Introduction
Lewy body (LB) disease is a leading neurodegenerative cause of cognitive impairment, second only to Alzheimer’s disease (AD), with which it often co-occurs (McAleese et al., Reference McAleese, Colloby, Thomas, Al‐Sarraj, Ansorge, Neal, Roncaroli, Love, Francis and Attems2021). This may manifest in dementia at the later stages (McKeith et al., Reference McKeith, Boeve, Dickson, Halliday, Taylor, Weintraub, Aarsland, Galvin, Attems, Ballard, Bayston, Beach, Blanc, Bohnen, Bonanni, Bras, Brundin, Burn, Chen-Plotkin and Kosaka2017), or mild cognitive impairment (MCI) at the earlier stages (McKeith et al., Reference McKeith, Ferman, Thomas, Blanc, Boeve, Fujishiro, Kantarci, Muscio, O’Brien, Postuma, Aarsland, Ballard, Bonanni, Donaghy, Emre, Galvin, Galasko, Goldman and Gomperts2020), when objective cognitive impairments appear, but independent function is still relatively maintained (Albert et al., Reference Albert, DeKosky, Dickson, Dubois, Feldman, Fox, Gamst, Holtzman, Jagust, Petersen, Snyder, Carrillo, Thies and Phelps2011).
Both dementia with Lewy bodies (DLB) and MCI with Lewy bodies (MCI-LB) often feature a pattern of cognitive impairments which differs from that typical of AD. Attention, executive, and visuospatial dysfunctions are often greater, while there is typically less amnestic memory impairment (Ciafone et al., Reference Ciafone, Thomas, Durcan, Donaghy, Hamilton, Lawley, Roberts, Colloby, Firbank, Allan, Petrides, Taylor, O’Brien and Gallagher2021; Hamilton et al., Reference Hamilton, Matthews, Donaghy, Taylor, O’Brien, Barnett, Olsen, Lloyd, Petrides, McKeith and Thomas2021b; Metzler-Baddeley, Reference Metzler-Baddeley2007).
However, the cognitive dysfunctions present in DLB may be distinctly characterized by their variability within individuals: cognitive fluctuations are a common clinical feature of MCI-LB and DLB (Donaghy et al., Reference Donaghy, Ciafone, Durcan, Hamilton, Barker, Lloyd, Firbank, Allan, O’Brien, Taylor and Thomas2022; Donaghy et al., Reference Donaghy, Taylor, O’Brien, Barnett, Olsen, Colloby, Lloyd, Petrides, McKeith and Thomas2018), characterized by variations from periods of poor alertness and unresponsiveness, to periods of relative lucidity (Matar et al., Reference Matar, Shine, Halliday and Lewis2019; O’Dowd et al., Reference O’Dowd, Schumacher, Burn, Bonanni, Onofrj, Thomas and Taylor2019). These overt clinically manifest fluctuations are typically recognized by clinical interview with a carer/informant who may report varying levels of confusion or consciousness over the course of minutes, hours, or days (Lee et al., Reference Lee, McKeith, Mosimann, Ghosh-Nodial, Grayson, Wilson and Thomas2014; Walker et al., Reference Walker, Ballard, Ayre, Wesnes, Cummings, McKeith and O’Brien2000). Cognitive fluctuations are crucial to recognize and understand, being associated with worse prognosis in MCI (Hamilton et al., Reference Hamilton, Matthews, Donaghy, Taylor, O’Brien, Barnett, Olsen, Durcan, Roberts, Ciafone, Barker, Firbank, McKeith and Thomas2021a) and greater healthcare utilization costs in dementia (Espinosa et al., Reference Espinosa, Davis, Johnson, Cline and Weintraub2020).
The fluctuating nature of cognitive impairments in DLB and MCI-LB are difficult to capture and quantify in routine cognitive assessment, due to their sporadic nature and the substantial barrier that severe periods of dysfunction may pose to testing. Further complicating this, overt fluctuations are not reported in all cases, either due to absence of an informant, or these symptoms being mild and difficult to distinguish.
Fluctuations in DLB have been related to broader neuropsychological deficits, specifically in attention (Ballard et al., Reference Ballard, Walker, O’Brien, Rowan and McKeith2001b). Underlying clinically evident severe reductions in alertness, there may be more subtle changes in attention over the short term (i.e. moment to moment) in LB disease. These may not be evident to an observer, but may be detectable through electrophysiological methods (Stylianou et al., Reference Stylianou, Murphy, Peraza, Graziadio, Cromarty, Killen and Taylor2018), or by assessing cognitive performance continuously for a period of time to examine variability in performance (Ballard et al., Reference Ballard, O’Brien, Gray, Cormack, Ayre, Rowan, Thompson, Bucks, McKeith, Walker and Tovee2001a) or errors (Phillips et al., Reference Phillips, Matar, Martens, Halliday, Moustafa and Lewis2020) reflecting sporadic attentional lapses.
Continuous assessment of cognitive performance and errors over the course of a single task may enable researchers to examine three key aspects of cognitive performance of relevance to cognitive symptoms in LB disease:
-
1. How an individual best performs at a given task (typically captured by common cognitive measures)
-
2. The rate and magnitude of deviations from the typical level of performance, including errors and “outlier” measurements
-
3. Changes in performance over the duration of the measurement, such as performance improving with practice, or worsening as compensatory resources are exhausted.
We therefore aimed to assess whether MCI-LB in general, and those with cognitive fluctuation symptoms in particular, would feature sporadically poorer performance on a continuous performance task (CPT). We hypothesized that sustained attention performance deficits in MCI-LB would be characterized by slowed response times to stimuli or higher error rates (failures to respond to targets, and erroneous responses to non-targets), and that this would worsen over the course of the task.
Methods
Participants
People with MCI and cognitively healthy older adults were drawn from the SUPErB study cohort. Full details on recruitment, diagnostics, and assessment criteria have been reported previously (Ciafone et al., Reference Ciafone, Thomas, Durcan, Donaghy, Hamilton, Lawley, Roberts, Colloby, Firbank, Allan, Petrides, Taylor, O’Brien and Gallagher2021; Donaghy et al., Reference Donaghy, Ciafone, Durcan, Hamilton, Barker, Lloyd, Firbank, Allan, O’Brien, Taylor and Thomas2022). In brief, MCI patients were recruited from older persons’ medical, psychiatric, neurology, and memory services in North East England. All had a recent health service diagnosis of MCI, which was ratified within the study.
Healthy controls were recruited from the families of MCI patients, and from local research involvement organizations.
All participants were aged 60+ and medically stable at baseline. Exclusion criteria for MCI patients were the presence of dementia at baseline, Parkinson’s disease for >12 months prior to cognitive symptoms, absence of objective cognitive impairment, Mini Mental Status Examination score <20, or suspected vascular or frontotemporal etiology. Exclusion criteria for controls were presence of any cognitive impairment or neurological disease.
Favorable ethical approval for this study was given by the National Research Ethics Service North East – Newcastle and North Tyneside 2 (15/NE/0420), and all participants gave their written informed consent to participate. All research was completed in accordance with the Helsinki Declaration.
Diagnosis and imaging
All participants underwent detailed cognitive and clinical assessment at baseline, and at approximately yearly follow-ups. These provided information on the presence and absence of cognitive and functional impairments, used by a clinical panel to rate presence of MCI (Albert et al., Reference Albert, DeKosky, Dickson, Dubois, Feldman, Fox, Gamst, Holtzman, Jagust, Petersen, Snyder, Carrillo, Thies and Phelps2011), dementia (McKhann et al., Reference McKhann, Knopman, Chertkow, Hyman, Jack, Kawas and Mayeux2011), or no cognitive impairment.
The panel also assessed the presence of core clinical features of DLB on the basis of the clinical interview: REM sleep behavior disorder, parkinsonism, cognitive fluctuations, and complex visual hallucinations (McKeith et al., Reference McKeith, Boeve, Dickson, Halliday, Taylor, Weintraub, Aarsland, Galvin, Attems, Ballard, Bayston, Beach, Blanc, Bohnen, Bonanni, Bras, Brundin, Burn, Chen-Plotkin and Kosaka2017). Polysomnography was not available to confirm presence of REM sleep without atonia, and so clinical judgement of RBD was based on clinical interview only.
Participants underwent dopaminergic FP-CIT imaging and metaiodobenzylguanidine (MIBG) cardiac scintigraphy at baseline (Roberts et al., Reference Roberts, Donaghy, Lloyd, Durcan, Petrides, Colloby and Thomas2021a; Roberts et al., Reference Roberts, Durcan, Donaghy, Lawley, Ciafone, Hamilton and Thomas2021b). Images were rated as normal/abnormal (FP-CIT) or quantified by the heart:mediastinum ratio (MIBG) blind to clinical information. Abnormal imaging was incorporated into diagnostic classification alongside core clinical features to provide a classification of MCI due to Alzheimer’s disease (MCI-AD), possible MCI-LB, or probable MCI-LB, in accordance with current consensus clinical or research criteria (Albert et al., Reference Albert, DeKosky, Dickson, Dubois, Feldman, Fox, Gamst, Holtzman, Jagust, Petersen, Snyder, Carrillo, Thies and Phelps2011; McKeith et al., Reference McKeith, Ferman, Thomas, Blanc, Boeve, Fujishiro, Kantarci, Muscio, O’Brien, Postuma, Aarsland, Ballard, Bonanni, Donaghy, Emre, Galvin, Galasko, Goldman and Gomperts2020).
Diagnoses were updated after each follow-up visit as new information came to light. Possible MCI-LB, being a small, diagnostically uncertain, and heterogeneous group, were not included in primary analyses but reported for context. MCI-LB, unless otherwise specified, therefore refers to probable MCI-LB only in this study.
Cognitive assessment and sustained attention task
Participants underwent a cognitive testing battery including Addenbrooke’s Cognitive Examination – Revised, trail making test parts A and B, Stroop color-word interference task, FAS phonemic fluency task, forward and backward digit span task, Rey Auditory Verbal Learning Test, Graded Naming Test, Modified Taylor Complex Figure, and Digit-Symbol Substitution Tests. These were supplemented by several computerized tests: simple reaction time, binary forced choice reaction time, line angle judgement task, Visual Patterns Test, and Corsi Block test (Ciafone et al., Reference Ciafone, Thomas, Durcan, Donaghy, Hamilton, Lawley, Roberts, Colloby, Firbank, Allan, Petrides, Taylor, O’Brien and Gallagher2021; Donaghy et al., Reference Donaghy, Ciafone, Durcan, Hamilton, Barker, Lloyd, Firbank, Allan, O’Brien, Taylor and Thomas2022). At baseline and repeated follow-up, participants were administered an AX CPT. Stimuli – capital letters from A to Z – were serially displayed in the center of a laptop screen for 85 ms each with an interstimulus interval (ISI) of 900 ms.
Participants were instructed to watch the letter stream for the cue (A). If the cue was followed by a target (X), they were to respond to the target with a button press as quickly as possible. If the cue was followed by a non-target (for brevity, labeled Y here, but inclusive of all non-X stimuli), they were instructed to not respond. Any X targets following a non-cue (labeled B here, but inclusive of all non-A stimuli), and any A cues themselves, were also not to be responded to.
Out of 480 total stimuli, there were 168 AX cued valid targets, 22 AY cued non-targets, 24 BX non-cued invalid targets, 71 BY non-cued non-targets, and 195 A cues. A cues could themselves directly follow a previous A cue, therefore allowing for more A cues in total (195) than the combined number of AX (168) and AY (22) pairs. Cue-target pairs were block-randomised throughout the experiment such that they were spread over the course of the task.
In addition to valid responses, different forms of errors were also assessed: omission errors, missed responses to valid cued targets, were considered to reflect attentional lapses leading to substantially delayed or entirely missed responses. Commission errors were also recorded, with several possible forms:
AY errors, responses to a non-target after an A cue, were considered as evidence of failures in inhibitory control.
BX errors, responses to an X stimulus without a valid cue, were considered as symptomatic of a failure of context maintenance.
BY errors, responses to any non-X and non-A stimulus in the absence of any preceding A cue, were considered as symptomatic of generalized issues in sustaining attention.
A errors, response to any A stimulus, also considered as symptomatic of generalized issues in sustaining attention.
Response times were recorded for each displayed stimulus – both for valid and invalid trials – with MATLAB R2012b. Participant responses were registered by the thumb of their dominant hand using a handheld button peripheral. Those unable to use their thumb to respond were permitted to use their index finger. Responses to invalid targets or cues were not included in analysis of response time.
The CPT was administered as part of the battery of computerized tests, provided in a consistent order: participants first completed a 40-item simple reaction task, a 40-item binary forced choice reaction task, a brief line angle judgement task, and finally the CPT. This order of administration ensured that participants would be familiar with the speed and accuracy demands of the response time experiment, and with the handheld response indicators. The Visual Patterns and Corsi Block tests followed the CPT at baseline, and were not administered at follow-up.
At baseline, this computerized battery was administered at the start of a separate day to pen-and-paper neuropsychological testing. At follow-up, this was administered at the end of the pen-and-paper battery in a single session.
Analysis
Data for each seen stimulus were extracted for each subject and analyzed with multi-level models incorporating subject-level intercepts and slopes for both time elapsed in the experiment (task progress) and time elapsed since baseline assessment (time in years).
Response times were estimated as an ex-Gaussian distributed outcome to account for the right-skew inherent in human reaction time. Error rates, both omission errors (failure to respond to a valid target after a cue) and commission errors (erroneous responses to a non-target, un-cued target, or cue itself), were each estimated with a Bernoulli logistic model for successes or failures.
All analyses were undertaken with the brms package as an interface to the Stan probabilistic programming language for R software. Response times were estimated with weakly informative priors and starting values derived from previous data comparing DLB, AD, and controls. Error rate analyses included weakly informative, zero-centered boundary avoiding priors for all parameter estimates.
Markov chain Monte Carlo samples were drawn by a Hamiltonian Monte Carlo/No-U-Turn Sampler running four parallel chains with 2000 iterations (1000 warmup) initially and any sampling pathologies addressed as needed. Final models were refit with 6000 iterations (2000 warmup) to ensure chains had not only converged at the local scale, and to provide sufficient effective sample sizes.
Secondary analysis of delayed responses
It has previously been noted that in tasks of this nature, responses to a valid target may be so delayed as to fall on the following stimulus (i.e. if the response time is greater than the display time and ISI) (Gallagher et al., Reference Gallagher, Nilsson, Finkelmeyer, Goshawk, Macritchie, Lloyd, Thompson, Porter, Young, Ferrier, McAllister-Williams and Watson2015). As all responses following a target must be a non-target, this would appear to be a commission error, while the delayed response would appear to be an omission error.
To assess whether such substantially delayed responses might account for observed effects, we conducted a sensitivity analysis with responses reprocessed in accordance with the criteria described previously (Gallagher et al., Reference Gallagher, Nilsson, Finkelmeyer, Goshawk, Macritchie, Lloyd, Thompson, Porter, Young, Ferrier, McAllister-Williams and Watson2015): when a commission error immediately followed an omission error, this was re-coded for the sensitivity analysis as a non-error with a delayed response. The overall time for this response was calculated as the response time to the non-target stimulus plus the display time of the target stimulus and the ISI.
Derived summary measures
To explore the clinical utility of the CPT and any relationship to other performance indicators, we extracted simple cross-sectional summary measures derived from this (mu, sigma, and tau RT parameters (see Supplementary Figure S1), omission error rate, and commission error rate). We examined the correlations between these and other measures of attention and executive functions, and compared their utility
Results
Across baseline and longitudinal follow-up, 89 persons with MCI (35 MCI-AD, 15 possible MCI-LB, 39 probable MCI-LB) and 31 cognitively healthy older adults completed the CPT (see Table 1).
Table 1. Baseline characteristics of cohort.

Note: Count (%), mean (SD), or median (min–max).
Response time
Initial analyses estimated baseline participant-level RT distribution parameters by maximum likelihood estimation; both mu and sigma parameters were broadly similar across MCI groups, with weak evidence of a faster response (lower mu) in controls (see Figure 1), and only weak evidence of a longer tau tail in MCI-LB than MCI-AD [Estimate = +22 [−18 to 62]). Within MCI-LB, there was weak evidence of an association between parkinsonism and slower mean response times (Estimate = +171ms [−37 to 386]).
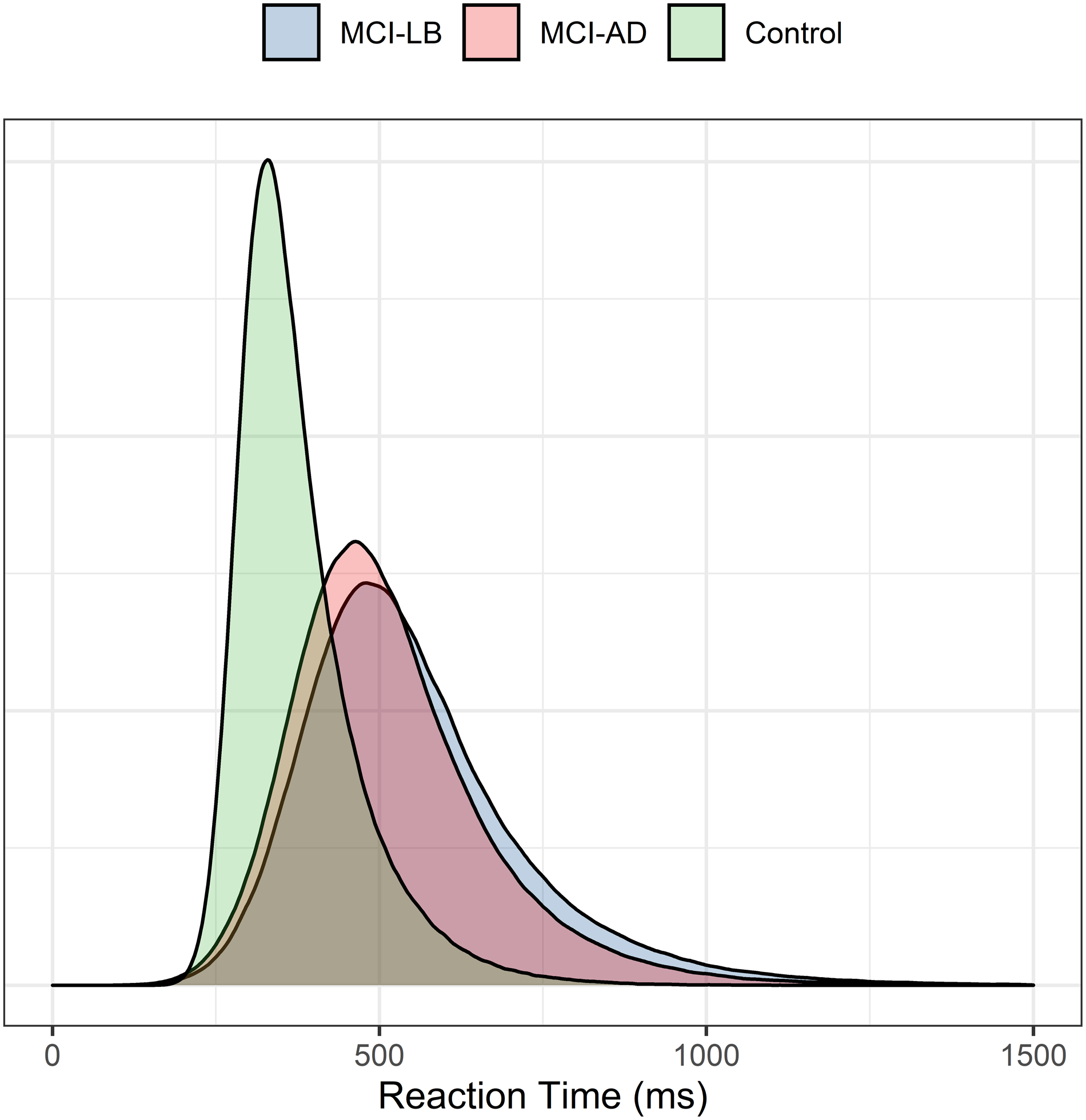
Figure 1. Estimated baseline ex-Gaussian distributions for healthy controls, MCI-AD and MCI-LB.
Omission errors
Developing on this analysis of RTs, there was a clear difference in response accuracy between groups. Omission errors were substantially more common in probable MCI-LB than MCI-AD and controls – this was evident from the start of the task to its conclusion. Practice effects were seen for all groups however, with omissions becoming less common by the end of the task (see Figure 2). This greater error rate in probable MCI-LB was already evident at study baseline and for up to 2 years afterwards, with greater uncertainty in the estimate beyond this point reflecting lack of longer-term data.
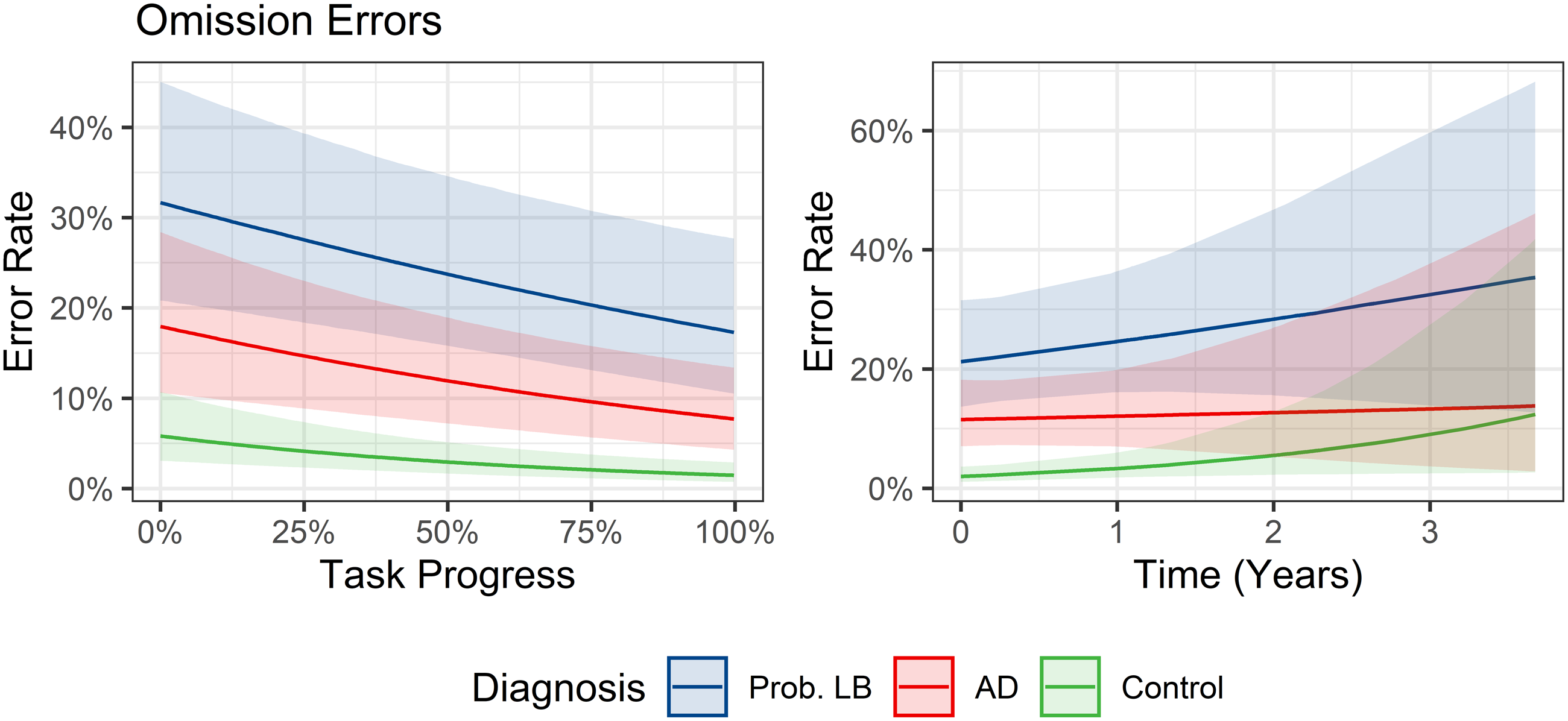
Figure 2. Marginal predicted rates of omission errors per target trial over duration of experiment (left) and longitudinal follow-up (right).
A sensitivity analysis was conducted to assess whether this increased omission rate was a consequence of valid responses being delayed into the following stimulus as previously described. Adjusting for this possibility did not meaningfully change this effect: the MCI-LB group consistently made more omission errors than MCI-AD (odds ratio = 2.3, 95% CI = 1.1–4.7 in primary analysis vs 2.3, 1.3–4.3 in the sensitivity analysis).
There was evidence that omission errors were more likely in MCI-LB cases with parkinsonism (OR = 1.9, 95% CI 1.3–2.9), and those with cognitive fluctuations (OR = 4.3, 95% CI 2.2–8.8) as rated by the clinical panel, with the latter carrying the strongest associations albeit with a large degree of uncertainty.
Commission errors
Several forms of incorrect responses were also assessed (see Figure 3).

Figure 3. Marginal predicted commission error rates per trial over experiment duration (left) and longitudinal follow-up (right).
AY error rates were similar across groups at the start of the experiment, with estimated marginal probabilities of an error of 14% per trial (10–20%) for MCI-AD and 13% (9–19%) for probable MCI-LB. However, these had diverged by the end of the experiment, with error rates of 11% (7–17%) per trial for MCI-AD vs. 17% (12–24%) for MCI-LB. Controls showed some improvement on average from year to year, though this was less evident in MCI.
There was only weak evidence of a higher rate of BX errors in MCI-LB at the start of the task, which had largely resolved by the end of the task (see Figure 4). Controls again showed some improvement year to year, while this was not evident in MCI.

Figure 4. Conditional effects of diagnosis (MCI-LB vs MCI-AD) on different error rates at experiment start vs. end.
As in AY errors, BY errors became more common in MCI-LB by the end of the task (see Figure 3). Unlike AY errors, this was not driven by worsening performance in MCI-LB, but rather performance not improving as quickly in this group as in MCI-AD. This difference in BY errors also emerged earlier than in AY errors, being evident by the midway point of the experiment. Overall rates of BY errors were low in contrast to other errors (i.e. typically occurring in ≤6% of valid trials).
There was weak evidence that A errors were more common in MCI-LB than MCI-AD, which converged toward the end of the task due to slightly greater improvement in MCI-LB (see Figure 3).
There was little evidence that any specific LB symptoms were associated with AY commission errors within MCI-LB.
As when assessing omission errors, there was little evidence of any group-trajectory differences in commission errors across longitudinal repeats, with the exception of a slightly reduced rate of BX and possibly AY errors at follow-up in healthy controls suggesting possible long-term practice effects (see Figure 3).
Associations with attention and executive measures
Residual correlations between summary measures derived from the CPT, Stroop test, and trail making tests were examined simultaneously in a multivariate model. Omission and commission error rates were not clearly positively correlated (β = 0.11 [−0.12 to 0.34]).
The data were most compatible with weak-to-moderate positive correlations between overall omission error rate and response time mu (β = 0.33 [0.13 to 0.50]), sigma (β = 0.27 [0.06 to 0.45]), and tau (β = 0.29 [0.08 to 0.49]) parameters, as well as time taken to complete the trail making test parts A (β = 0.40 [0.19 to 0.58]) and B (β = 0.27 [0.05 to 0.47]). Omission rate was not clearly associated with interference (β = −0.10 [−0.33 to 0.13]) or error rates (β = −0.02 [−0.24 to 0.21]) in the Stroop color-word interference task.
While commission error rate was also positively associated with mu (β = 0.42 [0.21 to 0.59]) and sigma (β = 0.50 [0.32 to 0.66]) parameters, this was not associated with the tau response time parameter (β = 0.01 [−0.22 to 0.24]). Commission error rates were also not associated with time taken to complete trail making test parts A (β = −0.01 [−0.23 to 0.20]) or B (β = 0.01 [−0.22 to 0.23]), nor interference (β = 0.05 [−0.18 to 0.27]) or errors (β = −0.02 [−0.24 to 0.22]) in the Stroop task.
Classification accuracy
The utility of derived CPT response time parameters (mu, sigma, and tau) and omission error rate in differentiating MCI-LB from healthy controls, and MCI-AD, was assessed with receiver-operating characteristic curves. These measures were compared to a common screening tool for cognitive impairment: the trail making test parts A and B.
Response time parameters were poor at differentiating MCI-LB from healthy controls (mu AUC = 0.55 [0.41–0.68], sigma AUC = 0.64 [0.51–0.77], tau AUC = 0.70 [0.57–0.82]). Trail making test parts A (AUC = 0.81 [0.71–0.91]) and B (AUC = 0.88 [0.80–0.96]) outperformed these. Omission error rates from the CPT had better discriminatory utility than all parameters but trail making test B (AUC = 0.92 [0.86–0.99]). Commission error rates had similar utility to the trail making test A (AUC = 0.83 [0.73–0.93]), outperforming response time measures but no other test.
All measures were poor at differentiating MCI-LB from MCI-AD, with only the CPT omission error rate (AUC = 0.74 [0.63–0.86]) having an AUC clearly greater than 0.50.
DLB progression
Of 39 probable MCI-LB cases, 17 had developed DLB during longitudinal follow-up at the time of data locking. In comparison to other MCI-LB cases, those who progressed to DLB were more susceptible to omission errors (OR = 2.59, 95% CI = 1.12–5.20) and A commission errors at baseline (OR = 2.39, 95% CI = 1.14–4.90). There was weak evidence supporting that DLB progressors also became more susceptible to AY (OR = 1.82, 95% CI = 1.04–3.17) and BY (OR = 2.65, 95% CI = 0.98–6.38) commission errors over the course of longitudinal follow-up.
Discussion
We hypothesized that cases of MCI-LB would feature poorer performance on this continuous sustained attention task than MCI-AD cases, and that this would be associated with the presence of parkinsonism and cognitive fluctuation symptoms specifically.
We found that performance in MCI-LB was worse than MCI-AD; however, this was not characterized by a generalized slowing of successful responses overall, but rather by frequent omission errors which may represent attentional lapses, and an increased rate of commission errors after extended testing, which may represent a breakdown of inhibitory control.
Omission errors were associated with motor parkinsonism and cognitive fluctuations in MCI-LB, and with general slowing overall in other metrics such as the trail making test. In contrast, commission errors appeared to be a more generalized issue, not related to any specific clinical phenotype examined within MCI-LB, nor to performance in other tasks.
These results provide important context as to the manifestation of cognitive impairments in MCI-LB. DLB is characterized by pronounced variability in overall alertness and responsiveness (Ballard et al., Reference Ballard, Walker, O’Brien, Rowan and McKeith2001b), and these results support several studies finding that this may manifest in greater variability in cognitive functioning, for example, attentional lapses (Phillips et al., Reference Phillips, Matar, Martens, Halliday, Moustafa and Lewis2020) and variability (Ballard et al., Reference Ballard, O’Brien, Gray, Cormack, Ayre, Rowan, Thompson, Bucks, McKeith, Walker and Tovee2001a; Webber et al., Reference Webber, Kiselica, Mikula and Woods2022). DLB also often features executive dysfunctions and failures of inhibitory control similar to those seen in frontotemporal dementias (Johns et al., Reference Johns, Phillips, Belleville, Goupil, Babins, Kelner, Ska, Gilbert, Inglis, Panisset, de Boysson and Chertkow2009). This research builds on this to demonstrate that similar performance lapses may be seen in the cognitive prodrome, MCI-LB, and also demonstrates that inhibitory failures may not be seen initially, but possibly emerge with sustained effort in longer cognitive tasks.
There was no clear evidence that any performance characteristics worsened over long-term follow-up across diagnostic groups, though some evidence of possible practice effects in healthy controls. This is however limited by a paucity of data beyond 2 years post baseline. With longer-term follow-up, groups may show clearer divergence up to the onset of dementia: in further exploratory analyses, there was some evidence of progressive susceptibility to commission errors in MCI-LB cases who developed DLB.
When examining derived response time parameters only, there was limited evidence of any clear differences between MCI diagnostic groups. Cognitive outcome measures which rely on a threshold of performance being met to provide data may underestimate cognitive deficits in MCI-LB in particular, due to data from periods of worse performance being systematically missing, for example, due to attentional lapses. Additionally, some performance deficits in MCI-LB may only be observable through sustained observation or recording of performance. Deviations from an individual’s usual level of performance may be informative, whether transient or sustained, with the former possibly being more pertinent in those with symptoms of cognitive fluctuations (due to cognitive lapses) or motor parkinsonism (due to movement slowing). Neuropsychological testing procedures which average across repeated trials, or which utilize only successful or best performing trials, may risk discarding valuable information on cognitive impairments in MCI-LB.
While the above methods may not be feasible for characterizing individual performance in clinical settings, characterizing variation in repeated-measures paradigms by measuring the difference between best and worst performance, missing data or error rates, or intra-individual variability, may hint at sporadic or fluctuating deficits within individuals. We provide some tentative support here that simplified metrics, such as the percentage of omitted responses over the course of the task, could more effectively screen for MCI-LB than response time parameters or other attention/executive measures.
It remains unclear whether these symptoms may also lead to variability in longer-term retesting, possibly obscuring measures of global cognitive decline: we have previously found that cognitive fluctuations in MCI were associated with greater risk of dementia (Hamilton et al., Reference Hamilton, Matthews, Donaghy, Taylor, O’Brien, Barnett, Olsen, Durcan, Roberts, Ciafone, Barker, Firbank, McKeith and Thomas2021a) but not of progressive cognitive decline on objective measures (Hamilton et al., Reference Hamilton, Matthews, Donaghy, Taylor, O’Brien, Barnett, Olsen, McKeith and Thomas2020). This could be explained by a high degree of year-to-year variability in cognitive performance obscuring the underlying disease progression.
These results also demonstrate that the length of cognitive tasks should also be considered as a factor when designing outcome measures for MCI in research and clinical trials. When impairments are mild, individuals may be more capable of compensating or masking underlying deficits for the duration of a brief assessment. We found indications that some deficits may only emerge later, after several minutes of sustained effort. However, longer tasks, or those which aim to push subjects to failure, may be disagreeable to participants, raise burden to an unacceptable level, or lead to missing data through task refusal or early withdrawal. Any possible benefits should therefore be carefully weighed against the likely drawbacks of more rigorous cognitive testing. Direct manipulation of cognitive load through adaptive designs (e.g. increasing task difficulty until performance breaks down) may be particularly beneficial in this population, with such methods previously used to test visuospatial performance (Wood et al., Reference Wood, Watson, Firbank, Mosimann, Barber, Blamire and O’brien2013).
The CPT was administered after brief introductory computer testing at baseline on a separate day to pen-and-paper testing, enabling participants to be familiar with the testing environment without being fatigued. At follow-up this was typically administered after pen-and-paper testing in a single session, and the order of administration was not counter-balanced. Variable levels of fatigue, particularly at follow-up, may therefore introduce confounding.
Presence of RBD was determined by clinical interview, polysomnography was not available to confirm REM sleep without atonia, a limitation of this cohort. In the absence of biomarker confirmation, some cases of RBD may be mistaken cases of other sleep disorders (e.g. non-REM sleep parasomnias, sleep apnea).
We considered that omission errors might stem from the same processes as long tails in RT distributions (i.e. the tau parameter of the ex-Gaussian), where a severely delayed reaction might be mistaken for an entirely missed response threshold, with corresponding commission error on the following trial. Sensitivity analyses adjusting for this possibility did not support this theory however – wholly absent responses were still seen to be more common in MCI-LB.
In summary, MCI-LB features a distinct pattern of responses to a sustained attention task, characterized by frequent omissions and errors rather than slowed responses. These may be related to both motor impairments and fluctuating attentional processes. Assessing sporadic lapses of performance in repeated-measures designs may therefore be beneficial in detecting cognitive deficits in MCI-LB with a fluctuating cognitive course. Assessing individuals’ best level of performance on successful trials may mask such deficits.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1355617723000772.
Acknowledgements
None.
Funding statement
This study was funded by Alzheimer’s Research UK (ARUK-PG2015-13) and supported by the NIHR Newcastle Biomedical Research Centre. GE Healthcare provided the FP-CIT ligand for this investigator-run study.
Competing interests
No relevant conflicts to report.







