Although the benefits of breast-feeding for both infants and mothers have been extensively documented, the breast-feeding rates in many countries still fall short of the WHO recommendations( 1 , Reference Bagci Bosi, Eriksen and Sobko 2 ). In China, although the breast-feeding rate started to increase in the 1990s, the national target of exclusive breast-feeding (EBF) of 80 % was not reached in most cities and provinces( Reference Xu, Qiu and Binns 3 ). In central and western China, less than one-third of infants younger than 6 months have been exclusively breast-fed( Reference Guo, Fu and Scherpbier 4 ). The low breast-feeding rates may be partly explained by the increase in obesity among pregnant women. In China, the prevalence of overweight/obesity is low (approximately 2·1 %), according to the criteria of the WHO( Reference Liu, Dai and Dai 5 ). However, the prevalence of obesity increased from 5·0 to 10·1 % in women during 1993–2009, according to the WHO suggestions for Chinese( Reference Xi, Liang and He 6 ).
Numerous studies on the association of pre-pregnancy BMI with breast-feeding have mainly focused on the populations of the USA, European countries and Australia( Reference Turcksin, Bel and Galjaard 7 ). Most reports have supported a negative association between pre-pregnancy obesity and the duration of EBF( Reference Hauff, Leonard and Rasmussen 8 , Reference Baker, Michaelsen and Sorensen 9 ) and any breast-feeding (ABF)( Reference Hauff, Leonard and Rasmussen 8 – Reference Li, Jewell and Grummer-Strawn 11 ). Previous studies have also revealed ethnic differences in breast-feeding success among American women of different pre-pregnancy BMI categories( Reference Masho, Cha and Morris 12 – Reference Liu, Smith and Dobre 14 ). Additional studies are required for a comprehensive understanding of the association between pre-pregnancy BMI and breast-feeding among different races/ethnicities( Reference Wojcicki 15 ). Moreover, little information is available on the current situation of breast-feeding in Asian women with pre-pregnancy obesity, including Chinese women.
Compared with pre-pregnancy BMI, which represents the level of fatness before conception, gestational weight gain (GWG) is an indicator of the fat gained during pregnancy. The role of GWG in breast-feeding initiation and duration remains unclear. Some studies have reported that excessive GWG exerts a negative effect on breast-feeding practice( Reference Winkvist, Brantsaeter and Brandhagen 16 , Reference Hilson, Rasmussen and Kjolhede 17 ). However, other studies have failed to support this effect( Reference Baker, Michaelsen and Sorensen 9 , Reference Castillo, Santos and Matijasevich 18 , Reference Manios, Grammatikaki and Kondaki 19 ). The inconsistent findings may be partly because of the number and type of confounders that are controlled for in statistical analyses. Additional studies are required to discern the unique and independent contribution of GWG to breast-feeding outcomes.
Moreover, the timing of the onset of copious milk secretion, known as lactogenesis II, is crucial for successful breast-feeding( Reference Brownell, Howard and Lawrence 20 ). Delayed lactogenesis II is usually defined as the onset of copious milk secretion 72 h postpartum. Previous studies have found an association between maternal postpartum obesity and delayed lactogenesis II( Reference Dewey, Nommsen-Rivers and Heinig 21 , Reference Nommsen-Rivers, Chantry and Peerson 22 ). Two studies with small sample sizes have also indicated that high pre-pregnancy BMI or having a ‘heavy body build’ is associated with delayed lactogenesis II( Reference Hilson, Rasmussen and Kjolhede 23 , Reference Chapman and Perez-Escamilla 24 ). In women with gestational diabetes mellitus, maternal pre-pregnancy obesity has been shown to be a key risk factor for delayed lactogenesis II( Reference Matias, Dewey and Quesenberry 25 ). Few reports have focused on the relationship between GWG and delayed lactogenesis II.
Thus, the purpose of the current prospective longitudinal cohort study was to examine the relationship among maternal pre-pregnancy BMI, onset time of lactogenesis II, and initiation and duration of breast-feeding in Chinese women after adjustment for known maternal and infant factors. The study also examined whether GWG is independently associated with breast-feeding.
Materials and methods
Study population
The study population for the present analysis was drawn from participants of the Ma’anshan Birth Cohort Study in China. The objective of the study was to investigate the effects of prenatal exposure on adverse pregnancy outcomes, child health and development. At recruitment, participants comprised women attending prenatal care clinics affiliated with Ma’anshan Maternity and Child Health Hospital in Ma’anshan City. Women eligible for inclusion into the study were those who initiated prenatal care prior to 16 weeks of gestation. Women were ineligible if they were younger than 18 years of age, did not plan to carry the pregnancy to term, or did not plan to deliver at the research hospital. From 9 October 2013 to 10 September 2014, 3474 eligible women were approached. As shown in the subject flow diagram (Fig. 1), 162 women with pregnancy terminations or stillbirths were excluded. Of the 3312 women who had a live birth, 116 were ineligible due to multiple pregnancies, maternal mortality, missing pre-pregnancy BMI, no delivery and neonatal records, missing breast-feeding data or giving up breast-feeding due to maternal disease. The final sample of postpartum women participating in the study was 3196. At 1, 3, 6 and 12 months postpartum, 99·6, 97·8, 96·6 and 95·3 % of breast-feeding data were available, respectively. The study was approved by the Biomedical Ethics Committee of Anhui Medical University and informed consent for participation was obtained from the mother at the commencement of the study.

Fig. 1 Flow diagram showing the selection of subjects from the Ma’anshan Birth Cohort Study
Pre-pregnancy BMI and gestational weight gain variables
Maternal pre-pregnancy weight was self-reported at the first prenatal visit and maternal height was measured. Pre-pregnancy BMI was calculated as kg/m2 and categorized according to the Chinese classification as underweight (<18·5 kg/m2), normal weight (18·5–23·9 kg/m2), overweight (24·0–27·9 kg/m2) and obese (≥28·0 kg/m2). GWG was calculated as the difference between pre-pregnancy weight and the last register of weight at delivery. GWG category was also according to Chinese-recommended weight gain( Reference Yang, Peng and Wei 26 ). Underweight women category should aim to gain 15·0–22·0 kg during pregnancy; those with normal weight, 13·0–21·0 kg; the overweight, 10·0–18·0 kg; and the obese, 9·5–17·0 kg. Maternal GWG categories were then defined as excessive, sufficient and insufficient weight gain.
Breast-feeding variables
The non-initiation of breast-feeding was defined as no breast-feeding postpartum at all and was assessed at 1 month postpartum. Maternal perception of the onset of lactation was also evaluated. Mothers were asked to indicate on what day postpartum they first felt that their breasts were noticeably full after delivery. The timing reported was categorized as three variables (<24 h, 1–3 d, >3 d). It is a valid method of determining timing of lactogenesis II and has been shown to correlate with actual timing of this stage( Reference Chapman and Perez-Escamilla 27 , Reference Perez-Escamilla and Chapman 28 ). Delayed lactogenesis II is defined as lactogenesis II onset more than 3 d postpartum( Reference Dewey, Nommsen-Rivers and Heinig 21 , Reference Chapman and Perez-Escamilla 24 ).
At the 1-, 3-, 6- and 12-month follow-ups, the frequency of EBF and ABF was evaluated by a questionnaire survey with the guardian. EBF included infants fed breast milk and allows the infant to receive oral rehydration salts, drops and syrups (vitamins, minerals, medicines), but nothing else, as described by WHO( 29 ). ABF was defined as breast-feeding in combination with infant formula, other milk and/or solid food.
Maternal and infant characteristics
A self-administered questionnaire was used to collect information on sociodemographic factors and reproductive history in the first trimester of pregnancy. Covariates were selected for their potential influence in the relationship between obesity and breast-feeding, including maternal age, ethnicity, education, parity, household income, smoking status and alcohol consumption. After delivery, the mothers’ and infants’ charts were systematically reviewed to collect data on pregnancy and delivery, including mode of delivery, pregnancy-induced hypertension and gestational diabetes status, infant sex, gestational age and birth weight. Diagnoses of pregnancy-induced hypertension or gestational diabetes were made by specialized physicians.
Statistical analysis
The characteristics of the cohort were summarized with unadjusted percentages for categorical data. Pearson’s χ 2 test was used to test differences between the three categorized BMI groups regarding characteristics of the study population. A stacked bar chart was utilized for the three ranges of onset time of lactogenesis II in women initiating breast-feeding based on different pre-pregnancy BMI groups. Unadjusted and adjusted risk ratios (RR) for onset of lactogenesis II and sustained EBF at 1, 3 and 6 months postpartum according to pre-pregnancy BMI category were calculated through the use of logistic regression models.
Kaplan–Meier curves and log-rank tests were used to evaluate the relationships between pre-pregnancy BMI and GWG groups and duration of breast-feeding. Then, the hazard ratios (HR) between BMI and breast-feeding outcomes after controlling for potential confounding variables were calculated by Cox proportional hazards models. We adjusted the logistic regression and Cox proportional hazards models for the following categorical variables: maternal age (<25, 25–35 or ≥35 years old), gestational age (<37 or ≥37 weeks), birth weight (<2500 or ≥2500 g), mode of delivery (vaginal or caesarean), infant sex (male or female), GWG (inadequate, adequate or excessive), ethnicity (Han or other), education (less than high school, high school, junior college or more than junior college), monthly income of family (<2500, 2500–3999 or ≥4000 RMB), parity (primiparous or multiparous), cigarette smoking (never or former/current), alcohol drinking (yes or no), pregnancy-induced hypertension (yes or no) and gestational diabetes (yes or no). All statistical analyses were performed using the statistical software package SPSS version 13.0. Statistical significance was defined as two-sided P<0·05.
Results
Characteristics
Among the 3196 pregnant women included in our analysis, 605 (18·9 %) were classified as underweight, 300 (9·4 %) as overweight and eighty-two (2·6 %) as obese. The mean age of the pregnant women was 26·4 (sd 3·6) years. The mean gestational age and birth weight of infants were 39·0 (sd 1·4) weeks and 3367 (sd 443) g, respectively. Maternal and infant characteristics according to BMI category are reported in Table 1. In higher BMI groups, socio-economic status, based on household income and maternal education, was lower. In contrast, in higher BMI groups, maternal age and the rate of caesarean delivery, multiparity, pregnancy-induced hypertension and gestational diabetes were higher. Compared with normal-weight women, the overweight or obese women were more likely to have an excessive GWG, premature or low-birth-weight infant.
Table 1 Characteristics of the included participants from the Ma’anshan Birth Cohort Study (n 3196) by pre-pregnancy BMI category

GWG, gestational weight gain.
* Categorized according to guidelines from Chinese-recommended weight gain: inadequate GWG=weight gain of <15·0, <13·0, <10·0 and <9·5 kg for underweight, normal-weight, overweight and obese women, respectively; excessive GWG=weight gain of >22·0, >21·0, >18·0 and >17·0 kg for underweight, normal-weight, overweight and obese women, respectively; adequate GWG=weight gain between the cut-off values for inadequate and excessive GWG.
Breast-feeding initiation and onset time of lactogenesis II
Overall, the rate of breast-feeding initiation was 95·9 %. No statistical difference was observed in the rates of breast-feeding initiation among women with pre-pregnancy underweight (95·0 %), normal weight (96·4 %), overweight (94·7 %) and obesity (93·9 %; P=0·22). Similarly, no statistical difference was found in the rates of breast-feeding initiation among women with insufficient (94·8 %), sufficient (95·7 %) and excessive (96·9 %; P=0·13) weight gain. In women initiating breast-feeding, the proportions of onset time of lactogenesis II were 33·0 % for <24 h postpartum, 58·3 % for 1–3 d postpartum and 8·7 % for >3 d postpartum. In higher pre-pregnancy BMI groups, the onset time of lactogenesis II was longer (Fig. 2). After adjusting for potential maternal and infant confounders, the RR of delayed lactogenesis II was 0·84 (95 % CI 0·58, 1·22) for underweight, 1·38 (95 % CI 0·90, 2·12) for overweight and 1·89 (95 % CI 1·04, 3·43) for obese, when compared with the normal-weight group. However, the rate of delayed lactogenesis II was not significantly different among insufficient (7·5 %), sufficient (7·4 %) and excessive (8·5 %; P=0·47) weight gain groups.

Fig. 2 Stacked bar chart for onset time of lactogenesis II (![]() , <24 h postpartum;
, <24 h postpartum; ![]() , 1–3 d postpartum;
, 1–3 d postpartum; ![]() , >3 d postpartum) according to pre-pregnancy BMI category in women initiating breast-feeding (n 3065) from the Ma’anshan Birth Cohort Study
, >3 d postpartum) according to pre-pregnancy BMI category in women initiating breast-feeding (n 3065) from the Ma’anshan Birth Cohort Study
Breast-feeding duration
At 1, 3 and 6 months postpartum, the rates of EBF were 43·8, 51·6 and 11·0 %, respectively. Among the different BMI groups, obese women had a higher rate of EBF than did normal-weight women only at 1 month postpartum (RR=1·62, 95 % CI 1·01, 2·59). Moreover, the difference became not significant after adjusting for antenatal characteristics (Table 2). Kaplan–Meier analysis also showed that no significant difference existed in the rate of EBF among the different BMI groups (P=0·739 for log-rank test). Overall, the median duration of ABF was 7·0 months. Figure 3 shows the Kaplan–Meier curves for the percentage of ABF across the 12 months of observation when the groups are stratified by BMI. The median duration of ABF for pre-pregnancy underweight, normal weight, overweight and obesity was 7·0, 7·0, 6·0 and 3·0 months, respectively. A significant difference was observed among the four BMI groups by log-rank test (P<0·01). The multivariable Cox proportional hazards regressions showed that the HR of weaning were 0·99 (95 % CI 0·90, 1·10) for underweight, 1·11 (95 % CI 0·95, 1·29) for overweight and 1·38 (95 % CI 1·09, 1·75) for obese, when compared with the normal-weight group. We also examined whether GWG was associated with the continuation of breast-feeding. However, in all analyses, GWG was not a significant predictor for early termination of EBF or ABF, and did not interact with pre-pregnancy BMI to affect the duration of breast-feeding (data not shown).
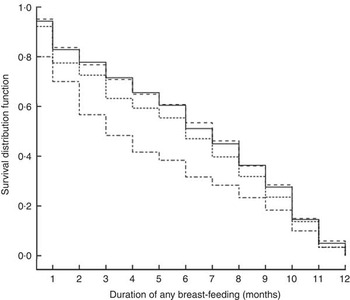
Fig. 3 Kaplan–Meier curves showing the proportion of infants who continued any breast-feeding according to pre-pregnancy BMI category (– – – – –, underweight; ———, normal weight; - - - - -, overweight; – · – · –, obese) in women initiating breast-feeding (n 3065) from the Ma’anshan Birth Cohort Study
Table 2 Relative risk (RR) of sustained exclusive breast-feeding at 1, 3 and 6 months postpartum according to pre-pregnancy BMI category in women initiating breast-feeding (n 3065) from the Ma’anshan Birth Cohort Study
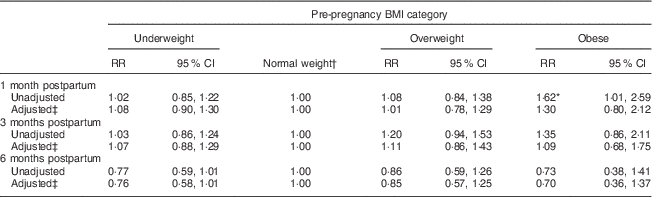
* P<0·05.
† Reference category.
‡ Adjusted for maternal age, gestational age, birth weight, mode of delivery, infant sex, gestational weight gain, ethnicity, education, monthly income of family, parity, cigarette smoking, alcohol drinking, pregnancy-induced hypertension and gestational diabetes.
Discussion
The present longitudinal study found that compared with normal-weight women, pre-pregnancy obese women had higher risks of delayed lactogenesis II and early termination of ABF. However, GWG was not associated with poor breast-feeding outcomes. The results mainly support previous findings on the association between pre-pregnancy BMI and the duration of ABF( Reference Hauff, Leonard and Rasmussen 8 – Reference Li, Jewell and Grummer-Strawn 11 ).
The rate of delayed lactogenesis II in the present study (8·7 %) is close to that in our previous report (9·8 %) in another city of China( Reference Zhu, Hao and Jiang 30 ), but much lower than that reported for American women (23–44 %) in studies with small sample sizes( Reference Dewey, Nommsen-Rivers and Heinig 21 , Reference Nommsen-Rivers, Chantry and Peerson 22 , Reference Chapman and Perez-Escamilla 24 ). Our study, which included a large sample from the general population, is one of the few studies to examine the effect of pre-pregnancy BMI on lactogenesis II( Reference Hilson, Rasmussen and Kjolhede 23 , Reference Matias, Dewey and Quesenberry 25 ). Many studies have primarily focused on the relationship between pre-pregnancy BMI and breast-feeding initiation, and most of them have indicated that pre-pregnancy obesity is associated with decreased breast-feeding initiation( Reference Hauff, Leonard and Rasmussen 8 , Reference Winkvist, Brantsaeter and Brandhagen 16 , Reference Manios, Grammatikaki and Kondaki 19 , Reference Mehta, Siega-Riz and Herring 31 – Reference Guelinckx, Devlieger and Bogaerts 33 ). In the present study, more than 95 % of mothers initiated breast-feeding postpartum, which is close to the rate in other reports in China( Reference Guo, Fu and Scherpbier 4 , Reference Liu, Qiao and Xu 34 ). Moreover, no statistical difference was observed in the rates of breast-feeding initiation among the women of different pre-pregnancy BMI categories. The high rates of breast-feeding initiation for all pre-pregnancy BMI groups mainly resulted from the Chinese government’s support for the Baby-Friendly Hospital Initiative( Reference Xu, Qiu and Binns 3 ), which promotes breast-feeding in hospitals. However, our findings indicate that pre-pregnancy obesity seemed to increase the risk of delayed lactogenesis II, which is consistent with the results of two studies with small sample sizes( Reference Hilson, Rasmussen and Kjolhede 23 , Reference Chapman and Perez-Escamilla 24 ).
The mean duration of ABF in the majority of cities or provinces of China is 7–9 months( Reference Xu, Qiu and Binns 3 ). Similarly, the median duration of ABF in our study was 7 months. We found that obese women were at a higher risk of earlier termination of ABF, compared with normal-weight women, and this finding is consistent with that of a large prospective study in American women( Reference Li, Jewell and Grummer-Strawn 11 ). Studies evaluating Danish( Reference Baker, Michaelsen and Sorensen 9 ), Norwegian( Reference Winkvist, Brantsaeter and Brandhagen 16 ) and Australian( Reference Oddy, Li and Landsborough 35 ) populations have also found that being overweight is related to a shorter duration of ABF. Moreover, previous studies have indicated a negative association between pre-pregnancy BMI and the duration of EBF( Reference Hauff, Leonard and Rasmussen 8 , Reference Guelinckx, Devlieger and Bogaerts 33 , Reference Martinez, Chapman and Perez-Escamilla 36 , Reference Mehta, Siega-Riz and Herring 37 ). However, this observation was not found in the present study. The possible reasons for such inconsistent results include the different study designs, measures of maternal BMI and adjustments for confounding factors. Moreover, ethnic or cultural variation may partly explain the difference. Many studies have shown that pre-pregnancy BMI is negatively associated with breast-feeding outcomes in Caucasian women, but not in African-American women( Reference Masho, Cha and Morris 12 – Reference Liu, Smith and Dobre 14 ).
The present study showed that GWG was not associated with the duration of ABF or EBF, which is supported by most observational studies( Reference Baker, Michaelsen and Sorensen 9 , Reference Castillo, Santos and Matijasevich 18 , Reference Manios, Grammatikaki and Kondaki 19 ). Although two studies have indicated that excessive GWG has a negative effect on full breast-feeding( Reference Winkvist, Brantsaeter and Brandhagen 16 ) and ABF( Reference Winkvist, Brantsaeter and Brandhagen 16 , Reference Hilson, Rasmussen and Kjolhede 17 ), significantly higher risks were found among most GWG categories of overweight or obese women only in comparison with the reference group of normal-weight women who gained the recommended weight. The residual confounding effect of pre-pregnancy BMI was not adequately controlled for. For example, the difference in breast-feeding duration between obese women with excessive GWG and normal-weight women with adequate GWG may be attributed to high BMI rather than excessive GWG. Another study showed that women with either insufficient or excessive weight gain had significantly shorter breast-feeding duration than did those who gained the recommended gestational weight( Reference Li, Jewell and Grummer-Strawn 11 ). Although the breast-feeding duration of the GWG groups differed by less than 1 week, the differences were statistically significant because of the large sample size (n 13 234).
The mechanisms underlying the association of maternal obesity with poor breast-feeding practice are still not clearly understood. Because of the effect of fat mass on prolactin and oxytocin levels, obese women are more likely to have delayed lactogenesis II than are normal-weight women( Reference Amir and Donath 38 ), which was confirmed in our study. Women with delayed lactogenesis II exhibit shorter breast-feeding duration( Reference Brownell, Howard and Lawrence 20 ). By contrast, some studies have shown that obese women are less confident of having sufficient milk supply( Reference Mok, Multon and Piguel 39 ) and exhibit a reduced prolactin response to suckling( Reference Rasmussen and Kjolhede 40 ), which may contribute to early lactation failure. Large breasts or flat nipples also make it difficult to establish copious milk production( Reference Jevitt, Hernandez and Groer 41 ). Furthermore, pre-pregnancy obese women have a significantly higher risk of adverse health outcomes, including gestational diabetes, pregnancy-induced hypertension, pre-eclampsia and caesarean delivery( Reference Rahman, Abe and Kanda 42 , Reference Yu, Han and Zhu 43 ), which can confound the relationship between maternal obesity and breast-feeding. In particular, caesarean delivery is being performed ever more frequently in China( Reference Feng, Xu and Guo 44 , Reference Feng, Wang and An 45 ). In our study population, obese women had a higher rate of caesarean delivery than did normal-weight women. However, the significance of the association between pre-pregnancy BMI and ABF did not change after this factor was added to the statistical models. This suggested that caesarean delivery, although more prevalent in obese women, did not play a determinant role in the association between pre-pregnancy BMI and termination of ABF in our study population.
The major strength of our study is the prospective cohort study design. This design enabled the measurement of the exposure and mediators before the outcome and thus facilitated the assessment of the risk. However, the current study has several limitations that influence the interpretation and generalizability of the results. Because of the low prevalence of pre-pregnancy overweight and obese women in our cohort, the lack of power may have limited our ability to study breast-feeding duration. In addition, the self-reported weight before pregnancy might be underestimated in overweight or obese women( Reference Hill and Roberts 46 , Reference Paxton, Sculthorpe and Gibbons 47 ), potentially resulting in non-differential misclassification bias. Despite this limitation, the self-reported pre-pregnancy weight is widely used in observational studies, because the reporting error is a small percentage of the total body weight( Reference Harris and Ellison 48 ). The present study did not include psychological factors such as body perception and social support of breast-feeding, which may affect the success of breast-feeding. Although the study also did not include other pregnancy-related psychological factors such as depressive symptoms, perceived stress and anxiety, most reports have indicated that these factors do not mediate the relationship between pre-pregnancy BMI and breast-feeding( Reference Mehta, Siega-Riz and Herring 31 , Reference Mehta, Siega-Riz and Herring 37 ). Finally, our study was limited to the use of geographic origin as a means of exploring Chinese women in our sample.
Conclusion
The present study showed that pre-pregnancy obesity, but not GWG, exerted a negative effect on breast-feeding in Chinese women. Pre-pregnancy obesity increased the risks of delayed lactogenesis II and early termination of ABF. The clear implication of our findings is that a healthy weight should be maintained for women before conception. Because of the increasing obesity rate in women, at-risk individuals should be identified for more targeted clinic interventions to support breast-feeding practices.
Acknowledgements
Acknowledgements: The authors acknowledge and extend their appreciation to all of the participants in the Ma’anshan Birth Cohort Study. Financial support: This research was funded by the National Natural Science Foundation of China (grant number NSCF−81573168). The National Natural Science Foundation of China had no role in the design, analysis or writing of this article. Conflict of interest: The authors declare that they have no conflicts of interest. Authorship: F.-B.T., X.-Y.T. and K.H. designed the study. F.-B.T., X.-Y.T., K.H., S.-Q.Y., A.-Z.Z., R.-W.T., H.C. and C.-L.G. generated, collected, analysed and/or interpreted the data. F.-B.T., X.-Y.T., A.-Z.Z. and R.-W.T. drafted or revised the initial manuscript. All authors read and approved the final manuscript. Ethics of human subject participation: All protocols, instruments and the process for obtaining informed consent for this study were approved by the Biomedical Ethics Committee of Anhui Medical University in China.








