks’ gestation in the ADUP Study.
In addition to diet and supplementation differences between obese and lean pregnant women, pre-pregnancy obesity may alter the absorption and metabolism of carotenoids and EFA. It has been previously hypothesized that fat-soluble nutrients, such as carotenoids, may also be sequestered by adipose tissue(Reference Wallstrom, Wirfalt and Lahmann25). However, among the individual nutritional biomarkers that had lower concentrations in obese women, we did not find a distinct pattern between the lipid-soluble and water-soluble markers.
Major strengths of our study include an analysis of a wide array of measured nutritional biomarkers. The use of factor analysis accounted for inherent correlations between nutrients and reduced the likelihood of type 1 error due to multiple comparisons. Our study was limited by self-reported weight to calculate BMI, which may have contributed to non-differential misclassification(Reference Bodnar, Siega-Riz and Simhan26). While we had a relatively small sample size of primarily white, well-educated, non-smoking women, we do not expect the biological associations between nutritional biomarkers and pre-pregnancy BMI to alter based on these factors. Our lack of longitudinal biomarker measurements limits our understanding of the temporality of these relationships. Large, prospective studies with repeated biomarker measures and detailed information on supplement use during pregnancy are needed to determine the extent of the association between maternal obesity and poor nutritional status.
Our results suggest that obese pregnant women have diminished EFA and carotenoid status during pregnancy. A better understanding of the effect of pre-pregnancy obesity on maternal micronutrient and EFA status may lead to specific nutritional interventions that can improve pregnancy and birth outcomes in this at-risk population.
Acknowledgements
Sources of funding : Support for this project came from the National Institutes of Health (grants K01 MH074092, R01 MH060335 and R24 HL076858) and the Eunice Kennedy Shriver National Institute of Child Health & Human Development (Reproductive, Perinatal and Pediatric Epidemiology Grant HD 055 162-03 NIH NRSA T32). Conflicts of interest: The authors have no conflicts of interest to declare. Ethics: The study was approved by the University of Pittsburgh Institutional Review Board. Authors’ contributions: L.E.T. analysed the data and wrote the manuscript; C.-C.H.C. provided valuable biostatistical assistance; P.K.N. provided significant assistance with pattern analysis; R.W.E. provided technical assistance for the biomarker assays and critically reviewed the manuscript; J.F.L. performed the principal component analysis; K.L.W. designed the research and provided the data; L.M.B. provided significant analytical assistance and critically edited the manuscript; all authors read and approved the final manuscript. Acknowledgements: The authors thank Ms Brandi Duffy and Ms Rona de la Vega for expert chemical analysis.





