- AMD
age-related macular degeneration
- NST
nucleus of the solitary tract
- ORN
olfactory receptor neurons
Advancements in the treatment of critical illnesses such as cancer, heart attack and stroke have dramatically lengthened life expectancy for patients who survive with these conditions. While medical science has substantially improved the prognosis for individuals who suffer from many critical illnesses, there are often negative physiological and perceptual effects that accompany prolonged survival that can have a serious impact on daily functioning and quality of life. For example, patient complaints of sensory alterations in vision, hearing, taste, smell and touch often occur during a period of critical care and persist with continued survival. These sensory changes can be qualitative, quantitative and/or affective. As experience of the world depends largely on the senses, losses in sensory perception can seriously affect overall health, nutritional status, activities of daily living and quality of life. Sensory impairments that reduce the ability to procure and appreciate food can lead to inadequate energy and nutrient intake, weight loss and ultimately increased risk of morbidity and mortality (Toth & Poehlman, Reference Toth and Poehlman2000). Thus, awareness of potential sensory impairments in the critically-ill patient by physicians and other health providers is crucial for making decisions about appropriate treatments that optimize medical (including nutritional) care.
The elderly are more likely than younger cohorts to suffer from critical illnesses, including strokes (Di Carlo et al. Reference Di Carlo, Lamassa, Baldereschi, Pracucci, Consoli, Wolfe, Giroud, Rudd, Burger, Ghetti and Inzitari2006), heart attacks (Hellermann et al. Reference Hellermann, Reeder, Jacobsen, Weston, Killian and Roger2002; Yawn et al. Reference Yawn, Wollan, Jacobsen, Fryer and Roger2004) and cancer (Cohen, Reference Cohen and Perry1998), and are also more likely to suffer from sensory losses (Schiffman & Zervakis, Reference Schiffman and Zervakis2002; Schiffman et al. Reference Schiffman, Rogers, Zervakis, Bales and Ritchie2003). For this reason, the present review article will provide an overview of changes in sensory perception that can occur in patients with critical illnesses, with a special focus on the elderly who as a group suffer disproportionately from sensory loss. Understanding the sensory impairments of an older cohort is important because man-life expectancy is increasing dramatically, with the number of elderly expected to reach 1·21 billion worldwide in 2025 (US Senate Special Committee on Aging, 1985–6). Many older patients are not consciously aware of age-related sensory changes that take place slowly over time; however, perceptual changes often reach consciousness if they are exacerbated during a critical illness.
It should be emphasized from the outset that sensory losses in critically-ill patients may or may not be related to their current medical condition. For this reason, the present article will first describe typical sensory losses that occur with advancing age as well as how disease states, including critical illnesses, impact on sensory perception. The effect of medications on the senses will also be addressed because use of medication is elevated in older individuals, especially during a period of critical care (Schiffman, Reference Schiffman1997; Noble, Reference Noble2003; Schiffman et al. Reference Schiffman, Rogers, Zervakis, Bales and Ritchie2003), and because adverse drug reactions are observed two to three times more frequently in geriatric patients compared with younger adults (Turnheim, Reference Turnheim1998, Reference Turnheim2004). Finally, the results of a research study are described that provide the first quantitative data on the amount of sensory loss for all five senses that can be expected with one type of critical condition, i.e. coronary artery bypass surgery.
Vision
Changes in the visual system with age
Many adverse physiological changes in the structure and function of the eye can occur during the aging process (Weale, Reference Weale, Evans, Williams, Beattie, Michel and Wilcock2000; Schiffman et al. Reference Schiffman, Rogers, Zervakis, Bales and Ritchie2003). Lacrimation (tear flow) decreases substantially over the lifespan causing ‘dry eye’ in many older individuals. As lacrimal fluids provide a smooth corneal surface for refraction, decreased tear production can reduce the clarity of an image. The pupil decreases in size with age (called ‘senile miosis’) so that the elderly need brighter illumination to see. There is reduced transparency of the lens with attenuation of light, particularly short wavelengths including blue and violet. The accommodative power of the lens decreases with age (presbyopia) so that most elderly require reading glasses. Alterations in both the aqueous humour and the vitreous humour also occur. The aqueous humour is the clear watery fluid that circulates in the chamber of the eye between the cornea and the lens. With increasing age the drainage network for the aqueous humour can become partially blocked, which leads to increased intraocular eye pressure and potential damage to vision. The gelatinous vitreous humour that fills the eyeball between the retina and the lens tends to liquefy, contract and separate from the retina. This process creates ‘floaters’ in front of the retina that can be very distressing for observant individuals. Age-related alterations in the structure of protein molecules in the cornea, lens and vitreous humour generate complaints of glare. Retinal changes also occur with advancing age, including a reduction in the number of nerve cells within the retina along with alterations in the retinal vasculature.
Perceptual visual losses accompany age-related ocular changes in the absence of disease (Faye, Reference Faye, Evans, Williams, Beattie, Michel and Wilcock2000; Sekuler & Sekuler, Reference Sekuler, Sekuler, Evans, Williams, Beattie, Michel and Wilcock2000; Schiffman et al. Reference Schiffman, Rogers, Zervakis, Bales and Ritchie2003). At approximately 65 years of age performance is diminished on tests of far visual acuity (resolution of spatial detail) and contrast sensitivity. Contrast sensitivity is the ability to recognize subtle differences in shading and colour between an object and its background. Evaluation of contrast sensitivity is considered a more sensitive measure for detecting losses in visual acuity than the standard graded symbols of the Snellen eye chart. A typical method for testing contrast sensitivity is the use of gratings with alternating light and dark bars (or stripes) from low frequencies (wider bars) to high frequencies (thin bars). Age-related losses are first seen at high spatial frequencies and low contrast.
Losses in peripheral vision also occur, with the visual field reduced to two-thirds by age 75 years and to half by age 90 years. The visual field is the entire area (both horizontal and vertical) that is seen while gazing straight ahead. The consequence of reduction of the size of the field is that the elderly do not recognize movement or objects at the periphery of the field. Loss of warning signals from peripheral vision makes the elderly more vulnerable to hazards while driving or walking. As the peripheral receptors are rods, these losses are especially severe in dim light. The loss in peripheral vision is a result in part of decreased pupil size (miosis) and in part of reduced sensitivity of the rods. The peripheral field can be screened simply by occluding one eye and determining when the patient can see an object (e.g. index finger) as it moves from outside the unoccluded lateral field into the visual field. Aging is also associated with changes in adaptation to light such that the older retina requires more time to adapt from light to dark and to adjust to bright light. Impairments in the rate and maximum level of dark adaptation can have profound effects on activities such as driving at night, reading a menu in a restaurant or reading a programme in a theatre.
Age-related eye diseases
Progression of degenerative changes beyond those of normal aging leads to a variety of age-related ocular diseases, including cataracts, macular degeneration and glaucoma (Weale, Reference Weale, Evans, Williams, Beattie, Michel and Wilcock2000). Diabetic retinopathy also increases in prevalence with progression of the disease. Cataracts (because of excessive lens opacity) impair vision because a cloudy lens does not transmit a sharply-focused image to the retina. Blurred vision and sensitivity to glare are common complaints of individuals with cataracts. Risk factors for the development of cataracts include age, trauma to the eye, sun exposure, malnutrition, use of steroids and smoking. The impaired vision from cataracts can be reversed by surgical removal of the clouded lens and replacement with a clear plastic intraocular lens. Age-related macular degeneration (AMD) is the gradual and irreversible loss of vision caused by degeneration of the macula, a small circular membrane at the centre of the retina that includes the fovea, which is responsible for visual acuity (detail vision), daytime (photopic) vision and colour vision (the macula is a cone-enriched area of the retina). AMD impairs central vision that is required for reading, driving and other activities that involve fine detail and visual contrast. Individuals with AMD report that objects disappear perceptually behind a blind spot, that faces are difficult to recognize and that straight lines appear wavy. In the worst cases there is a complete loss of central vision; however, total blindness does not occur because peripheral vision is unaffected. Heredity, smoking, obesity, CVD, exposure to sunlight, high blood pressure, high cholesterol, ethnicity, diabetes and oxidation of the macula as a result of inadequate intake of antioxidants can play a role in the development of AMD. AMD is the most common cause of blindness in individuals >50 years of age. There are two forms of AMD, wet and dry. Wet AMD occurs with the growth (as well as haemorrhage) of abnormal blood vessels behind the retina. Dry AMD involves the breakdown of the light-sensitive cells in the macula but no leakage of fluid. A common early sign of dry AMD is drusen, i.e. yellow deposits in the retina that can be detected during a comprehensive dilated-eye examination even before there is vision loss. The central visual field is screened with an Amsler grid (a checkerboard of horizontal and vertical lines). The straight lines of the grid appear wavy to an individual with AMD.
Glaucoma results from damage to the optic disc and optic nerve in the eye that typically occurs from a rise in intraocular pressure. This increase in pressure is caused by an imbalance in fluid production and drainage of the aqueous humour as a result of partial blockage in the trabecular meshwork (situated in the angle where the iris and cornea meet). The most common type of glaucoma is open-angle glaucoma, which develops slowly with gradual and painless loss of sight. Gradual loss of outer (peripheral) vision occurs first, with central vision being the last to be affected because the optic nerve fibres mediating peripheral vision are the first to be compressed and damaged; i.e. loss of visual function in glaucoma is the opposite of that found in AMD. The peripheral visual field loss in end-stage glaucoma is analogous to looking down the barrel of a gun. Overall, the prevalence of open-angle glaucoma increases exponentially from <1% during the fifth decade of life to 10% in the ninth decade, with some variation depending on ethnicity. Sudden increases in intraocular pressure can also occur (acute closed-angle glaucoma) with immediate symptoms of blurred vision, halos around lights, reddening of the eye and severe eye pain, which lead to permanent vision loss. Aging, genetics, myopia, diabetes, hypertension, previous eye surgery and steroid use have all been implicated as risk factors in the development or exacerbation of glaucoma.
Critical illnesses that affect the eye
Visual disturbances have been reported in many critical medical conditions, including cancer, stroke, cardiovascular surgery and infectious diseases. These visual losses can be caused or exacerbated by the medical condition itself or by its treatment. Chemotherapy for a broad range of cancer types has been associated with visual disturbance (Al-Tweigeri et al. Reference Al-Tweigeri, Nabholtz and Mackey1996; Gianni et al. Reference Gianni, Panzini, Li, Gelber, Collins and Holmberg2006). One study shows the prevalence of ocular toxicity from chemotherapy to be 10·9% (Gianni et al. Reference Gianni, Panzini, Li, Gelber, Collins and Holmberg2006). Fenretinide, a synthetic derivative of retinoic acid that has been studied clinically for cancer prevention, causes alterations in dark adaptation (Baglietto et al. Reference Baglietto, Torrisi, Arena, Tosetti, Gonzaga, Pasquetti, Robertson and Decensi2000). The probable cause is that fenretinide lowers circulating retinol and thus affects night vision. Cisplatin, which is used in the treatment of solid tumours, has been associated with sudden onset of blindness. This severe visual loss appears to be a manifestation of Mg deficiency because the visual symptoms are reversed with correction of the electrolyte abnormality (Al-Tweigeri et al. Reference Al-Tweigeri, Magliocco and DeCoteau1999). Visual functioning can be profoundly impaired after a posterior cerebral artery stroke (Schoenfeld et al. Reference Schoenfeld, Noesselt, Poggel, Tempelmann, Hopf, Woldorff, Heinze and Hillyard2002). Stroke often exacerbates age-related loss of peripheral vision associated with decreased pupil size (miosis) and reduced sensitivity of the rods. Visual memory, including total time to complete a test as well as the number of correct answers, can also be impaired in patients who have had a stroke (Yaretzky et al. Reference Yaretzky, Raviv, Netz and Jacob1995). Anterior ischaemic optic neuropathy with subsequent visual loss occurs in some patients who have undergone cardiac surgery with cardiopulmonary bypass (Tidow-Kebritchi & Jay, Reference Tidow-Kebritchi and Jay2003). Cortical blindness and visual disorientation occurs in some patients who have sustained bilateral occipital and parietal lobe ischaemia during coronary artery bypass surgery (Russell & Bharucha, Reference Russell and Bharucha1978). Altered visual functioning that is associated with vascular hypertension in the absence of eye disease (Eisner & Samples, Reference Eisner and Samples2003) can be exacerbated by cardiac surgery. Infections and malnutrition are also well known to impact on visual functioning (Oduntan, Reference Oduntan2005). Interestingly, visual functioning has been reported to be impaired in patients undergoing long-term total parenteral nutrition (Vinton et al. Reference Vinton, Heckenlively, Laidlaw, Martin, Foxman, Ament and Kopple1990).
Medications that affect visual functioning
There are a vast number of medications that induce visual side effects (Fraunfelder & Fraunfelder, Reference Fraunfelder and Fraunfelder2001; Physicians' Desk Reference, 2005). Blurred vision is a common side effect of hundreds of medications, including antihistamines and antihypertensive drugs. There are multiple causes of blurred vision, including anticholinergic properties that impair accommodation. Conversely, other drugs alter vision by constricting the pupil, such as miotic eye drops for glaucoma (e.g. pilocarpine and carbachol); heroin usage is also associated with constricted pupils. Drugs can also directly damage the eye, e.g. retinal toxicity from antimalarial drugs and cataract formation from steroids. Bisphosphonates (such as alendronate, clodronate, etidronate, ibandronate, pamidronate, risedronate and zolendronate), which are used to treat osteoporosis as well as hypercalcaemia of malignancy, osteolytic bone metastases of both breast cancer and multiple myeloma and Paget's disease of the bone, as well as other indications depending on the product, are associated with adverse changes in visual perception (Canadian Family Physician, 2004). These alterations include blurred or double vision, decreased vision and ‘floaters’, as well as eye pain and redness. The anticonvulsant drug topiramate that is used to treat epilepsy can cause a syndrome consisting of acute myopia associated with secondary angle-closure glaucoma, decreased visual acuity and ocular pain (Physicians' Desk Reference, Reference Pavani, Ladavas and Driver2005). This loss can be transient (reversible) or permanent. The antiarrhythmic drug amiodarone can cause visual disturbance, including blurred vision, coloured halos around lights, bright lights, glare and general decreased vision (Johnson et al. Reference Johnson, Krohel and Thomas2004; US National Library of Medicine and the National Institutes of Health, Medline Plus, 2005). Hydroxychloroquine, which has antimalarial actions and is also used to treat rheumatoid arthritis and lupus erythematosis, can cause a vast number of ocular reactions, including blurred vision, halos around lights, photophobia, loss of foveal reflex, scotomas, retinal changes that can be irreversible, colour vision loss and decreased vision (Physicians' Desk Reference, Reference Pavani, Ladavas and Driver2005). Similar losses are caused by the related drug chloroquine. Antipsychotic drugs of the phenothiazine class, such as chlorpromazine, thioridazine, fluphenazine, perphenazine and trifluoperazine, some of which are used in the treatment of nausea and vomiting, cause visual effects with prolonged usage, in part because of the development of opacities in the anterior lens and cornea (Pouget et al. Reference Pouget, Blayac, Largey, Alric, Castelnau and Boulet1976). Visual hallucinations have been associated with a broad range of drugs, including atropine, dopamine agonists, antidepressants, anticonvulsants, cardiovascular drugs and anti-inflammatory agents (Brown et al. Reference Brown, Salmon and Rendell1980; Fisher, Reference Fisher1991; Holroyd, Reference Holroyd1996; Faye, Reference Faye, Evans, Williams, Beattie, Michel and Wilcock2000; Physicians' Desk Reference, Reference Pavani, Ladavas and Driver2005).
Hearing
Changes in the auditory system with age
Both physiological and perceptual changes in the auditory system occur with advancing age (Gulya, Reference Gulya, Evans, Williams, Beattie, Michel and Wilcock2000; Schiffman et al. Reference Schiffman, Rogers, Zervakis, Bales and Ritchie2003). The pinna, or outer ear, which channels sound waves to the tympanic membrane (eardrum), demonstrates several physiological changes, including loss of elasticity, but these changes have little impact on auditory perception. The vibrations of the eardrum are transmitted to the three bones of the middle ear (malleus, incus and stapes), which become more fixed with age and less effective in deforming the oval window. Vibrations of the oval window set up similar vibrations in the internal fluid of the cochlea (the snail-shaped structure in the inner ear) that cause mechanical waves to travel down the basilar membrane and bend the cilia of hair cells. Sensory presbycusis (hearing loss in the elderly) involves degeneration of hair cells and supporting cells of the cochlea, while neural presbycusis is a loss of cochlear neurons. Degenerative neural changes have also been reported in central auditory pathways, including the brain stem as well as the cortex.
Hearing impairment in the elderly typically involves mild-to-moderate high-frequency sensorineural hearing loss along with difficulty understanding conversational speech (Moscicki et al. Reference Moscicki, Elkins, Baum and McNamara1985). The impairment in speech comprehension is because much of the energy of consonant sounds is concentrated in the higher frequencies. Difference thresholds increase so that larger changes are needed to detect a difference in the pitch of tones. The ability to locate sound in space becomes more difficult. Overall, hearing loss has many consequences beyond the ability to hear and understand speech, including inability to hear or locate warning signals (emergency sirens, fire alarms, honking horns) as well as withdrawal and social isolation, loneliness, cognitive impairment, depression and compromised mobility.
Hearing loss cannot be reversed because man lacks the ability to regenerate cochlear sensory cells. In spite of the progressive hearing loss that occurs during aging, it is noteworthy that only a minority of hearing-impaired elderly utilize a hearing aid for amplification (Popelka et al. Reference Popelka, Cruickshanks, Wiley, Tweed, Klein and Klein1998). Yet, use of a hearing aid to amplify sounds has been shown to improve functional status. One possible reason for the low utilization of hearing aids among the elderly is that while amplification has considerable benefit in quiet listening situations, it is less beneficial in natural environments with noisy backgrounds and multiple talkers. The simple whispered voice test (Eekhof et al. Reference Eekhof, de Bock, de Laat, Dap, Schaapveld and Springer1996) can be used in the clinic or hospital room to determine whether a patient needs audiology testing.
Many factors contribute to hearing loss during aging. They include noise exposure (typically prolonged occupational exposures), genetic predisposition, dietary factors, vascular disease, systemic disease, cancer, infections and inflammation, head injuries, ototoxic drugs and environmental pollutants such as solvents and pesticides. Routine use of many medications (e.g. loop diuretics for hypertension) can cause hearing loss.
Critical illness
Cancer and its treatment can affect auditory perception. Inner ear radiation for treatment of head-and-neck cancer causes high-frequency hearing loss (≥2000 Hz) in a dose-dependent fashion (Pan et al. Reference Pan, Eisbruch, Lee, Snorrason, Ten Haken and Kileny2005). Many cancer drugs are ototoxic, including cisplatin (Boyer et al. Reference Boyer, Raghavan, Harris, Lietch, Bleasel, Walsh, Anderson and Tsang1990) and carboplatin (Takeno et al. Reference Takeno, Harrison, Mount, Wake and Harada1994). Patients who after a stroke have left neglect (failure to recognize the left side of the body in the absence of major visual problems) have impaired ability on an auditory task that requires speeded discrimination of sound elevation (Pavani et al. Reference Pavani, Ladavas and Driver2005). Selective auditory deficits occur in patients with focal right-hemispheric lesions (Clarke et al. Reference Clarke, Bellmann, Maeder, Adriani, Vernet, Regli, Cuisenaire and Thiran2002). High fever, such as meningitis, can cause damage to the cochlea. Many medications that are used during a period of critical care can impair hearing (Shine & Coates, Reference Shine and Coates2005). For example, the antibiotic gentamicin A causes hearing loss, and high doses of aspirin can cause tinnitus. Clonidine can cause auditory hallucinations (Physicians' Desk Reference, Reference Pavani, Ladavas and Driver2005).
Taste
Changes in the taste system with age
Perceptual losses in taste perception in the elderly have been well documented, but the cause of these decrements in the absence of medications and disease is not fully understood (Schiffman, Reference Schiffman1997; Schiffman & Zervakis, Reference Schiffman and Zervakis2002). The earliest studies that have quantified the number of taste buds and/or papillae (elevated lingual structures on which taste buds are located) in older individuals using large sample sizes have revealed substantial losses (Arey et al. Reference Arey, Tremaine and Monzingo1935; Mochizuki, Reference Mochizuki1937, Reference Mochizuki1939; Moses et al. Reference Moses, Rotem, Jagoda, Talmor, Eichhorn and Levin1967). Furthermore, later studies have found striking localized deficits (i.e. regional losses) in taste perception on the tongues of elderly individuals (Bartoshuk et al. Reference Bartoshuk, Desnoyers, Hudson, Marks, O'Brien, Catalanotto, Gent, Williams and Ostrum1987; Matsuda & Doty, Reference Matsuda and Doty1995). The reports of perceptual loss of regional sensitivity are consistent with anatomical studies of the lingual surface that have found entirely flat areas on the surface of the tongue without papillae in old age (Kobayashi et al. Reference Kobayashi, Kumakura, Yoshimura and Shindo2001). However, there are other studies by established researchers using smaller numbers of elderly that suggest such losses of papillae and taste buds are minimal (Arvidson, Reference Arvidson1979; Miller, Reference Miller1986, Reference Miller1988; Bradley, Reference Bradley1988). When losses do occur, reduced renewal of the taste cells in the taste buds may be a factor. Taste cells normally replicate every 10–10·5 d, but delayed cell renewal has been reported in taste buds of aged mice (Fukunaga, Reference Fukunaga2005). Reduced levels of oestrogen and testosterone in the elderly may contribute to the blunting of taste cell renewal since lowering of these hormones is known to depress cell proliferation in other tissues (Eartly et al. Reference Eartly, Grad and Leblond1951; Hopper et al. Reference Hopper, Rose and Wannemacher1972; Williamson, Reference Williamson1978; Zhang et al. Reference Zhang, Laping, Glasser, Day and Mulholland1998). Testosterone has been shown to increase taste bud number in a rat model (Cano et al. Reference Cano, Machado, Roza and Rodriguez-Echandia1982). Some taste losses from normal aging may occur at taste-cell membranes (e.g. altered functioning of ion channels and receptors) rather than from losses of taste buds or papillae. Alterations in neurotransmitter levels may also be involved.
Taste signals from the taste buds are relayed to nucleus of the solitary tract (NST) in the medulla and then to the thalamus and cortical taste centres with ipsilateral projections from the taste buds to the thalamus. Signals from taste buds on the tongue and in the throat are transmitted to the rostral portion of NST via the VIIth, IXth and Xth cranial nerves. The caudal portion of the NST receives visceral sensory information originating in the oesophagus, stomach, intestines and liver. The confluence of taste and digestive information in the NST explains why taste losses can have an impact on a range of digestive processes; i.e. the NST is the first processing area in which taste signals can affect digestion by impacting on gastric secretion, pancreatic exocrine secretion and secretion of insulin. Imaging studies suggest some taste loss with age may be a result of shrinkage of taste projection areas in the upper part of the medulla oblongata in the brainstem (Yamamoto et al. Reference Yamamoto, Shibata, Goto, Ezure, Ito and Suzuki2005). Inflammatory processes that occur in many medical conditions can potentially induce neural damage and apoptosis along taste pathways and may account for some complaints of reduced taste sensitivity in the elderly. Environmental pollutants also contribute to some taste losses (see Schiffman & Nagle, Reference Schiffman and Nagle1992).
Perceptual changes in taste with aging are more fully documented than physiological changes (Schiffman, Reference Schiffman1993; Schiffman & Zervakis, Reference Schiffman and Zervakis2002; Schiffman et al. Reference Schiffman, Rogers, Zervakis, Bales and Ritchie2003, Reference Schiffman, Sattely-Miller, Taylor, Graham, Landerman, Campagna and Garst2007). Detection and recognition thresholds for tastes are moderately elevated in older individuals compared with a younger cohort; i.e. more molecules (or ions) are needed by the elderly to perceive or recognize a tastant compared with younger individuals. Taste losses occur for individual compounds (e.g. NaCl) in simple aqueous solutions as well as in mixtures in which other ingredients mask important food components. The average loss, as measured by detection threshold (elderly):detection threshold (young), for a range of compounds (including Na salts with different anions, bitter compounds, sweeteners, acids, astringent compounds, amino acids (including glutamate salts), fats and polysaccharides and gums) is 4·7. The average loss in recognition, as measured by recognition threshold (elderly):recognition threshold (young), across this same qualitative range of compounds is also 4·7. The extent of loss within a group (such as Na salts), however, is not uniform but rather varies with the chemical structure of the compounds. For both the young and the elderly thresholds for compounds with high energy or nutritional value such as sugars, fats and amino acids are higher than thresholds for many noxious bitter compounds that can be detected in minute amounts. Losses in perception also occur at suprathreshold concentrations. Suprathreshold tastants are perceived to be weaker by the elderly compared with the young (e.g. amino acids taste half as strong). There is reduced ability to discriminate between different suprathreshold intensities of the same stimuli with age, with the elderly needing a 2- to 3-fold greater concentration change to perceive a difference in intensity. The elderly also perceive more off-tastes than younger subjects, which is further exacerbated by medications and medical conditions. The terms used to describe these taste losses are hypogeusia (reduction in taste intensity) and dysgeusia (distortion of taste).
Medical conditions associated with taste complaints
Taste changes have been reported in a vast range of medical conditions (Schiffman, Reference Schiffman1983a, Reference Schiffmanb, Reference Schiffman1997; Schiffman & Zervakis, Reference Schiffman and Zervakis2002). Examples of specific medical conditions associated with taste alterations include: nervous system disorders (Alzheimer's disease, Bell's palsy, damage to chorda tympani, Guillain-Barre syndrome, familial dysautonomia, head trauma, multiple sclerosis, Raeder's paratrigeminal syndrome, tumours and lesions); endocrine disorders (adrenal cortical insufficiency, congenital adrenal hyperplasia, cretinism, Cushing's syndrome, panhypopituitarism, hypothyroidism, diabetes mellitus, gonadal dysgenesis, pseudohypoparathyroidism); nutritional disorders (niacin and Zn deficiency); amyloidosis and sarcoidosis; cancer; chronic renal failure; cystic fibrosis; facial hypoplasia; glossitis and other oral disorders; hypertension; influenza-like infections; laryngectomy; leprosy; liver disease including cirrhosis; major depressive disorder; oral Crohn's disease; radiation therapy; Sjögren's syndrome; thermal burn. In most of the studies that have reported gustatory alterations in specific medical conditions medication usage was not controlled, so it is impossible to determine the relative contribution of disease status and medications to taste changes.
Medications implicated in taste complaints
Community-dwelling elderly individuals aged >65 years who are not critically ill consume an average of 2·9–3·7 medications daily (Lewis et al. Reference Lewis, Hanlon, Hobbins and Beck1993), and this number increases substantially for critically-ill elderly patients in hospitals and nursing homes. Hundreds of medications, including all major drug classes, have been associated clinically with taste complaints such as ‘loss of taste’, ‘altered taste’ and ‘metallic taste’ (Schiffman & Zervakis, Reference Schiffman and Zervakis2002; Schiffman et al. Reference Schiffman, Rogers, Zervakis, Bales and Ritchie2003). These medications include: AIDS- and HIV-related therapeutic drugs; amebicides; anthelmintics; anticholesteraemics and antilipidaemics; anticoagulants; antihistamines; antimicrobial agents; antiproliferative and immunosuppressive agents; antirheumatic, antiarthritic, analgaesic, antipyretic and anti-inflammatory agents; antiseptics; antispasmodics; antithyroid agents; antiulcerative drugs; antivirals; agents for dental hygiene; bronchodilators and antiasthmatic drugs; cardiovascular medications including diuretics, antiarrhythmic, antihypertensive and antifibrillatory agents; hyper- and hypoglycaemic drugs; hypnotics and sedatives; muscle relaxants and drugs for the treatment of Parkinson's disease; psychopharmacological drugs including antiepileptic drugs; sympathomimetic drugs; vasodilators (Schiffman, Reference Schiffman1983a, Reference Schiffmanb, Reference Schiffman1997; Schiffman et al. Reference Schiffman, Rogers, Zervakis, Bales and Ritchie2003).
The actual prevalence of medication-induced losses is not yet known because quantitative studies of taste perception in large populations taking specific medications have not yet been performed. Thus, it is not yet clear whether adverse taste complaints reported clinically are indeed gustatory (or olfactory) or whether they are actually caused in part by the medical conditions they are designed to treat. Furthermore, little is known about the sites of action or cascade of cellular events by which medications induce taste complaints. However, medications can potentially impact on taste perception at several levels of the nervous system, including the peripheral receptors, chemosensory neural pathways and/or the brainstem and brain. At the peripheral receptors drugs can induce a taste of their own or modify transduction mechanisms (e.g. intracellular messengers of the adenylate cyclase system) in taste receptor cells. Drugs can also alter activity along the gustatory pathways when they permeate into the brainstem or brain itself. Some areas of the NST have complexes of microvessels that are permeable to blood-borne compounds (Gross et al. Reference Gross, Wall, Pang, Shaver and Wainman1990), and both extracellular and intracellular pathways are known to exist that circumvent the blood–brain fluid barrier (Broadwell & Sofroniew, Reference Broadwell and Sofroniew1993; Broadwell et al. Reference Broadwell, Baker-Cairns, Friden, Oliver and Villegas1996). Some drugs interfere with cell turnover of taste receptors (e.g. by blocking mitosis); examples include chemotherapeutic agents and some antibiotics.
When the taste of the medication itself is the cause of the complaint, the drug may reach the receptors in three different ways: oral ingestion of the dosage; excretion into the saliva; diffusion from lingual blood vessels to activate receptors on the basolateral and even apical side of taste cells via permeation (termed intravascular ‘taste’; see Holland et al. Reference Holland, Zampighi and Simon1991). An example of a drug that can induce unpleasant tastes by salivary excretion as well as by intravascular taste is the protease inhibitor saquinavir. After a 600 mg dose the concentrations of saquinavir in saliva and in plasma are approximately 0·0127 mm and 0·22 mm respectively (Hoetelmans et al. Reference Hoetelmans, van Essenberg, Meenhorst, Mulder and Beijnen1997). Both these salivary and plasma levels of saquinavir exceed the taste detection thresholds of saquinavir of 0·0029 mm in uninfected patients and 0·0061 mm in unmedicated patients with HIV (Schiffman et al. Reference Schiffman, Zervakis, Heffron and Heald1999). For most drugs, however, the salivary or plasma levels tend to be lower than the taste threshold values (see Schiffman et al. Reference Schiffman, Graham, Sattely-Miller, Zervakis and Welsh-Bohmer2002). Yet, drugs as well as their metabolites can accumulate in taste buds over time to reach concentrations that are greater than taste detection thresholds.
Transient complaints of dysgeusia (e.g. metallic taste) sometimes occur on injection of drugs at concentrations below the taste threshold, and this treatment can be accompanied by emesis. The cause of dysgeusia in this case is likely to be a result of activation (by injection) of pressure receptors in the arterial system that are innervated by the IXth cranial nerve and that project to the NST, which also receives taste input.
Critical illness
Taste changes have been reported in many critical illnesses, and clinical studies of patients with wasting indicate that these losses are especially severe (Schiffman & Wedral, Reference Schiffman and Wedral1996). For cancer, taste alterations occur in untreated patients (Brewin, Reference Brewin1980; Ovesen et al. Reference Ovesen, Srensen, Hannibal and Allingstrup1991) as well as in those undergoing chemotherapy (Nielsen et al. Reference Nielsen, Theologides and Vickers1980; Fetting et al. Reference Fetting, Wilcox, Sheidler, Enterline, Donehower and Grochow1985) and radiation (Conger, Reference Conger1973). An overview of studies suggests that cancer and its treatment impair the ability to detect the presence of the taste of sweet, sour, salty and bitter taste qualities; in addition, there is reduced ability to perceive suprathreshold concentrations of tastants and thus to discriminate and identify tastes (Schiffman & Graham, Reference Schiffman and Graham2000). Distortions of taste (i.e. dysgeusia) also commonly occur. DeWys & Walters (Reference DeWys and Walters1975) have suggested that ≥50% of patients with cancer have impaired taste functioning at some point during the course of their disease and treatment. The duration of losses can last from several weeks to 6 months, or longer (Conger, Reference Conger1973; Mossman & Henkin, Reference Mossman and Henkin1978; Ophir et al. Reference Ophir, Guterman and Gross-Isseroff1988; Maes et al. Reference Maes, Huygh, Weltens, Vandevelde, Delaere, Evers and Van den Bogaert2002; Schiffman et al. Reference Schiffman, Sattely-Miller, Taylor, Graham, Landerman, Campagna and Garst2007). Altered taste perception in cancer can be caused by metabolic changes induced by the presence of a neoplasm as well as damage to the sensory receptors by radiotherapy and chemotherapy. Oral infections (fungal, viral, bacterial), ulcers, drug-induced stomatitis and dry mouth can also play a role in taste complaints. Some of the many chemotherapeutic drugs associated with altered taste perception include cisplatin, carboplatin, cyclophosphamide, doxorubicin, 5-fluorouracil, levamisole and methotrexate (Epstein et al. Reference Epstein, Phillips, Parry, Epstein, Nevill and Stevenson-Moore2002; Schiffman & Zervakis, Reference Schiffman and Zervakis2002). It is probable that chemotherapeutic medications that target rapidly-dividing cancer cells also impact on the turnover and replication of taste cells. Alterations in taste acuity can also occur after allogeneic bone-marrow transplantation (Mattsson et al. Reference Mattsson, Arvidson, Heimdahl, Ljungman, Dahllof and Ringden1992; Epstein et al. Reference Epstein, Phillips, Parry, Epstein, Nevill and Stevenson-Moore2002). It should be noted that some of the taste complaints in cancer are not a result of altered perception per se but are due to learned aversions that arise during the noxious effects of radiotherapy and chemotherapy (Schiffman & Graham, Reference Schiffman and Graham2000). Patients associate the taste of foods and beverages consumed shortly before or after treatment with the negative side-effects of radiotherapy and chemotherapy.
Taste disorders frequently occur after a stroke, with hypogeusia being the most-frequent gustatory symptom (Heckmann et al. Reference Heckmann, Stossel, Lang, Neundorfer, Tomandl and Hummel2005). However, left posterior insular stroke can lead to increased taste sensitivity contralateral to the lesion (Mak et al. Reference Mak, Simmons, Gitelman and Small2005). Taste disorders also occur in end-stage liver disease (Bloomfeld et al. Reference Bloomfeld, Graham, Schiffman and Killenberg1999). Malnutrition in the critically-ill patient, independent of the specific type of medical condition, can clearly affect the turnover/reproduction of taste cells and reduce their numbers (Schiffman, Reference Schiffman1983a, Reference Schiffmanb, Reference Schiffman1997). Furthermore, the burden of taste disorders from medications is elevated in a critically-ill geriatric population because of the extensive use of prescription drugs. It will be shown (see pp. 339–341) that hypogeusia and dysgeusia are more prevalent in one group of critically-ill patients than in non-medicated or even medicated community-living controls.
Smell
Changes in the olfactory system with age
Odour sensations are initiated when odorants bind to olfactory receptors on the cilia of olfactory receptor neurons (ORN) situated in the olfactory epithelium high within the nasal vault (Schiffman, Reference Schiffman1997; Schiffman & Zervakis, Reference Schiffman and Zervakis2002). There are extensive anatomical and physiological changes in the olfactory epithelium with age, including an increase in ORN apoptosis (programmed cell death), decreases in the rate of basal cell proliferation, decreased thickness of the olfactory epithelium, decreased number of cilia and supporting microvilli and increased accumulation of electron-dense granules in supporting cells (Schiffman, Reference Schiffman, Evans, Williams, Beattie, Michel and Wilcock2000). Over time, the olfactory epithelium is gradually replaced by non-olfactory (i.e. respiratory) epithelium with a subsequent loss in the receptor area. The axons of the bipolar ORN pass through small openings in a bone known as the cribriform plate where they synapse in neural masses termed glomeruli in the olfactory bulb. During the aging process the olfactory bulb takes on a ‘moth-eaten’ appearance as glomeruli atrophy and fibres degenerate and disappear. Central olfactory projection areas are especially vulnerable to aging, with many anatomical and physiological changes in the hippocampus, amygdaloid complex and hypothalamus, which include reductions in cell number, damage to cells and diminished levels of neurotransmitters.
An overview of odour threshold studies (Schiffman et al. Reference Schiffman, Moss and Erickson1976) indicates that detection and recognition thresholds for a broad range of individual volatile compounds (as well as odour mixtures) are elevated for elderly individuals compared with their younger counterparts. This loss in olfactory sensitivity at the threshold level typically begins in the 6th decade of life, with progressively greater losses into the 7th and 8th decades. Impairment of odour perception at suprathreshold levels in the elderly is generally more pronounced than that for taste. The prevalence of impaired ability to identify odours increases from 17·3% for individuals aged 60–69 years, to 29·2% for individuals aged 70–79 years and 62·5% for the 80–97-year-olds according to a population-based cross-sectional study in the USA (Murphy et al. Reference Murphy, Schubert, Cruickshanks, Klein, Klein and Nondahl2002). This impairment or diminished sensitivity of smell is termed hyposmia. Total absence of smell is termed anosmia. Given these US prevalence data along with UN projections of global population growth (United Nations, 1997), the number of individuals projected to have olfactory loss worldwide by 2050 is ≥230 million. Loss of the ability to identify odours for most individuals tends to occur gradually over the years, and some elderly individuals are not aware of the problem until it becomes very troubling. Older individuals also lose the ability to make discriminations among different odours. It is estimated that young adults with a normal sense of smell can distinguish ⩽10 000 different chemicals, although there appear to be some genetic differences among individuals in the qualitative range (Menashe et al. Reference Menashe, Man, Lancet and Gilad2003). The number of compounds that can be differentiated is greatly reduced in the elderly, who first lose the ability to make fine discriminations between odours with similar qualities (e.g. different types of coffees) and ultimately, in more extensive loss, between odours with different qualities (e.g. beef v. lemon).
Medical conditions and environmental exposures
Smell losses that occur from normal aging are compounded by a wide range of medical conditions (e.g. endocrine, neurological, nutritional, psychiatric) as well as environmental exposures. Numerous medications such as antianginal drugs (diltiazem, nifedipine), antimicrobial agents (allicin, streptomycin) and antithyroid agents (carbimazole, methimazole, methylthiouracil, propylthiouracil), as well as radiation therapy and chemotherapy, have been reported to affect the sense of smell (Schiffman, Reference Schiffman1983a, Reference Schiffmanb, Reference Schiffman1997; Schiffman & Zervakis, Reference Schiffman and Zervakis2002; Schiffman et al. Reference Schiffman, Rogers, Zervakis, Bales and Ritchie2003). Conductive losses in which there is obstruction of nasal airflow to the olfactory epithelium, e.g. sinusitis, polyps, tumours, adenoid hypertophy and allergic rhinitis lead to hyposmia and/or anosmia. Sensory and/or neural losses (e.g. from upper respiratory infections, head trauma and inhaled environmental toxins) often result from damage to the ORN, the olfactory bulb and/or central projection areas. ORN are especially vulnerable because they are exposed to the external environment and thus can be directly impacted on by inhaled environmental agents, including viruses. Damage to the olfactory bulb (and even higher cortical areas) can also occur because ORN serve as a direct conduit for the transport of substances from the nasal cavity to the brain. When ORN are damaged by injury (e.g. infectious agents and toxins in inhaled air) the regenerating olfactory axons are generally unable to traverse the holes in the cribriform plate to reach the olfactory bulb. This breakdown in appropriate connections of regenerating neurons can create aberrant synaptic associations that lead to dysosmia (distortion of smell). Ineffectual regeneration (e.g. after a virus) can also result in the replacement of olfactory epithelium by respiratory epithelium, scarring of the olfactory epithelium, resulting in gradual reduction in olfactory receptor area.
Olfactory losses are especially profound in certain disease states such as Alzheimer's disease and Parkinson's disease that are prevalent in an older population (Schiffman et al. Reference Schiffman, Clark and Warwick1990, Reference Schiffman, Graham, Sattely-Miller, Zervakis and Welsh-Bohmer2002; Schiffman & Zervakis, Reference Schiffman and Zervakis2002). Meta-analyses of olfactory studies of patients with Alzheimer's disease indicate that severe losses can occur in five olfactory domains: threshold detection; recognition; discrimination; identification; olfactory memory. With the possible exception of losses in sensitivity at the threshold level, all these olfactory domains are impaired relative to age-matched controls in the earliest stages of Alzheimer's disease. Neural degeneration along olfactory pathways (e.g. olfactory bulb, anterior olfactory nucleus, olfactory tubercle, amygdala, prepiriform cortex, hippocampus) is also more pronounced in Alzheimer's disease compared with age-matched controls.
Critical illness
Loss of smell occurs during the course of cancer and its treatment (Lehrer et al. Reference Lehrer, Levine and Bloomer1985; Schiffman & Graham, Reference Schiffman and Graham2000). Olfactory function in patients with nasopharyngeal carcinoma deteriorates after radiotherapy (Ho et al. Reference Ho, Kwong, Wei and Sham2002), and anticancer drugs such as tegafur cause olfactory dysfunction (Nakamura et al. Reference Nakamura, Nonomura, Fujiwara and Nakano1995). As with taste, left posterior insular stroke can lead to increased sensitivity to smell contralateral to the lesion (Mak et al. Reference Mak, Simmons, Gitelman and Small2005), and olfactory disorders occur in end-stage liver disease (Bloomfeld et al. Reference Bloomfeld, Graham, Schiffman and Killenberg1999). Furthermore, the sense of smell is severely impaired in chronic renal failure (Griep et al. Reference Griep, Van der Niepen, Sennesael, Mets, Massart and Verbeelen1997).
Somatosensory system
Changes in the somatosensory system with age
The somatosensory system comprises a variety of receptors in the skin that transmit sensations of touch, temperature, pressure and pain. Skin receptors can be divided into two principal types: free nerve endings; encapsulated nerve endings, such as Meissner corpuscles, Pacinian corpuscles and Merkel discs. Studies of elderly individuals (Bolton et al. Reference Bolton, Winkelman and Dyck1965; Cauna, Reference Cauna and Montagna1965) indicate that there are reduced numbers of various touch receptors in the skin with age; Meissner and Pacinian corpuscles have the greatest loss, both in numbers and morphology, and free nerve endings have the least loss. The physiological loss of Meissner's corpuscles is correlated with the perceptual loss of skin sensitivity with aging (Kenshalo, Reference Kenshalo1986).
Perceptual decreases in touch sensitivity with age have been reported using a variety of tasks for measuring touch perception, including the two-point discrimination task, orientation of line grating, point localization, detection of a gap in a disc, line length discrimination and line or point orientation (Schiffman et al. Reference Schiffman, Rogers, Zervakis, Bales and Ritchie2003). Although these tests of touch quantify different facets of touch perception and have different thresholds, the loss appears to be generic because scores decline at about the same rate (1%/year) with age. Acuity declines faster at the fingertip than at the forearm or lip (Stevens & Patterson, Reference Stevens and Patterson1995). Thermal sensitivity also deteriorates with age, with the greatest reduction at the extremities such as the fingers, toes and sole of the foot (Stevens & Choo, Reference Stevens and Choo1998). Vibratory sensitivity also declines with age, with preferential loss at higher frequencies (Verrillo, Reference Verrillo1980).
Diseases and medications
Peripheral neuropathies (attributed to diabetes, thyroid disorders, rheumatoid arthritis, HIV infection, alcoholism and vitamin B12 deficiency) as well as peripheral vascular disease exacerbate age-related somatosensory impairments (Schiffman et al. Reference Schiffman, Rogers, Zervakis, Bales and Ritchie2003). Many drugs and medical treatments have somatosensory side effects, including HIV drugs (zalcitabine, stavudine), statins and chemotherapy. Examples of somatosensory symptoms attributed clinically to numerous medications include numbness and tingling.
Critical illness
Patients with unilateral oral or pharyngeal cancer who are treated with bilateral radiotherapy (to reduce the potential for metastases) have deterioration of intra-oral sensation even on the non-tumour side 6 months after treatment, and there is no recovery 1 year after radiotherapy (Bodin et al. Reference Bodin, Jaghagen and Isberg2004). The antineoplastic agent cisplatin causes sensory neuropathies in a dose-dependent fashion (Krarup-Hansen et al. Reference Krarup-Hansen, Fugleholm, Helweg-Larsen, Hauge, Schmalbruch, Trojaborg and Krarup1993). Somatosensory deficits following stroke are common (Kim & Choi-Kwon, Reference Kim and Choi-Kwon1996); examples of losses include impaired fingertip texture discrimination and wrist position sense (Carey et al. Reference Carey, Matyas and Oke2002) and referred sensations such as misplaced localization of touch (Turton & Butler, Reference Turton and Butler2001). Upper-extremity neuropathies (Honet et al. Reference Honet, Raikes, Kantrowitz, Pursel and Rubenfire1976; Seyfer et al. Reference Seyfer, Grammer, Bogumill, Provost and Chandry1985) have been reported after cardiac surgery, including sensory neuropathies involving the ulnar nerve.
Effect of history of critical care on sensory performance (all five senses) in patients with cardiovascular conditions: review of study
It has long been known that unusual sensory complaints are reported clinically after cardiac surgery (Ellis, Reference Ellis1972). Remarkably, however, neither the extent nor type of loss has yet been quantified across all five senses concomitantly in individuals with a history of cardiovascular surgery. The purpose in performing the research that will be reviewed briefly was to evaluate and compare sensory performance (all five senses as well as cognition) in three groups of subjects: (1) patients who had undergone coronary artery bypass surgery (n 38); (2) patients with cardiovascular conditions but with no history of surgery (n 125); (3) healthy non-medicated age-matched controls (n 65). The study design allowed the determination of whether the combined impact of cardiovascular surgery plus cardiovascular drugs on sensory performance was greater than the impact of the use of cardiovascular drugs alone. The three groups tested were statistically equivalent in age (mean 73 (range 65–84) years) and duration of education (16 (range 12–21) years). The mean number of prescription medications taken by patients who had undergone coronary artery bypass surgery was 4·5; for the patients with cardiovascular conditions but with no history of surgery the mean number was 3·6. The healthy non-medicated age-matched controls took no medications other than over-the-counter vitamins. The exclusion criteria for all groups included: poor general health at the time of testing; current use of antibiotics; known problems with sensory functioning (e.g. AMD, glaucoma, hearing aids in both ears, chemosensory disorders unassociated with medications); current dental problems; current cancer; peripheral neuropathy; a score <25 on the mini mental status examination (Folstein et al. Reference Folstein, Folstein and McHugh1975), which assesses cognitive functioning. Subjects in the coronary artery bypass surgery group were tested between 3 and 6 months post surgery to ensure adequate enrolment of patients who met both the inclusion and exclusion criteria. All subjects were tested in triplicate over a 2-month period, with each evaluation separated by approximately 1 month. Multiple testing was performed to investigate and compare short-term intra-individual fluctuation or lability of the five senses and cognition, with repeated testing over a short time period when little change in perception was expected.
The scores on the sensory and cognitive tests along with brief descriptions of the test protocols are given in Table 1. Analysis of the scores reveals several major findings. First, the greatest losses for the patients who had undergone coronary bypass (who were prescreened to eliminate those with serious cognitive dysfunctioning) are for taste and smell thresholds. Taste thresholds for this group of patients range from 1·6 to 2·5 times higher than thresholds for the patients who were taking cardiovascular drugs but had no history of surgery. Taste thresholds for the patients who had undergone coronary bypass range from 1·6 to 3·7 times higher than thresholds for non-medicated subjects. Smell thresholds for the bypass patients are 8·1 times higher than thresholds for non-medicated subjects and 1·8 times higher than those for patients taking cardiovascular drugs without a history of surgery. Patients taking Ca-channel blockers tend to have the greatest losses. Furthermore, elderly patients who had undergone coronary artery bypass surgery reported more off-tastes for each of the taste stimuli tested (e.g. bitter in addition to sweet for sucrose and metallic in addition to salty for NaCl). Taking all tests together, the numerical value of the mean scores for the patients who had undergone coronary artery bypass surgery on sixteen or seventeen tests in Table 1 are in the direction of poorer performance compared with scores for patients with cardiovascular conditions without a history of surgery or the healthy non-medicated controls (P<0·000015). Furthermore, the intra-individual variability is also numerically greater for all tests for the patients who had undergone coronary artery bypass surgery (P<0·0000008). The probable cause of numerically-lower scores and greater variability in the elderly patients who had undergone coronary artery bypass surgery is likely to be multifactorial, involving the consequences of surgery (e.g. type and duration of anaesthesia, polypharmacy in conjunction with anaesthesia, invasiveness of the surgery, microemboli, post-operative hyperthermia and periods with reduced cerebral perfusion), other medical conditions and current medications. Statistical analysis reveals that the reduced performance on sensory tests is only weakly correlated or not correlated with cognitive performance. This result is probably, in part, because none of the subjects in the study were demented; all participants had been prescreened on the mini mental status examination to ensure their intellectual competence in order to eliminate potential subjects with cognitive impairments triggered by coronary artery bypass surgery (see Russell & Bornstein, Reference Russell and Bornstein2005). Thus, while sensory losses are not necessarily a consequence of cognitive loss, sensory and cognitive losses in elderly patients who have undergone coronary artery bypass surgery may be a result of a common cause or unrelated causes. Furthermore, the absence of significant differences between the mean values for the three repetitions of the tests in patients who had undergone coronary artery bypass surgery suggests that mean sensory and cognitive performance for the group was stable from 3 months to 6 months in spite of substantial intra-individual variation.
Table 1. Mean scores and intra-individual variability over three sessions on tests of the five senses and cognition for three groups of subjects
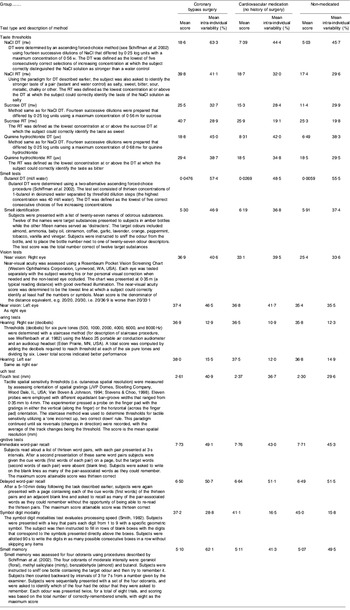
DT, detection threshold; RT, recognition threshold.
Consequences of sensory losses for nutrition
The sensory losses described earlier can have profound effects on procurement of food, food choices, appetite, nutrient intake and ultimately nutritional or health status. Loss of appetite is of special concern for patients with critical illnesses who are at a higher risk of developing protein–energy malnutrition as well as micronutrient deficiencies. Impaired vision interferes with mobility (including driving), activities of daily living, food preparation and use of utensils. Ocular diseases such as AMD impair the ability to monitor food quality and safety by appearance, to visually identify food or to discriminate eating utensils. Loss of visual cues such as colour and arrangement reduces the enjoyment of the eating experience and motivation to eat. Hearing loss and deafness can lead to social isolation and depression, both of which are associated with increased risk of malnutrition in the elderly. Hearing loss also reduces sensory input from textural cues such as crispiness (high-frequency sounds) and crunchiness (low-frequency sounds). Losses and distortions of the chemical senses (e.g. taste and smell) can reduce the motivation to eat as well as interfere with the ability to select appropriate foods and to modulate intake as nutritional requirements vary over time. Since taste and smell signals initiate responses that prepare the body for food, cephalic-phase responses, including salivary, gastric, pancreatic and intestinal secretions, are blunted, which can affect digestion of food and absorption of nutrients. When taste and smell losses no longer play a major role in initiating, sustaining and terminating ingestion, the quantity of food that is eaten and the size of meals can be affected. The inability to detect noxious tastes and odours renders the elderly patient vulnerable to food-borne illness. Losses in somatosensory perception also impact on nutrition. Reduced oral sensitivity impairs the ability to discriminate different textures of food. The inability to perceive heat can reduce pleasure from food and becomes a safety issue if foods or beverages are served at boiling temperatures (e.g. inducing oral burns). Reduced temperature perception in the fingers is a risk factor for burns during cooking. Tactile losses make food preparation and manipulation of utensils more difficult. Overall, losses of visual, auditory, taste, smell and somatosensory input from food can dramatically alter the eating behaviour and profoundly interfere with quality of life. In conclusion, the ability of the medical professional to identify a patient's sensory losses during a period of critical illness has the potential to improve treatment and reduce costs of prolonged sickness.
Acknowledgements
Research described in this paper was supported in part by NIH AG00443.



