Introduction
This paper describes rich Middle and Late Jurassic radiolarian faunas from four localities in the Hallstatt Mélange near Bad Mitterndorf in Austria. Radiolarian assemblages are well preserved, suitable for taxonomic studies, and precise biostratigraphy.
Middle and Late Jurassic low-latitude radiolarians have been relatively well studied in terms of species-level systematics and biochronology (Baumgartner et al., Reference Baumgartner, Bartolini, Carter, Conti, Cortese, Danelian, De Wever, Dumitrica, Dumitrica-Jud, Goričan, Guex, Hull, Kito, Marcucci, Matsuoka, Murchey, O’Dogherty, Savary, Vishnevskaya, Widz and Yao1995a, Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and Steigerb); however, the systematics of genera and families has not been sufficiently elaborated yet. O’Dogherty et al. (Reference O’Dogherty, Carter, Dumitrica, Goričan, De Wever, Bandini, Baumgartner and Matsuoka2009) presented an illustrated catalogue of type species of Jurassic and Cretaceous genera described so far. All genera were revised, and many were considered as invalid (synonyms, homonyms, nomina dubia). Another group of radiolarians that needs taxonomic revision are those Mesozoic species that still bear inappropriate names of Recent genera. The structure of the catalogue, consisting exclusively of illustrations and synonymy, did not allow us to discuss the distinguishing characteristics of the valid genera or to describe new taxa. The primary aim of this paper is to move towards a more natural taxonomy of Jurassic radiolarians. Six new genera are described, a replacement name for a homonym is proposed, the diagnoses of six valid genera are emended, and remarks for several other genera are provided to clarify their definition. For all new and revised genera, a list of included species is presented. Two new families are erected and two new species are described.
In addition to radiolarian taxonomy, this paper contributes to the biostratigraphic data of the Hallstatt Mélange in the Northern Calcareous Alps. Extensive radiolarian dating over the last 15 years has provided a wealth of age constraints in deep-water sediments of the Tirolic units and the Hallstatt Mélange (Suzuki and Gawlick, Reference Suzuki and Gawlick2003, Reference Suzuki and Gawlick2009; Missoni and Gawlick, Reference Missoni and Gawlick2011a, Reference Missoni and Gawlickb and references therein). These data had important implications for the reconstruction of the Jurassic tectonostratigraphy and distinguished several trench-like basins that formed progressively during the propagation of thrusting (see Missoni and Gawlick, Reference Missoni and Gawlick2011a, Reference Missoni and Gawlickb; Gawlick et al., Reference Gawlick, Missoni, Schlagintweit and Suzuki2012 for the latest reviews). However, the structure of this area, especially that of the Hallstatt Mélange, is extremely complex and the proposed tectonostratigraphic model can still be refined with additional biostratigraphic data.
Geological overview
The study area is located in the central Northern Calcareous Alps around 100 km southeast of Salzburg (Fig. 1.1). The Northern Calcareous Alps represent a far travelled nappe system in the highest structural position of the Eastern Alps (Tollmann, Reference Tollmann1985) and belong palaeogeographically to the Austroalpine domain (Fig. 1.1).

Figure 1 Tectonic and paleogeographic maps. (1) Tectonic sketch map of the Eastern Alps and study area (after Tollmann, Reference Tollmann1987; Frisch and Gawlick, Reference Frisch and Gawlick2003); GPU Graz Palaeozoic Unit; GU Gurktal Unit; GWZ Greywacke Zone; RFZ Rhenodanubian Flysch Zone. Star indicates study area (Fig. 3). (2) Late Triassic paleogeographic position and facies zones of the Austroalpine domain as part of the northwestern Neotethys passive margin; IAZ=Iberia-Adria Zone transform fault, AAT=future Austroalpine-Adria transform fault, TTT=future Tisza-Tatra transform fault, TMT=future Tisza-Moesia transform fault, AA=Austroalpine, BI=Bihor, BR=Briançonnais, BU=Bükk, C=Csovar, Co=Corsica, DI=Dinarides, DO=Dolomites, DR=Drau Range, HA=Hallstatt Zone, JU=Juvavicum, JL=Julian Alps, ME=Meliaticum, MK=Mecsek, MO=Moma unit, MP=Moesian platform, P=Pilis-Buda, R=Rudabanyaicum, SI=Silicicum, SL=Slovenian trough, SM=Serbo-Macedonian unit, TA=Tatricum, TO=Tornaicum, TR=Transdanubian Range, VA=Vascau unit, WC=central West Carpathians (modified after Haas et al., Reference Haas, Kovacs, Krystyn and Lein1995; Gawlick et al., Reference Gawlick, Frisch, Vecsei, Steiger and Böhm1999, Reference Gawlick, Frisch, Hoxha, Dumitrica, Krystyn, Lein, Missoni and Schlagintweit2008). (3) Schematic cross section (for position, see line a-b in 2) showing the typical passive continental margin facies distribution across the Austroalpine domain in Late Triassic time (after Gawlick and Frisch, Reference Gawlick and Frisch2003). (4) Palaeogeographic position of the Northern Calcareous Alps as part of the Austroalpine domain in Late Jurassic time (after Frisch, Reference Frisch1979; Gawlick et al., Reference Gawlick, Frisch, Hoxha, Dumitrica, Krystyn, Lein, Missoni and Schlagintweit2008). In this reconstruction the Northern Calcareous Alps are part of the Jurassic Neotethyan Belt (orogen) striking from the Carpathians to the Hellenides. The Neotethys suture is equivalent to the obducted West-Vardar ophiolite complex (e.g., Dinaric Ophiolite Belt) in the sense of Schmid et al. (Reference Schmid, Bernoulli, Fügenschuh, Matenco, Schefer, Schuster, Tischler and Ustaszewski2008)=far-travelled ophiolite nappes of the western Neotethys Ocean in the sense of Gawlick et al. (Reference Gawlick, Frisch, Hoxha, Dumitrica, Krystyn, Lein, Missoni and Schlagintweit2008) (see Robertson, Reference Robertson2012 for discussion). The eastern part of the Neotethys Ocean remained open=Vardar Ocean (Missoni and Gawlick, Reference Missoni and Gawlick2011a). Toarcian to Early Cretaceous Adria-Apulia carbonate platform and equivalents according to Golonka (Reference Golonka2002), Vlahović et al. (Reference Vlahović, Tišljar, Velić and Matičec2005), and Bernoulli and Jenkyns (Reference Bernoulli and Jenkyns2009). (5) Schematic cross section reconstructed for Middle to Late Jurassic times showing the passive continental margin of the Lower Austroalpine domain facing the Penninic Ocean to the northwest (e.g., Tollmann, Reference Tollmann1985; Faupl and Wagreich, Reference Faupl and Wagreich2000) and the lower plate position and imbrication of the Austroalpine domain in relation to the obducted Neotethys oceanic crust (after Gawlick et al., Reference Gawlick, Frisch, Hoxha, Dumitrica, Krystyn, Lein, Missoni and Schlagintweit2008; compare with Frisch, Reference Frisch1979). Star indicates position of study area (compare Figure 3).
In Middle–Late Triassic times the Austroalpine domain as part of the central and southeastern European shelf show a typical carbonate passive continental margin facies distribution (Fig. 1.2–1.3), whereas in Jurassic times the Austroalpine realm was situated between the Penninic Ocean to the northwest and the Neotethys Ocean in the southeast (Fig. 1.4–1.5).
Contemporaneous with progressive Jurassic extension and opening of the Alpine Atlantic Ocean (and the Penninic realm as part of it; see Missoni and Gawlick, Reference Missoni and Gawlick2011a for details) as an eastward continuation of the Central Atlantic Ocean, closure of parts of the Neotethys Ocean with formation of the Neotethys ophiolite imbricates started in the late Early Jurassic and prevailed until the early Late Jurassic (Karamata, Reference Karamata2006). Obduction of the Neotethys ophiolite imbricates started in the Middle Jurassic and the Austroalpine and its northern and southern equivalents attained a lower plate position (Frisch and Gawlick, Reference Frisch and Gawlick2003, Gawlick et al., Reference Gawlick, Frisch, Hoxha, Dumitrica, Krystyn, Lein, Missoni and Schlagintweit2008, Schmid et al., Reference Schmid, Bernoulli, Fügenschuh, Matenco, Schefer, Schuster, Tischler and Ustaszewski2008). Westward to northwestward propagating Middle to early Late Jurassic compression led to the imbrication of the Austroalpine domain and its equivalents along the Neotethys suture and resulted in the Neotethyan orogeny (Missoni and Gawlick, Reference Missoni and Gawlick2011a). A characteristic feature of this orogenesis is the formation of deep-water radiolaritic basins in front of the westward propagating nappe stack. Sediment supply in these basins derived from the nappe fronts.
Gawlick et al. (Reference Gawlick, Frisch, Vecsei, Steiger and Böhm1999) interpreted this sedimentation pattern as a reflection of nappe movements in the Northern Calcareous Alps in the late Middle to early Late Jurassic and related it to the Kimmeric orogeny according to earlier authors (see ‘Jurassic gravitational tectonics’ in Plöchinger, Reference Plöchinger1974, Reference Plöchinger1976; Tollmann, Reference Tollmann1981, Reference Tollmann1985, Reference Tollmann1987; Mandl, Reference Mandl1982). This orogenic event (Lein, Reference Lein1985, Reference Lein1987a, Reference Leinb) was related to the closure of the western half of the Neotethys Ocean, today named the Neotethyan orogeny (Missoni and Gawlick, Reference Missoni and Gawlick2011a).
In the southern and therefore highest nappe group (Tirolic units and equivalents, Fig. 1.2–1.3) of the Northern Calcareous Alps the remains of this orogenic event are well preserved with a series of deep-water radiolaritic basins which were formed in sequence in front of the advancing nappe front (e.g., Gawlick et al., Reference Gawlick, Frisch, Vecsei, Steiger and Böhm1999, Missoni and Gawlick, Reference Missoni and Gawlick2011b). These southernmost radiolarite basins contain the Hallstatt Mélange as an erosional product of the Juvavic nappes, which are mainly eroded today. Subsequently the trench-like basins became overthrusted and incorporated into the accretionary prism (Missoni and Gawlick, Reference Missoni and Gawlick2011b). This Hallstatt Mélange was therefore formed in the late Early to early Late Jurassic interval as a result of successive shortening of the Triassic to Jurassic distal shelf area (Hallstatt Zone).
Jurassic evolution of the southern Northern Calcareous Alps
In the early Early Jurassic, sedimentation was generally controlled by the topography of the Late Triassic Hauptdolomit/Dachstein carbonate platform (Böhm, Reference Böhm2003, Gawlick and Frisch, Reference Gawlick and Frisch2003; Figs. 1, 2). On top of the Rhaetian shallow-water carbonates, red condensed limestones of the Adnet Group (?late Hettangian/Sinemurian to Toarcian: Böhm, Reference Böhm1992, Reference Böhm2003) were deposited, mostly separated by a gap of sedimentation (mainly early Hettangian, partly also late Hettangian; Fig. 2). On top of the Rhaetian Kössen Formation (e.g., Eiberg Basin, Restental Basin, Fig. 2) cherty and marly bedded limestones (Kendlbach Formation; Scheibelberg Formation: Böhm, Reference Böhm1992, Reference Böhm2003; Krainer and Mostler, Reference Krainer and Mostler1997; Ebli, Reference Ebli1997) were deposited, while in marginal areas of the basins crinoidal or sponge-spicule rich limestones of the Enzesfeld Formation were laid down (Böhm, Reference Böhm1992). In the late Pliensbachian to early Toarcian a horst-and-graben morphology developed (Bernoulli and Jenkyns, Reference Bernoulli and Jenkyns1974, Krainer et al., Reference Krainer, Mostler and Haditsch1994) and triggered breccia formation along submarine slopes and escarpments (Böhm et al., Reference Böhm, Dommergues and Meister1995). The Toarcian and most of the Middle Jurassic are characterized by starved sedimentation, ferro-manganese crusts, or a hiatus on the horsts, whereas the grabens were filled with deep-water carbonates and breccias, which latter formed near fault scarps. Neptunian dykes are found on the horsts. In the newly formed basinal areas gray bedded limestones of the younger Allgäu Formation were deposited, while condensed red limestones of the Klaus Formation formed on the top of the topographic highs (Krystyn, Reference Krystyn1971, Reference Krystyn1972; Fig. 2).
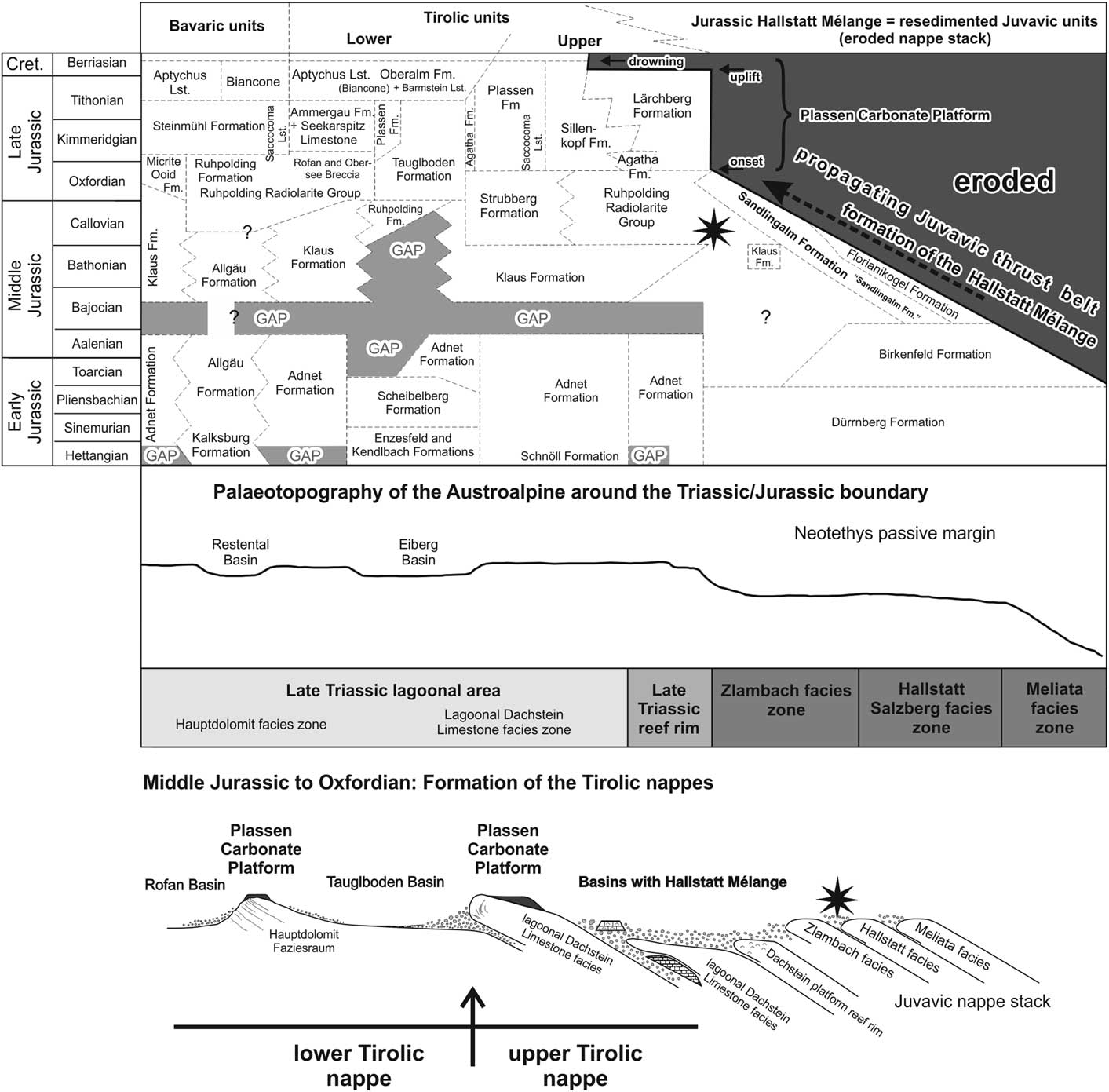
Figure 2 Stratigraphic table with lithostratigraphic names and main tectonic events of the Jurassic in the Austroalpine realm with their variations depending on the palaeogeographic position (after Gawlick et al., Reference Gawlick, Missoni, Schlagintweit, Suzuki, Frisch, Krystyn, Blau and Lein2009); star indicates investigated sequence. Note that this sequence is thrust further northward to its present position during younger shortening events. Bavaric units, Tirolic units, and the Hallstatt Mélange belong to the Northern Calcareous Alps.
This sedimentation pattern changed dramatically in the late Middle Jurassic (Gawlick and Frisch, Reference Gawlick and Frisch2003). Sedimentation resumed with the deposition of radiolarian cherts and radiolaria-rich marls, shales, and limestones of the Ruhpolding Radiolarite Group (Diersche, Reference Diersche1980; Gawlick and Frisch, Reference Gawlick and Frisch2003; Fig. 2).
In the Bajocian the sedimentary evolution in the southern (palaeogeographically southeastern, Fig. 1.4–1.5) part of the Tirolic realm as well as in the Hallstatt realm (Fig. 2) differed from that in the northern part (palaeogeographically in the northwestern, see Fig. 1.4–1.5). Deep-water trench-like basins formed in front of advancing nappes. The first basin group in the southern parts of the Northern Calcareous Alps received mass-flow deposits and large slides up to nappe size, which derived from the Hallstatt Zone (= Hallstatt Mélange; Gawlick and Frisch, Reference Gawlick and Frisch2003). The thickness of the basin fills may reach 2000 meters (Gawlick, Reference Gawlick1996, Reference Gawlick1997, Gawlick et al., Reference Gawlick, Schlagintweit and Suzuki2007b). The nappe stack carrying the Hallstatt Mélange is defined as the upper Tirolic nappe (group) (Frisch and Gawlick, Reference Frisch and Gawlick2003). The second basin group (Fig. 2), the Tauglboden and the Rofan trench-like basins in the north, was subjected to high subsidence and sedimentation rates in the Oxfordian to earliest Kimmeridgian (Schlager and Schlager, Reference Schlager and Schlager1973, Gawlick and Frisch, Reference Gawlick and Frisch2003). The nappe stack carrying the Tauglboden Mélange is defined as the lower Tirolic nappe (Frisch and Gawlick, Reference Frisch and Gawlick2003). These two basin groups are different: the huge mass flows of the Hallstatt Mélange trench-like basins formed earlier from material derived from the outer shelf facing the Neotethys Ocean (Hallstatt Zone, Fig. 1.2–1.5), whereas the Tauglboden Mélange trench-like basin formed later from material derived of the lagoonal part of the Hauptdolomit/Dachstein carbonate platform (Fig. 1.4, 1.5). However, both basins formed syntectonically and suggest a substantial relief between the basin axis and the source area. A third type of radiolarite basin, the Sillenkopf Basin (Missoni et al., Reference Missoni, Steiger and Gawlick2001), remained in the southern part of the Northern Calcareous Alps as a starved basin in the Kimmeridgian (Fig. 2). This basin contains the earliest ophiolitic detritus from the accreted and obducted Neotethys Ocean floor (Missoni, Reference Missoni2003).
In the Tirolic units of the Northern Calcareous Alps the establishment of the shallow-water Plassen Carbonate Platform started at the frontal parts of the rising and advancing nappes (Gawlick et al., Reference Gawlick, Frisch, Missoni and Suzuki2002, Reference Gawlick, Schlagintweit and Missoni2005). From this position, the progradation of several independent platforms took place towards the adjacent radiolarite basins (Gawlick and Frisch, Reference Gawlick and Frisch2003; Gawlick and Schlagintweit, Reference Gawlick and Schlagintweit2006; Gawlick et al., Reference Gawlick, Schlagintweit and Missoni2005, Reference Gawlick, Schlagintweit and Missoni2007a, Reference Gawlick, Missoni, Schlagintweit and Suzuki2012). This resulted in a complex basin-and-rise topography with different types of sediments in shallow-water and deep-water areas (Gawlick and Schlagintweit, Reference Gawlick and Schlagintweit2006). In the Kimmeridgian a huge carbonate platform was formed in the upper Tirolic unit, whereas in the lower Tirolic unit shallow-water carbonates were restricted to its northern part (Gawlick et al., Reference Gawlick, Schlagintweit and Missoni2007a). The whole Plassen Carbonate Platform cycle lasted from the Kimmeridgian until the late early Berriasian platform drowning (Gawlick and Schlagintweit, Reference Gawlick and Schlagintweit2006).
Description of the studied localities
All studied localities belong to the Hallstatt Mélange around the village of Obersdorf north of Bad Mitterndorf (Fig. 3). For a more detailed description of the geology of the area, the Late Triassic to Late Jurassic sedimentary succession, and radiolarian and conodont dating, see O’Dogherty and Gawlick (Reference O’Dogherty and Gawlick2008).
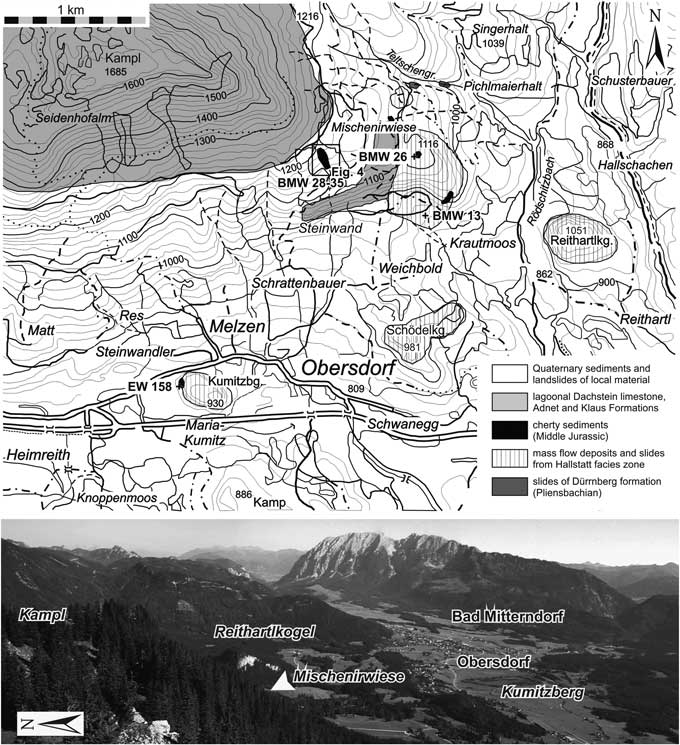
Figure 3 Topography and simplified geology of the study area, showing sample locations (after O’Dogherty and Gawlick, Reference O’Dogherty and Gawlick2008). The plus signs indicate positions of the investigated samples below the Kumitzberg, northwest of Krautmoos, and southeast of the Mischenirwiese and north of the Steinwand. Photo below the map shows the study area as viewed from Mount Kampl to the southwest. The hilly area with dense forest and grassland consists of Jurassic cherty sediments with incorporated mass flows and slides of Hallstatt Limestones. The contact between matrix and blocks or complete sections is visible only in areas with steeper slopes or valleys, or anthropogenic excavations.
Kumitzberg
The Late Triassic Hallstatt Limestone block of Mt. Kumitzberg is surrounded by a grassland area without outcropping sedimentary rocks (Fig. 3). Only small pieces of dark-gray to black radiolarites can be found at the western base of Mt. Kumitzberg. During the reconstruction of a small bus station in the excavation hole the contact between the Hallstatt Limestone block and the underlying dark-gray to black radiolarite was visible. The contact between the Hallstatt Limestone block and the radiolarite is erosive. This clearly indicates that the massive limestone block cut deep into the radiolarite succession. Therefore the age of the radiolarites is slightly older than the time of its emplacement. The unlaminated and massive dark-gray radiolarite beds are intercalated by thin layers of cherty shales. Only one sample (EW-158) was collected from this locality.
Steinwand north
A slightly folded, relative thick radiolarite succession is preserved in a valley between the Steinwand and the Mischenirwiese (Fig. 3), on the southeastern slope of Mount Kampl. This succession occurs on top of the Late Triassic (Rhaetian) lagoonal Dachstein Limestone of the Steinwand (Fig. 4). The overlying Early Jurassic interval is covered by Quaternary deposits, but in rare cases some relics of the red nodular limestones of the Adnet Formation occur in the grassland below the dark-gray bedded radiolarite succession. Lower to Middle Jurassic condensed red limestones are well preserved on the southeastern slope of Mount Kampl on top of the Norian/Rhaetian lagoonal Dachstein Limestone. This series represents the northeastern part of the syncline structure between the Steinwand and Mount Kampl.
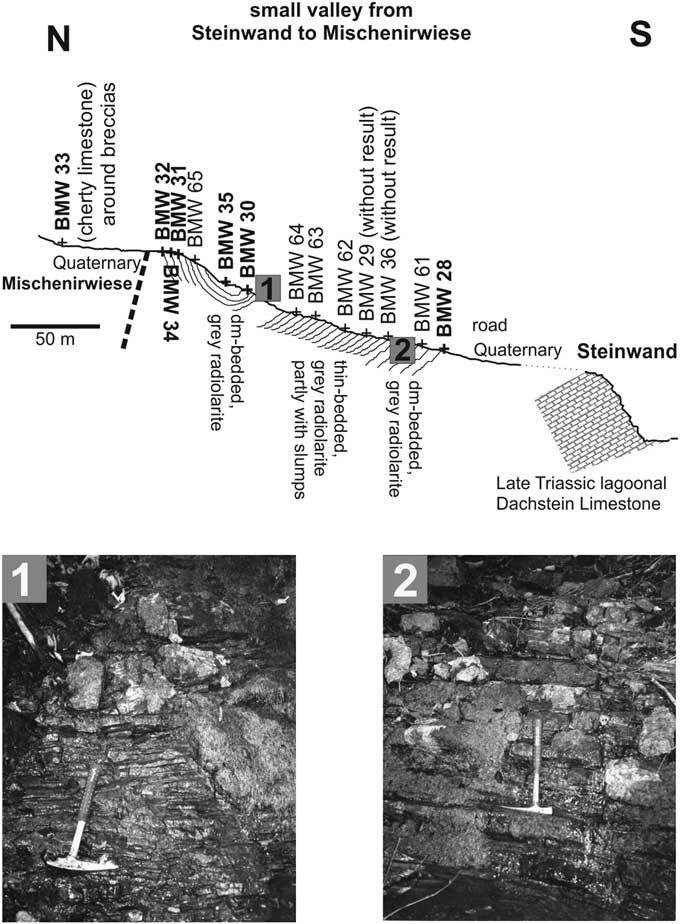
Figure 4 Cross-section in the small valley from Steinwand to Mischenirwiese, with the location of studied samples. Pictures 1 and 2 are details of the radiolaritic facies at lower (uppermost Bajocian–lower Bathonian) and upper (Oxfordian–?Kimmeridgian) part, respectively.
The lowermost part of the radiolarite succession (sample BMW-28, Fig. 4) outcrops near the entrance of the valley and yielded the oldest assemblage, whereas the youngest part is preserved in the core of the syncline (sample BMW-35, Fig. 4). The thickness of this black radiolarite succession is nearly 100 meters. Intercalated mass-flow deposits are missing in contrast to equivalent successions to the east. Radiolarian dating proves a continuous radiolarite deposition from Bathonian to the Oxfordian. At the end of the valley, near a spring, a small outcrop of gray bioturbated cherty limestones yielded the youngest radiolarians in this area (sample BMW-33). This clearly demonstrates that the radiolarite succession in the valley is separated from the area of the Mischenirwiese by a young fault.
Area between Krautmoos and Mischenirwiese
The area northwest of Krautmoos (Fig. 3) is characterized by a thick succession of mass-flow deposits with intercalated radiolarite matrix. In a few outcrops, below and between the amalgamated mass-flows matrix, radiolarians are well preserved, mainly in gray massive radiolarites. Two productive samples were taken, one from below the first mass flow (a massive dark-gray radiolarite, BMW-26) and another derived from the higher part of the mass-flow succession (sample BMW-13c).
The components of the different mass-flow deposits consist exclusively of different (gray and red) Hallstatt Limestone components, predominantly of Late Triassic age. Whereas in the lower part of the succession (near the top of the “Krautmoos hill”) the size of the components does not exceed a few decimeters (e.g., near the sample BMW-26), the component size increases upsection. The succession is finally topped by slide blocks of half a kilometer in size, also near Krautmoos. Interestingly in these higher mass-flow deposits radiolarite components occur in addition to the Hallstatt Limestone clasts.
Methods
Samples were selected by examination of thin sections for well-preserved radiolarians. Radiolarite samples were crushed to fragments <2–3 cm in size and placed in 1-liter plastic jars with concentrated hydrochloric acid (32%) until the reaction ceased and all the exposed calcium carbonate was dissolved. Then, the samples were rinsed and processed in the lab with diluted (4%) hydrofluoric acid for a period of 24 hours to extract radiolarians. The samples were wet-sieved through 200-μm and 63-μm sieves and the residues were rinsed and dried in an oven at 40°C. Eleven samples yielded diverse and relatively well-preserved assemblages (see Table 1 for species inventory), in part due to the low thermal overprint of these rocks (estimated by Conodont Color Alteration Index: CAI 1,0, see O’Dogherty and Gawlick, Reference O’Dogherty and Gawlick2008).
Table 1 Chart showing radiolarian taxa occurrences in studied samples; only absence or presence is noted. Solid circles indicate figured specimens; stars indicate unfigured specimens.

Generic and suprageneric systematics used in this work follows De Wever et al. (Reference De Wever, Dumitrica, Caulet and Caridroit2001) and O’Dogherty et al. (Reference O’Dogherty, Carter, Dumitrica, Goričan, De Wever, Bandini, Baumgartner and Matsuoka2009). Radiolarian taxonomy is based on the Middle Jurassic–Early Cretaceous catalogue of the InterRad Jurassic–Cretaceous Working Group (Baumgartner et al., Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and Steiger1995b) for 44 species. Age assignment is based on the Unitary Association Zones (UAZ) established by Baumgartner et al. (Reference Baumgartner, Bartolini, Carter, Conti, Cortese, Danelian, De Wever, Dumitrica, Dumitrica-Jud, Goričan, Guex, Hull, Kito, Marcucci, Matsuoka, Murchey, O’Dogherty, Savary, Vishnevskaya, Widz and Yao1995a), but in order to increase the resolution of this zonation a significant quantity of supplementary species (75 described species and 33 in open nomenclature) are introduced.
Systematic paleontology
Class Radiolaria Müller, Reference Müller1858
Subclass Polycystina Ehrenberg, Reference Ehrenberg1838
Order Nassellaria Ehrenberg, Reference Ehrenberg1876
Monocyrtids
Family Poulpidae De Wever, Reference De Wever1981
Genus Saitoum Pessagno, Reference Pessagno1977a
Type species
Saitoum pagei Pessagno, Reference Pessagno1977a.
Occurrence
Lower Pliensbachian to upper Barremian.
Saitoum pagei Pessagno, Reference Pessagno1977a
1977a Saitoum pagei Reference PessagnoPessagno, p. 98, pl. 12, figs. 11–14.
2003 Saitoum pagei; Reference Dumitrica and ZügelDumitrica and Zügel, p. 28, figs. 16A–B.
2003 Saitoum pagei; Reference Suzuki and GawlickSuzuki and Gawlick, p. 175, fig. 5.38.
2006 Saitoum pagei; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 458, pl. 11, figs. 6–8. [See for complete synonymy]
Saitoum trichylum De Wever, Reference De Wever1981
1981 Saitoum trichylum Reference De WeverDe Wever, p. 11, pl. 1, figs. 5–8.
1995b Saitoum trichylum; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 488, pl. 3021, figs. 1–6. [See for complete synonymy]
2002 Saitoum trichylum; Reference Beccaro, Baumgartner and MartireBeccaro et al., pl. 2, fig. 14.
2003 Saitoum trichylum; Reference Suzuki and GawlickSuzuki and Gawlick, p. 176, fig. 5.37.
‘Dicyrtids’
Family Gongylothoracidae Bak, Reference Bak1999
Genus Gongylothorax Foreman, Reference Foreman1968
Type species
Dicolocapsa verbeeki Tan, Reference Tan1927.
Occurrence
Upper Bajocian to upper Maastrichtian.
Gongylothorax favosus Dumitrica, Reference Dumitrica1970
1970 Gongylothorax favosus Reference DumitricaDumitrica, p. 56, pl. 1, figs. 1a–c, 2.
1995b Gongylothorax favosus; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 230, pl. 6131, figs. 1–7.
2003 Gongylothorax favosus; Reference Suzuki and GawlickSuzuki and Gawlick, p. 205, fig. 6.96. [See for complete synonymy]
2009 Gongylothorax favosus favosus; Reference Suzuki and GawlickSuzuki and Gawlick, p. 180, fig. 5.31A–C, 32A–B; fig. 6.21A–B. [See for complete synonymy]
Gongylothorax marmoris Kiessling in Kiessling and Zeiss, Reference Kiessling and Zeiss1992
1992 Gongylothorax (?) marmoris Reference KiesslingKiessling in Reference Kiessling and ZeissKiessling and Zeiss, p. 190, pl. 2, figs. 8−10.
2003 Gongylothorax cf. marmoris; Reference Suzuki and GawlickSuzuki and Gawlick, fig. 5.47.
Remarks
Originally this species was questionally attributed to Gongylothorax because the internal structure of the cephalis was not observed, but otherwise all characteristics match with the diagnosis of the genus.
Gongylothorax sp. A
Remarks
The ridges around the hexagonal areas are more elevated in relief than the pores commonly observed in Gongylothorax (see Gongylothorax sp. aff. G. favosus in Baumgartner et al., Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and Steiger1995b, p. 232=Gongylothorax favosus oviformis Suzuki and Gawlick Reference Suzuki and Gawlick2009, p. 180, fig. 5.33A–34C; fig. 6.22A–26B).
Genus Kilinora Hull, Reference Hull1997
Type species
Stylocapsa? spiralis Matsuoka, Reference Matsuoka1982.
Occurrence
Lower Bathonian to middle Callovian.
Kilinora? oblongula (Kocher in Baumgartner et al., Reference Baumgartner, De Wever and Kocher1980)
1980 Stylocapsa oblongula Reference KocherKocher in Reference Baumgartner, De Wever and KocherBaumgartner et al., p. 62, pl. 6, fig. 1.
2006 Kilinora (?) oblongula; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 443, pl. 8, figs. 25–29. [See for complete synonymy]
2007b Stylocapsa oblongula; Reference Gawlick, Schlagintweit and SuzukiGawlick et al., fig. 17.25.
2013 Kilinora (?) oblongula; Reference Chiari, Baumgartner, Bernoulli, Bortolotti, Marcucci, Photiades and PrincipiChiari et al., fig. 13c.
Remarks
According to O’Dogherty et al., Reference O’Dogherty, Bill, Goričan, Dumitrica and Masson2006, the genus is queried because this species does not exhibit a linear arrangement of pores, and clearly lacks costae. It may belong to a new genus.
Kilinora? sp. aff. K. oblongula (Kocher in Baumgartner et al., Reference Baumgartner, De Wever and Kocher1980)
Remarks
This form strongly resembles Kilinora (?) oblongula as illustrated by O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006 on pl. 8, figures 25−26, but possesses a somewhat pointed basal appendage.
‘Multicyrtids’
Family Diacanthocapsidae O’Dogherty, Reference O’Dogherty1994
Genus Theocapsomella O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006.
Type species
Theocapsomma cordis Kocher, Reference Kocher1981.
Remarks
This genus was misspelled as Theocapsommella in all descriptions of the species originally included under this genus (O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006). According to ICZN Art. 24.2.3, the selection of the correct original spelling should be Theocapsomella. This was the original name followed by the expression n. gen. (O’Dogherty et al., Reference O’Dogherty, Bill, Goričan, Dumitrica and Masson2006, p. 454) and was also fixed as the correct original spelling by the first reviser (O’Dogherty et al., Reference O’Dogherty, Carter, Dumitrica, Goričan, De Wever, Bandini, Baumgartner and Matsuoka2009).
In the original description (O’Dogherty et al., Reference O’Dogherty, Bill, Goričan, Dumitrica and Masson2006), Theocapsomella included also some four-segmented nassellarians (e.g., Stichocapsa himedaruma Aita). On the other hand, a phylogenetic relationship of Stichocapsa himedaruma Aita and Stichocapsa convexa Yao was also assumed (see O’Dogherty et al., Reference O’Dogherty, Bill, Goričan, Dumitrica and Masson2006, p. 441). New findings are in favor of the latter opinion (see Šegvić et al., Reference Šegvić, Kukoć, Dragičević, Vranjković, Brčić, Goričan, Babajić and Hrvatović2014, pl. 1, figs. 21, 22).
Occurrence
Lower Bathonian to lower Berriasian.
Theocapsomella cordis (Kocher in Baumgartner et al., Reference Baumgartner, De Wever and Kocher1980)
1980 Stylocapsa cordis Reference KocherKocher in Reference Baumgartner, De Wever and KocherBaumgartner et al., p. 62, pl. 6, fig. 1.
2006 Theocapsommella cordis; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 443, pl. 8, figs. 25–29. [See for complete synonymy]
2009 Theocapsomma cordis; Reference Suzuki and GawlickSuzuki and Gawlick, p. 181, figs. 5.37A–B, 6.28A–B.
2013 Theocapsomella cordis; Reference Chiari, Baumgartner, Bernoulli, Bortolotti, Marcucci, Photiades and PrincipiChiari et al., fig. 14e.
Theocapsomella medvednicensis (Goričan in Halamić et al., Reference Halamić, Goričan, Slovenec and Kolar-Jurkovsek1999)
1999 Theocapsomma medvednicensis Reference GoričanGoričan in Reference Halamić, Goričan, Slovenec and Kolar-JurkovsekHalamić et al., p. 37, pl. 1, figs. 12–16.
2003 Theocapsomma medvednicensis; Reference Suzuki and GawlickSuzuki and Gawlick, p. 206, fig. 6.87.
2006 Theocapsommella medvednicensis (Goričan); Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 456, pl. 8, figs. 30, 33–37. [See for complete synonymy]
2008 Theocapsomma sp. aff. T. medvednicensis; Reference Baumgartner, Flores, Bandini, Girault and CruzBaumgartner et al., pl. 4, fig. 10.
2009 Theocapsomma medvednicensis; Reference Suzuki and GawlickSuzuki and Gawlick, p. 181, figs. 5.38A–B.
Family Minocapsidae new family
Type genus
Minocapsa Matsuoka, Reference Matsuoka1991a.
Other genera
Crococapsa new genus; Doliocapsa new genus; Hemicryptocephalis Li, Reference Li1988; Hiscocapsa O’Dogherty, Reference O’Dogherty1994; Praewilliriedellum Kozur, Reference Kozur1984 (syn. Hemicryptocephalis); and Quarkus Pessagno, Blome, and Hull in Pessagno et al., Reference Pessagno, Blome, Hull and Six1993.
Diagnosis
Pyriform to ovoidal shell consisting in usually four segments. Last segment with or without an aperture. Lattice shell composed of small rounded to large polygonal pore frames.
Etymology
After type genus.
Occurrence
Early Pliensbachian to Middle Albian.
Remarks
This family is erected for genera included in the unnamed family pro Stichocapsidae used by O’Dogherty et al., Reference O’Dogherty, Carter, Dumitrica, Goričan, De Wever, Bandini, Baumgartner and Matsuoka2009. The genus Aitaum Pessagno and Hull, Reference Pessagno and Hull2002 is an exception because it has a multisegmented test composed of three to four post-abdominal segments. Aitaum has more affinity with the genus Lantus Yeh, Reference Yeh1987a and other eucyrtidiid-type nassellarians.
Genus Crococapsa new genus
Type species
Sethocapsa hexagona Hori, Reference Hori1999.
Other species
Cyrtocapsa asseni Tan, Reference Tan1927; Minocapsa aitai Hull, Reference Hull1997; Minocapsa truncata Wu, Reference Wu2000; Minocapsa? tansinhoki Hull, Reference Hull1997; Sethocapsa accincta Steiger, Reference Steiger1992; Sethocapsa horokanaiensis Kawabata, Reference Kawabata1988; Sethocapsa kitoi Jud, Reference Jud1994; Sethocapsa lagenaria Wu and Li, Reference Wu and Li.1982; Sethocapsa pseudouterculus Aita in Aita and Okada, Reference Aita and Okada1986; Sethocapsa? subcrassitestata Aita in Aita and Okada, Reference Aita and Okada1986; Sethocapsa? zweilii Jud, Reference Jud1994; Sethocapsa hashimotoi Tumanda, Reference Tumanda1989; Theocapsa simplex Tan, Reference Tan1927; and Theocapsa uterculus Parona, Reference Parona1890.
Diagnosis
Tetracyrtid nassellarian having a globose postabdominal segment. Lattice meshwork with polygonal pore frames lacking nodes or tubercles on its surface. Closed antapically or having a discrete number of small pores grouped into a depression (see Sethocapsa uterculus in Baumgartner et al., Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and Steiger1995b, p. 505, pl. 5462, fig. 2). Collar and lumbar strictures slightly recognizable or indistinct externally. Large and globose postabdominal segment is well distinguished from the abdomen by a marked stricture.
Etymology
From the latin croco (saffron) and capsa (box); feminine gender.
Occurrence
Bathonian to middle Albian.
Remarks
O’Dogherty (Reference O’Dogherty1994) erected the genus Hiscocapsa for a heterogeneous group of tetracyrtid nassellarians displaying a wide variety of pore frame arrangement. However, many species assigned originally to Hiscocapsa are clearly separated by lacking a distal aperture and bearing no tubercles on the surface.
Crococapsa n. gen. differs from Minocapsa Matsuoka, Reference Matsuoka1991a, by its distinct fourth segment, which is globose and well differentiated from the abdomen by a marked stricture. Crococapa n. gen. also differs from Lantus Yeh, Reference Yeh1987a by having only one postabdominal chamber.
Crococapsa tansinhoki (Hull, Reference Hull1997)
1997 Minocapsa(?) tansinhoki Reference HullHull, p. 148, pl. 38, figs. 4, 6.
2006 Minocapsa(?) tansinhoki; O’Dogherty et al., Reference O’Dogherty, Bill, Goričan, Dumitrica and Masson2006, p. 444, pl. 6, figs. 16, 17. [See for complete synonymy]
Remarks
O’Dogherty et al. (Reference O’Dogherty, Goričan and Dumitrica2006, p. 444) first questioned the placement of this species to Minocapsa because the basal aperture was absent and the phylogentic relationship with early Jurassic representatives was not demonstrated. This species fits well in the general description of the new genus Crococapsa.
Crococapsa sp. aff. C. tansinhoki (Hull, Reference Hull1997)
Remarks
These forms differ from Hull’s material by having smaller and more numerous pores on the abdomen.
Crococapsa sp. aff. C. truncata (Wu, Reference Wu2000)
aff. 2000 Minocapsa truncata Reference WuWu, p. 304, pl. 2, figs. 1, 5–6.
Remarks
This specimen differs from the Tibetan specimens by displaying a proportionally larger last segment.
Crococapsa sp. A
Remarks
These specimens resemble Crococapsa sp. aff. C. truncata (Wu, Reference Wu2000), but the proximal part of the shell is relatively longer and elongated.
Crococapsa sp. B
Remarks
This specimen is closely related to other congeneric forms included in this paper, but differs by the well-marked polygonal pore frames on almost all the shell surface.
Crococapsa sp. C
Remarks
These specimens are very close to Crococapsa tansinhoki but they display more cylindrical and smoother thorax.
Genus Doliocapsa new genus
Type species
Stichomitra (?) stecki O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006.
Other species
Hiscocapsa acuta Hull, Reference Hull1997; Hiscocapsa minuta Yeh, Reference Yeh2011; Hiscocapsa planata Wu, Reference Wu2000 (syn. Stichocapsa magnipora); Sethocapsa lugeoni O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006; Sethocapsa taukhaensis Kemkin and Taketani, Reference Kemkin and Taketani2004; Solenotryma keni Kocher, Reference Kocher1981; Stichocapsa magnipora Chiari, Marcucci, and Prela, Reference Chiari, Marcucci and Prela2002; Stichomitra doliolum Aita in Aita and Okada, Reference Aita and Okada1986; and ?Quarticella hunzikeri O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006.
Diagnosis
Shell with four segments (rarely more), generally thick-walled. Cephalis small, spherical, poreless, without apical horn. Other segments latticed with polygonal pore frames and lacking tubercles or nodes. Last segment somewhat inflated and having an aperture.
Etymology
From dolium (barrel) and capsa (box); feminine gender.
Occurrence
Lower Toarcian to Tithonian.
Remarks
This new genus is differentiated from Praewilliriedellum Kozur, Reference Kozur1984, by having very distinct strictures and well-differentiated segments.
Doliocapsa matsuokai (Yeh, Reference Yeh2009)
1998 Stichocapsa sp. F Reference ArakawaArakawa, p. 63, pl. 6, fig. 274.
2005 Stichocapsa sp. Reference Šmuc and GoričanŠmuc and Goričan, pl. 3, figs. 22a−b.
2009 Hiscocapsa matsuokai Reference YehYeh, p. 67, pl. 21, figs. 1, 8, 20, 22.
2011 Hiscocapsa matsuokai; Reference YehYeh, p. 16, pl. 7, figs. 10−13.
2011 Hiscocapsa cf. matsuokai; Reference YehYeh, p. 16, pl. 5−7.
Remarks
The proximal part in our specimens is slightly longer than in the type material. This species differs from all others representatives herein illustrated by having smaller and more numerous polygonal pores, less marked strictures at joints, and longer proximal conical part.
Doliocapsa keni (Kocher, Reference Kocher1981)
1981 Solenotryma keni Reference KocherKocher, p. 91, pl. 16, figs. 11−12.
2006 Stichomitra (?) keni (Kocher); Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 442, pl. 5, fig. 6.
Doliocapsa planata (Wu, Reference Wu2000)
2000 Hiscocapsa planata Reference WuWu, p. 303, pl. 1, figs 7–8.
2002 Stichocapsa magnipora Chiari, Marcucci and Prela, p. 76, pl. 3, figs. 13−17.
Genus Hiscocapsa O’Dogherty, Reference O’Dogherty1994
Type species
Cyrtocapsa grutterinki Tan, Reference Tan1927.
Other species
Sethocapsa aitai Chiari, Marcucci, and Prela, Reference Chiari, Marcucci and Prela2002; Sethocapsa kodrai Chiari, Marcucci, and Prela, Reference Chiari, Marcucci and Prela2002; Stichocapsa pseudornata Tan, Reference Tan1927; and Stichocapsa rutteni Tan, Reference Tan1927.
Emended diagnosis
Hiscocapsa is emended in order to include only tetracyrtid nassellarians having a globose postabdominal segment with tubercles. A large distal aperture is also presented and sometimes, when preserved, an appendage. We note that terminally closed species with tubercles (e.g., Sethocapsa kaminogoensis Aita in Aita and Okada, Reference Aita and Okada1986; Sethocapsa spp., pl. 5, fig. 15 in Aita and Okada, Reference Aita and Okada1986; Sethocapsa sp. 8, pl. 2, fig. 32 in Matsuoka, Reference Matsuoka1998) should belong to a new genus, which is not described herein.
Occurrence
Upper Bathonian to upper Aptian.
Hiscocapsa kodrai (Chiari, Marcucci, and Prela, Reference Chiari, Marcucci and Prela2002)
2002 Sethocapsa kodrai Reference Chiari, Marcucci and PrelaChiari, Marcucci and Prela, p. 74, pl. 3, figs. 1−7.
2003 Quarticella ovalis Takemura; Reference Suzuki and GawlickSuzuki and Gawlick, p. 199, fig. 5.40.
2005non Sethocapsa kodrai; Reference Nakae and KomuroNakae and Komuro, fig. 4.47.
2012 Hiscocapsa kodrai; Reference Djerić, Schmid and GerzinaDjeric et al., pl. 1, fig. 23.
Remarks
This species is very similar to Quarticella ovalis Takemura, but its aperture is much narrower. The larger postabdominal segment also displays well-differentiated tubercles instead of the typical spiny surface of Quarticella ovalis. Hiscocapsa kodrai might belong to Quarticella, but study of the internal structure is required before this new generic assignation is proposed. In the type material, a flattened base with a constricted aperture is clearly distinct (see Chiari et al., Reference Chiari, Marcucci and Prela2002, pl. 3, fig. 3). This flattened base is never present in representatives of Quarticella.
Genus Praewilliriedellum Kozur, Reference Kozur1984
Type species
Praewilliriedellum cephalospinosum Kozur, Reference Kozur1984.
Other species
Stichocapsa? pseudoconvexa Kemkin and Taketani, Reference Kemkin and Taketani2004; Stichocapsa convexa Yao, Reference Yao1979; Stichocapsa robusta Matsuoka, Reference Matsuoka1984; and Theocorys renzae Schaaf, Reference Schaaf1981.
Emended diagnosis
Praewilliriedellum is emended in order to allocate clear tetracyrtid forms (e.g., Stichocapsa robusta Matsuoka). Many representatives of this genus have an abdomen partially encased in the postabdominal segment, making it hard to differentiate externally. The degree of encasement is nevertheless much lower than in Williriedellum and other genera assigned to Williriedellidae.
Occurrence
Upper Aalenian to upper Barremian.
Praewilliriedellum convexum (Yao, Reference Yao1979)
1979 Stichocapsa convexa Reference YaoYao, p. 35, pl. 5, figs. 14–16; pl. 6, figs. 1–7.
2003 Stichocapsa convexa; Reference Suzuki and GawlickSuzuki and Gawlick, p. 212, fig. 6.51.
2006 Stichocapsa convexa; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 441, pl. 6, fig. 35. [See for complete synonymy]
2007b Stichocapsa convexa; Reference Gawlick, Schlagintweit and SuzukiGawlick et al., figs. 7.15, 8.32, 17.23, 18.10.
2008 Praewilliriedellum convexum; Reference Beccaro, Diserens, Goričan and MartireBeccaro et al., pl. 2, fig. 26.
2009 Stichocapsa convexa; Reference Suzuki and GawlickSuzuki and Gawlick, p. 186, figs. 5.54A–B.
2013 Praewilliriedellum convexum; Reference Chiari, Baumgartner, Bernoulli, Bortolotti, Marcucci, Photiades and PrincipiChiari et al., fig. 13n.
Praewilliriedellum robustum (Matsuoka, Reference Matsuoka1984)
1984 Stichocapsa robusta Reference MatsuokaMatsuoka, p. 146, pl. 1, figs. 6–13; pl. 2, figs. 7–12.
2003 Stichocapsa robusta; Reference Suzuki and GawlickSuzuki and Gawlick, p. 213, fig. 5.44.
2006 Stichocapsa robusta; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 441, pl. 6, figs. 31–34. [See for complete synonymy]
2007b Stichocapsa robusta; Reference Gawlick, Schlagintweit and SuzukiGawlick et al., fig. 8.34.
2008 Praewilliriedellum robustum; Reference Beccaro, Diserens, Goričan and MartireBeccaro et al., pl. 2, fig. 27.
2013 Praewilliriedellum robustum; Reference Chiari, Baumgartner, Bernoulli, Bortolotti, Marcucci, Photiades and PrincipiChiari et al., fig. 13p.
Genus Quarkus Pessagno, Blome, and Hull in Pessagno et al., Reference Pessagno, Blome, Hull and Six1993
Type species
Quarkus madstonensis Pessagno, Blome, and Hull in Pessagno et al., Reference Pessagno, Blome, Hull and Six1993.
Other species
Stichocapsa japonica Yao, Reference Yao1979.
Occurrence
Lower Bajocian to upper Callovian. A very similar specimen, but questionable to this genus, was found in upper Berriasian material of the Mariana Trench (Stichocapsa sp. 8 Matsuoka, Reference Matsuoka1998, pl. 2, fig. 24). We do not assign this species to Quarkus because continuous record from the Callovian to the Berriasian has not been demostrated yet.
Quarkus japonicus (Yao, Reference Yao1979)
1979 Stichocapsa japonica Reference YaoYao, p. 36, pl. 6, figs. 8−12; pl. 7, figs. 1−15.
2006 Stichocapsa japonica; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 441, pl. 6, fig. 6. [See for complete synonymy]
2009 Stichocapsa japonica; Reference Suzuki and GawlickSuzuki and Gawlick, p. 186, figs. 5.53.
2012 Praewilliriedellum japonicum; Reference Goričan, Pavšič and RožičGoričan et al., pl. 1, fig. 20.
2013 Praewilliriedellum japonicum; Reference Chiari, Baumgartner, Bernoulli, Bortolotti, Marcucci, Photiades and PrincipiChiari et al., fig. 13o.
Remarks
This species has always been assigned to the genus Stichocapsa (considered as a nomen dubium by O’Dogherty et al., Reference O’Dogherty, Carter, Dumitrica, Goričan, De Wever, Bandini, Baumgartner and Matsuoka2009, p. 330), however we note that this commonly illustrated species is very close to Quarkus madstonensis, the only difference being the less-expanded fourth segment. Judging from the stratigraphic distribution, Quarkus japonicus may be the ancestor of Quarkus madstonensis. An intermediate form (Stichocapsa sp. aff. S. japonica Yao) was illustrated by Chiari et al., Reference Chiari, Marcucci and Prela2002 (pl. 3, figs. 18−22) from the upper Bajocian−lower Bathonian of Albania.
Quarkus madstonensis Pessagno, Blome, and Hull in Pessagno et al., Reference Pessagno, Blome, Hull and Six1993
1993 Quarkus madstonensis Reference Pessagno, Blome and HullPessagno, Blome, and Hull in Reference Pessagno, Blome and HullPessagno et al., p. 159, pl. 8, figs. 9, 13–15, 24.
2006 Williriedellum madstonense; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 446, pl. 6, figs. 9–10.
2008 Williriedellum madstonense; Reference Danelian, Asatryan, Sosson, Person, Sahakyan and GaloyanDanelian et al., pl. 1, fig. 11.
Family Arcanicapsidae Takemura, Reference Takemura1986
Subfamily Arcanicapsinae Takemura, Reference Takemura1986
Included genera
Arcanicapsa Takemura, Reference Takemura1986; Fultacapsa Ožvoldová in Ožvoldová and Frantová, Reference Ožvoldová and Frantová1997; Religa Whalen and Carter, Reference Whalen and Carter2002; Squinabollum Dumitrica, Reference Dumitrica1970; Trisyringium Vinassa de Regny, Reference Vinassa de Regny1901; and the group of species assigned to Dorypyle Squinabol, Reference Squinabol1904. Dorypyle should be regarded as a nomen dubium that requires a new genus for taxonomic stability.
The genera Arcanicapsa Takemura, Reference Takemura1986 and Yamatoum Takemura, Reference Takemura1986 were questionably placed under this family in the recent revision by O’Dogherty et al., Reference O’Dogherty, Carter, Dumitrica, Goričan, De Wever, Bandini, Baumgartner and Matsuoka2009, but, as stated by Takemura Reference Takemura1986, the cephalic structure has more affinities with the genus Unuma Ichikawa and Yao, Reference Ichikawa and Yao1976. In this paper we follow the same opinion. In the same revision by O’Dogherty et al., Reference O’Dogherty, Carter, Dumitrica, Goričan, De Wever, Bandini, Baumgartner and Matsuoka2009, the genus Solidea Whalen and Carter in Carter et al., Reference Carter, Whalen and Guex1998 was erroneously placed in this subfamily, but its correct placement should be in Favosyringiinae.
Genus Arcanicapsa Takemura, Reference Takemura1986
Type species
Arcanicapsa sphaerica Takemura, Reference Takemura1986.
Other species
Sethocapsa aculeata Cortese, Reference Cortese1993; Sethocapsa congduensis Li and Wu, Reference Li and Wu1985; Sethocapsa echinata Li and Wu, Reference Li and Wu1985; Sethocapsa leiostraca Foreman, Reference Foreman1973b; Sethocapsa trachyostraca Foreman, Reference Foreman1973b; Sethocapsa funatoensis Aita, Reference Aita1987; and Zhamoidellum? exquisita Hull, Reference Hull1997.
Occurrence
Lower Toarcian to lower Aptian.
Remarks
Arcanicapsa as originally described has a stout apical horn (Takemura Reference Takemura1986, p. 54). In this paper we broaden the definition to include morphologically closely similar species without apical horn. Such tricyrtids, having a closed abdomen ornamented with spines or nodes, have been traditionally ascribed to Sethocapsa (e.g., Sethocapsa funatoensis Aita), which is considered nomen dubium (O’Dogherty et al., Reference O’Dogherty, Carter, Dumitrica, Goričan, De Wever, Bandini, Baumgartner and Matsuoka2009). Some species were questionably assigned to Zhamoidellum (e.g., Zhamoidellum? exquisita Hull). Here we assign to Zhamoidellum only species without tubercles (see discussion under Zhamoidellum). Species with a clear distinction between upward-directed spines proximally and downward-directed spines distally belong to Trisyringium Vinassa de Regny (see O’Dogherty, Reference O’Dogherty1994, p. 208; O’Dogherty et al., Reference O’Dogherty, Carter, Dumitrica, Goričan, De Wever, Bandini, Baumgartner and Matsuoka2009).
Arcanicapsa exquisita (Hull, Reference Hull1997)
1997 Zhamoidellum (?) exquisita Reference HullHull, p. 132, pl. 38, figs. 5, 16–17, 21.
2000 Zhamoidellum (?) exquisita; Reference WuWu, pl. 1, figs. 14–15.
2003 Zhamoidellum exquisita; Reference Suzuki and GawlickSuzuki and Gawlick, p. 204, fig. 6.55.
Arcanicapsa funatoensis (Aita, Reference Aita1987)
1987 Sethocapsa funatoensis Reference AitaAita, p. 73, pl. 2, figs. 6a–7b.
2005b Arcanicapsa funatoensis; Reference Nishihara and YaoNishihara and Yao, fig. 5.1.
2006 Zhamoidellum funatoense; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 445, pl. 10, fig. 32. [See for complete synonymy]
2009 non Zhamoidellum funatoense; Reference YehYeh, p. 68, pl. 21, figs. 10–11, 17, 25.
2011 Hiscocapsa funatoense; Reference YehYeh, p. 16, pl. 7, figs. 19–20, 23, 26.
Arcanicapsa sp. aff. A. funatoensis (Aita, Reference Aita1987)
Remarks
The specimens resemble A. funatoensis, but differ by having stronger spines and larger shell size.
Arcanicapsa undulata (Heitzer, Reference Heitzer1930)
1930 Lithobotrys undulata Reference HeitzerHeitzer, p. 390, pl. 28, fig. 22.
2003 Tricolocapsa undulata; Reference Suzuki and GawlickSuzuki and Gawlick, figs. 5.41, 6.39.
2006 Tricolocapsa undulata; Reference Auer, Gawlick and SuzukiAuer et al., fig. 6.44.
2007b Tricolocapsa undulata; Reference Gawlick, Schlagintweit and SuzukiGawlick et al., fig. 8.41.
2008 Tricolocapsa undulata; Reference Auer, Gawlick, Suzuki and SchlagintweiAuer et al., fig. 9.78.
2009 Tricolocapsa undulata; Reference Suzuki and GawlickSuzuki and Gawlick, p. 183, figs. 5.44A–B, 5.45A–B, 6.18A–B, 6.19A–B.
Remarks
Arcanicapsa undulata differs from A. funatoensis by having a smoother surface. Circular pores are present only on tubercles.
Arcanicapsa sp. A
Remarks
This specimen is similar to Arcanicapsa exquisita but possesses a smaller abdomen; the constriction between thorax and abdomen (lumbar stricture) is less pronounced.
Subfamily Favosyringiinae Steiger, Reference Steiger1992
Genus Spinosicapsa Ožvoldová, Reference Ožvoldová1975
Type species
Spinosicapsa ceblienica Ožvoldová, Reference Ožvoldová1975.
Occurrence
Upper Carnian to lower Aptian.
Spinosicapsa basilica (Hull, Reference Hull1997)
1997 Podobursa basilica Reference HullHull, p. 100, pl. 41, figs. 7–8, 10, 18, 20–21.
Spinosicapsa lata (Yang, Reference Yang1993)
1993 Syringocapsalata lata Reference YangYang, p. 132, pl. 24, figs. 1–2, 16, 20–21; pl. 26, figs. 7, 11, 15.
1997 Podobursa lata; Reference HullHull, p. 102, pl. 42, fig. 6.
2007b Syringocapsa lata; Reference Gawlick, Schlagintweit and SuzukiGawlick et al., fig. 19.37.
Spinosicapsa spinosa (Ožvoldová, Reference Ožvoldová1975)
1975 Heitzeria spinosa Reference OžvoldováOžvoldová, p. 78, pl. 101, fig. 2.
1995b Podobursa spinosa; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 426, pl. 3230, figs. 1–4. [See for complete synonymy]
Spinosicapsa sp. cf. S. triacantha (Fischli, Reference Fischli1916)
2006Podobursa triacantha; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 437, pl. 7, fig. 4. [See for complete synonymy]
Remarks
The assignment to S. triacantha is queried because the lateral spines are broken off in this specimen.
Family Eucyrtidiellidae Takemura, Reference Takemura1986
Genus Eucyrtidiellum Baumgartner, Reference Baumgartner1984
Type species
Eucyrtidium? unumaensis Yao, Reference Yao1979.
Occurrence
Lower Pliensbachian to upper Tithonian.
Eucyrtidiellum nodosum Wakita, Reference Wakita1988
1988 Eucyrtidiellum nodosum Reference WakitaWakita, p. 408, pl. 4, fig. 29; pl. 5, fig. 16.
2003 Eucyrtidiellum nodosum; Reference Suzuki and GawlickSuzuki and Gawlick, p. 217, figs. 5.20, 6.24–6.25, 6.29.
2006 Eucyrtidiellum nodosum; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 442, pl. 3, fig. 3. [See for complete synonymy]
2007b Eucyrtidiellum nodosum; Reference Gawlick, Schlagintweit and SuzukiGawlick et al., fig. 8.9; fig. 17.8.
Eucyrtidiellum ptyctum (Riedel and Sanfilippo, Reference Riedel and Sanfilippo1974)
1974 Eucyrtidium ptyctum Reference Riedel and SanfilippoRiedel and Sanfilippo, p. 778, pl. 5, fig. 7; pl. 12, fig. 14, not fig. 15.
2003 Eucyrtidiellum ptyctum; Reference Suzuki and GawlickSuzuki and Gawlick, p. 218, figs. 6.26–6.27.
2006 Eucyrtidiellum ptyctum; Reference Gawlik, Suzuki and SchlagintweitGawlick et al., figs. 8.10, 9.7.
2006 Eucyrtidiellum ptyctum; Reference Auer, Gawlick and SuzukiAuer et al., fig. 6.14.
2006 Eucyrtidiellum ptyctum; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 442, pl. 3, figs. 1–2. [See for complete synonymy]
2007b Eucyrtidiellum ptyctum; Reference Gawlick, Schlagintweit and SuzukiGawlick et al., fig. 7.3; fig. 19.18.
2009 Eucyrtidiellum ptyctum; Reference Suzuki and GawlickSuzuki and Gawlick, p. 188, fig. 5.63.
Eucyrtidiellum pustulatum Baumgartner, Reference Baumgartner1984
1984 Eucyrtidiellum pustulatum Reference BaumgartnerBaumgartner, p. 765, pl. 4, figs. 4–5.
2003 Eucyrtidiellum unumaense pustulatum; Reference Suzuki and GawlickSuzuki and Gawlick, p. 217, figs. 5.20, 6.24–6.25, 6.29. [See for complete synonymy]
2007b Eucyrtidiellum unumaense pustulatum; Reference Gawlick, Schlagintweit and SuzukiGawlick et al., figs. 8.13, 17.12.
Eucyrtidiellum pyramis (Aita in Aita and Okada, Reference Aita and Okada1986)
1986 Eucyrtidium (?) pyramis Reference AitaAita in Reference Aita and OkadaAita and Okada, p. 109, pl. 6, figs. 8–13; pl. 7, figs. 1a–b.
1995b Eucyrtidiellum pyramis; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 216, pl. 3019, figs. 1–2. [See for complete synonymy]
2010 Eucyrtidiellum pyramis; Reference Robin, Goričan, Guillocheau, Razin, Dromart and MosaffaRobin et al., fig. 4.14.
Eucyrtidiellum unumaense (Yao, Reference Yao1979)
1979 Eucyrtidium (?) unumaensis Reference YaoYao, p. 39, pl. 9, figs. 1–11.
2003 Eucyrtidiellum unumaense unumaense; Reference Suzuki and GawlickSuzuki and Gawlick, p. 216, fig. 6.28.
2006 Eucyrtidiellum unumaense; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 443, pl. 3, figs. 4–6. [See for complete synonymy]
2007b Eucyrtidiellum unumaense unumaense; Reference Gawlick, Schlagintweit and SuzukiGawlick et al., figs. 7.5, 17.11, 19.19.
2009 Eucyrtidiellum unumaense; Reference Suzuki and GawlickSuzuki and Gawlick, p. 188, fig. 5.62.
Superfamily Williriedelloidea Dumitrica, Reference Dumitrica1970
Family Williriedellidae Dumitrica, Reference Dumitrica1970
Genus Hemicryptocapsa Tan, Reference Tan1927
Type species
Hemicryptocapsa capita Tan, Reference Tan1927.
Other species
Hemicryptocapsa nonaginta new species; Praezhamoidellum yaoi Kozur, Reference Kozur1984; Praezhamoidellum buekkense Kozur, Reference Kozur1984; Tricolocapsa tetragona Matsuoka, Reference Matsuoka1983; Williriedellum? marcuccii Cortese, Reference Cortese1993; Williriedellum carpathicum Dumitrica, Reference Dumitrica1970.
Emended diagnosis
Cryptothoracic tricyrtids lacking nodose outer surface are included in this genus. The shell surface can be ornamented with regular pore frames as in the type species, or smooth as in Hemicryptocapsa buekkensis. Also see extended discussion under the genus Williriedellum.
Occurrence
Upper Tithonian to upper Aptian.
Hemicryptocapsa buekkensis (Kozur, Reference Kozur1984)
1984 Praezhamoidellum buekkenses Reference KozurKozur, p. 54, pl. 3, figs. 1a−b.
1998 Tricolocapsa? bukkense; Reference CordeyCordey, p. 128, pl. 27, fig. 9.
2006 Williriedellum buekkense; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 446, pl. 9, figs. 1−3
2008 Williriedellum buekkense; Reference Auer, Gawlick, Suzuki and SchlagintweiAuer et al., fig. 9.52.
2009 Praezhamoidellum buekkense; Reference Gawlick, Missoni, Schlagintweit, Suzuki, Frisch, Krystyn, Blau and LeinGawlick et al., figs. 5.50A–B.
2012 Hemicryptocapsa buekkensis; Reference Goričan, Pavšič and RožičGoričan et al., pl. 1, figs. 26, 34.
2012 Williriedellum buekkense; Reference Djerić, Schmid and GerzinaDjerić et al., pl. 3, fig. 2.
Hemicryptocapsa carpathica (Dumitrica, Reference Dumitrica1970)
1970 Williriedellum carpathicum Reference DumitricaDumitrica, p. 70, pl. 9, figs. 56a−b; 57−59; pl. 10, fig. 61.
1995b Williriedellum carpathicum; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p 626, pl. 4055, figs. 1−3.
2003 Williriedellum carpathicum; Reference Suzuki and GawlickSuzuki and Gawlick, p. 200, fig. 6.74. [See for complete synonymy]
2008 Williriedellum carpathicum; Reference Beccaro, Diserens, Goričan and MartireBeccaro et al., pl. 3, fig. 29.
2012 Hemicryptocapsa carpathica; Reference Goričan, Pavšič and RožičGoričan et al., pl. 2, fig. 20.
Hemicryptocapsa marcucciae (Cortese, Reference Cortese1993)
1993 Williriedellum (?) marcuccii Reference CorteseCortese, p. 180, pl. 7, figs. 6−7.
2006 Williriedellum marcucciae; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 446, pl. 9, figs. 28−36. [See for complete synonymy]
2008 Williriedellum marcucciae; Reference Auer, Gawlick, Suzuki and SchlagintweiAuer et al., fig. 11.46.
2008 Williriedellum (?) marcucciae; Reference Beccaro, Diserens, Goričan and MartireBeccaro et al., pl. 3, fig. 30.
2009 Williriedellum marcucciae; Reference Suzuki and GawlickSuzuki and Gawlick, fig. 5.25; fig. 6.28.
2010 Williriedellum marcucciae; Reference Robin, Goričan, Guillocheau, Razin, Dromart and MosaffaRobin et al., pl. 3, fig. 11.
2014 Hemicryptocapsa marcucciae; Reference Šegvić, Kukoć, Dragičević, Vranjković, Brčić, Goričan, Babajić and HrvatovićŠegvić et al., pl. 1, figs. 28A–B.
Remarks
Like other species in this paper, the absence of tubercles or nodes on the surface of this species justifies the transfer from Williriedellum to Hemicryptocapsa.
Hemicryptocapsa nonaginta new species
1993 Tricolocapsa(?) sp A. Reference Pessagno, Blome and HullPessagno et al., p. 160, pl. 8, fig. 27.
1995b Tricolocapsa sp. S Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 602, pl. 4057, figs. 1−3.
2004 Tricolocapsa sp. S; Reference Ziabrev, Aitchison, Abrajevitch, Badengzhu, Davis and LuoZiabrev et al., fig. 5.25.
2006 Tricolocapsa sp. S; Reference Auer, Gawlick and SuzukiAuer et al., fig. 6.45.
?2008 Tricolocapsa sp. S; Reference Auer, Gawlick, Suzuki and SchlagintweiAuer et al., fig. 9.79.
2008 Williriedellum sp. cf. W. sp. S Reference Baumgartner, Flores, Bandini, Girault and CruzBaumgartner et al., pl. 1, fig. 4.
2009 Tricolocapsa sp. S; Reference Kokubo and MatsuokaKokubo and Matsuoka, figs. 4.4−4.6.
2009non Tricolocapsa sp. S; Reference NishiharaNishihara, pl. 11, fig. 270.
?2009 Tricolocapsa aff. ruesti Tan; Reference NishiharaNishihara, pl. 11, fig. 268.
2009 Tricolocapsa sp. S; Reference Suzuki and GawlickSuzuki and Gawlick, p. 183, figs. 5.47A–B.
Holotype
Specimen MA7647 from sample MKS7A (illustrated in Baumgartner et al., Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and Steiger1995b pl. 4057, fig. 3) from Kashibara section (MA9), south of Hichiso town, Japan (see Matsuoka, Reference Matsuoka1995).
Diagnosis
Large cryptothoracic tricyrtid with very well defined lumbar stricture and a large abdomen. Outer surface without nodes and covered by hexagonal pore frames. Distal aperture constricted circular without rim.
Occurrence
Upper Bajocian to lower Bathonian.
Description
Cryptothoracic subspherical form composed of three distinct segments. Cephalis and thorax partially incased in the abdomen, with a very well defined lumbar stricture. The thorax is truncate-conical and covered by small pores whereas the abdomen is quite large and spherical. The entire surface of the abdomen is covered by a latticed meshwork of polygonal pore frames and a constricted aperture is visible.
Etymology
The name Nonaginta means ninety, in honor of the prodigious decade in contemporary history of radiolarian research.
Measurements
(in micrometers; µm) maximum width of shell 112−142, mean 118; maximum length 113−133, mean 123, based on four specimens.
Remarks
Hemicryptocapsa nonaginta n. sp. was published in open nomenclature in the Mesozoic radiolarian atlas (Baumgartner et al., Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and Steiger1995b) and since then it has been illustrated in many publications. This species differs from H. yaoi by having larger circular pores in the middle of polygonal areas. It differs from H. carpathica by having a simple circular aperture without a rim.
Hemicryptocapsa yaoi (Kozur, Reference Kozur1984)
1984 Praezhamoidellum yaoi Reference KozurKozur, p. 53, pl. 3, fig. 3a−b.
2003 Williriedellum dierschei Suzuki and Gawlick; Reference Suzuki and GawlickSuzuki and Gawlick, p. 201, fig. 6.73. [premature name]
2004 Williriedellum dierschei Reference Gawlick and SuzukiSuzuki and Gawlick in Reference Gawlick, Schlanginweit, Ebli and SuzukiGawlick et al., p. 311, pl. 4, figs. 1–6.
2006 Williriedellum dierschei; Reference Auer, Gawlick and SuzukiAuer et al., fig. 6.52.
2006 Williriedellum yaoi; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 446, fig. 9, figures 6−12. [See for complete synonymy]
2007b Williriedellum dierschei; Reference Gawlick, Schlagintweit and SuzukiGawlick et al., fig. 8.45, 17.32.
2009 Williriedellum dierschei; Reference Suzuki and GawlickSuzuki and Gawlick, p. 179, figs. 5.27A–B, 5.28, 6.48A–B.
2010 Williriedellum yaoi; Reference Robin, Goričan, Guillocheau, Razin, Dromart and MosaffaRobin et al., pl. 3, figs. 18, 20.
2012 Hemicryptocapsa yaoi; Reference Goričan, Pavšič and RožičGoričan et al., pl. 1, fig. 25; pl. 2, fig. 12.
Genus Williriedellum Dumitrica, Reference Dumitrica1970
Type species
Williriedellum crystallinum Dumitrica, Reference Dumitrica1970.
Included species
CryptamphoreIla crepida O’Dogherty, Reference O’Dogherty1994; Hemicryptocapsa polyhedra Dumitrica, Reference Dumitrica1970; Hemicryptocapsa prepolyhedra Dumitrica, Reference Dumitrica1970; Hemicryptocapsa tuberosa Dumitrica, Reference Dumitrica1970; Sethocapsa yahazuensis Aita, Reference Aita1987; Tricolocapsa clivosa Aliev, Reference Aliev1967; Tricolocapsa formosa Chiari, Marcucci, and Prela, Reference Chiari, Marcucci and Prela2002; Williriedellum? gilkeyi Dumitrica, Reference Dumitrica1970; Williriedellum crystallinum Dumitrica, Reference Dumitrica1970; Williriedellum nodosum Chiari, Marcucci, and Prela, Reference Chiari, Marcucci and Prela2002; Williriedellum peterschmittae Schaaf, Reference Schaaf1981; Williriedellum sujkowskii Widz and De Wever, Reference Widz and De Wever1993.
Emended diagnosis
The genus Williriedellum originally contained cryptothoracic tricyrtids with an aperture regardless of the external ornamentation of the shell. We restrict the name Williriedellum to species with raised ridges or nodes on the outer surface of the abdomen. Species with a regular distribution of circular pores (each pore may be bounded by a simple polygonal pore frame) are assigned to Hemicryptocapsa. The same differentiation between these two genera was applied by O’Dogherty et al. (Reference O’Dogherty, Carter, Dumitrica, Goričan, De Wever, Bandini, Baumgartner and Matsuoka2009). The concept of that publication, however, did not allow for written definitions and comments.
Occurrence
Upper Aalenian to lower Coniacian.
Williriedellum crystallinum Dumitrica, Reference Dumitrica1970
1970 Williriedellum crystallinum Reference DumitricaDumitrica, p. 69, pl. 10, figs. 60 a–c, 62–63.
2009 Williriedellum crystallinum; Reference Suzuki and GawlickSuzuki and Gawlick, p. 178, fig. 5.24. [See for complete synonymy]
Williriedellum sp. cf. W. formosum (Chiari, Marcucci, and Prela, Reference Chiari, Marcucci and Prela2002)
2002 Tricolocapsa formosa Reference Chiari, Marcucci and PrelaChiari, Marcucci, and Prela, p. 83, pl. 5, figs. 3−8.
2011 Williriedellum formosum; Reference Bandini, Baumgartner, Flores, Dumitrica, Hochard, Stampfli and JackettBandini et al., pl. 5, figs. 27–28.
2012 Williriedellum formosum; Reference Goričan, Pavšič and RožičGoričan et al., pl. 1, fig. 23.
Remarks
The external ornamentation of this species is very close to W. formosum but has a rounded aperture whereas in the type material the aperture is covered by a dish-like appendage. Moreover, some of our specimens also have groups of four small pores per frame area like occurring in W. gilkeyi.
Williriedellum yahazuense (Aita, Reference Aita1987)
1987 Sethocapsa yahazuense Reference AitaAita, p. 73, pl. 2, figs. 8a−9b; pl. 9, figs. 16−17.
1993 Williriedellum sujkowskii Reference Widz and De WeverWidz and De Wever, p. 88, pl. 1, figs. 7−10.
2005 Williriedellum yahazuense; Reference Šmuc and GoričanŠmuc and Goričan, p. 62, pl. 3, fig. 19.
Williriedellum sp. A
Remarks
These specimens show very small and pointed nodes on the outer surface of the abdomen.
Williriedellum sp. B
Remarks
This morphotype has very faintly developed ridges on the surface. These ridges do not build well-defined frame areas characteristic of W. crystallinum.
Williriedellum? sp. C
Remarks
The genus is queried because the presence of the aperture is not confirmed. The ridges on the surface of the illustrated specimens are only very faintly developed compared to other species of this genus.
Genus Zhamoidellum Dumitrica, Reference Dumitrica1970
1992 Complexapora Reference KiesslingKiessling in Kiessling and Zeiss.
Type species
Zhamoidellum ventricosum Dumitrica, Reference Dumitrica1970.
Included species
Complexapora kozuri Hull, Reference Hull1997; Complexapora tirolica Kiessling in Kiessling and Zeiss Reference Kiessling and Zeiss1992; Zhamoidellum argandi O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006; Zhamoidellum boehmi Kiessling, Reference Kiessling1999; Zhamoidellum calamin O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006; Zhamoidellum mikamense Aita, Reference Aita1986; Zhamoidellum ovum Dumitrica, Reference Dumitrica1970; Zhamoidellum ventricosum Dumitrica, Reference Dumitrica1970; Zhamoidellum yehae Dumitrica in Goričan et al., Reference Goričan, Carter, Dumitrica, Whalen, Hori, De Wever, O’Dogherty, Matsuoka and Guex2006.
Remarks
As stated by O’Dogherty et al., Reference O’Dogherty, Bill, Goričan, Dumitrica and Masson2006, the presence or absence of a sutural pore in Zhamoidellum can be regarded as character related to the intraspecific variability. Only species without tubercles are included. Species with spines and nodes are placed in Arcanicapsa.
Occurrence
Lower Pliensbachian? to upper Tithonian.
Zhamoidellum ovum Dumitrica, Reference Dumitrica1970
1970 Zhamoidellum ovum Reference DumitricaDumitrica, p. 79 pl. 9, figs. 52a–b, 53–54.
2009 Zhamoidellum ovum; Reference Suzuki and GawlickSuzuki and Gawlick, p. 179, fig. 5.30A–B, 6.33A–B. [See for complete synonymy]
Zhamoidellum sp. aff. Z. ovum Dumitrica, Reference Dumitrica1970
Remarks
These specimens are considered only affinis because they display less-pronounced constrictions and more elongated outline of the shell.
Zhamoidellum ventricosum Dumitrica, Reference Dumitrica1970
1970 Zhamoidellum ventricosum Reference DumitricaDumitrica, p. 79, pl. 9, figs. 55a–b.
2003 Zhamoidellum ventricosum; Reference Suzuki and GawlickSuzuki and Gawlick, p. 205, fig. 6.96.
2006 Zhamoidellum ventricosum; Reference Auer, Gawlick and SuzukiAuer et al., fig. 6.57.
2006 Zhamoidellum ventricosum; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 445, pl. 9, figs. 13–25. [See for complete synonymy]
2009 Zhamoidellum ventricosum; Reference Suzuki and GawlickSuzuki and Gawlick, p. 179, fig. 5.29.
Zhamoidellum sp. A
Remarks
This morphotype differs from other Zhamoidellum by its smaller size. The cephalis and thorax are imperforate, but the abdomen is covered by small circular pores set in polygonal pore frames. The collar and lumbar strictures are well marked on these specimens.
Zhamoidellum sp. B
Remarks
This morphotype differs from Z. ovum by having a more deeply encased thorax. In addition, the collar stricture is very distinct and the thorax is not porous. The illustrated specimens display enough characters to be considered a new species. However, pictures showing the basal aperture are not available and for this reason this morphotype is not described as a new taxon in this paper.
Zhamoidellum sp. C
Remarks
These specimens are very close to Zhamoidellum sp. B, but they differ by having a porous thorax and a less-marked collar stricture. The pores are more widely open and the surrounding ridges are thinner than in Zhamoidellum sp. B.
Family Japonocapsidae Kozur, Reference Kozur1984
Genus Striatojaponocapsa Kozur, Reference Kozur1984
Type species
Tricolocapsa plicarum Yao, Reference Yao1979.
Occurrence
Lower Bajocian to upper Callovian.
Striatojaponocapsa conexa (Matsuoka, Reference Matsuoka1983)
1983 Tricolocapsa conexa Reference MatsuokaMatsuoka, p. 20, pl. 3, figs. 3–7; pl. 7, figs. 11–14.
2003 Tricolocapsa conexa; Reference Suzuki and GawlickSuzuki and Gawlick, p. 208, figs. 5.42, 6.43–45.
2006 Striatojaponocapsa conexa; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 447, pl. 10, figs. 18–20. [See for complete synonymy]
2007 Striatojaponocapsa conexa; Reference Hatakeda, Suzuki and MatsuokaHatakeda et al., pl. 2, figs. 1–10.
2007 Striatojaponocapsa conexa; Suzuki and Gawlick, p. 182, figs. 5.40, 6.32A–B.
Striatojaponocapsa riri O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006
2006 Striatojaponocapsa riri Reference O’Dogherty, Goričan and DumitricaO’Dogherty, Goričan, and Dumitrica in Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 447, pl. 8, figs. 14–15.
2007 Striatojaponocapsa riri; Reference Hatakeda, Suzuki and MatsuokaHatakeda et al., pl. 2, figs. 11–20.
Striatojaponocapsa synconexa O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006
2006 Striatojaponocapsa synconexa Reference O’Dogherty, Goričan and DumitricaO’Dogherty, Goričan, and Dumitrica in Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 447, pl. 10, figs. 9–17. [See for complete synonymy]
2007 Striatojaponocapsa synconexa; Reference Hatakeda, Suzuki and MatsuokaHatakeda et al., pl. 1, figs. 11–20.
2008 Striatojaponocapsa synconexa; Reference Baumgartner, Flores, Bandini, Girault and CruzBaumgartner et al., pl. 4, fig. 18.
2012 Striatojaponocapsa synconexa; Reference Goričan, Pavšič and RožičGoričan et al., pl. 1, figs. 15, 35.
2013 Striatojaponocapsa synconexa; Reference Chiari, Baumgartner, Bernoulli, Bortolotti, Marcucci, Photiades and PrincipiChiari et al., p. 416, fig. 14c.
Striatojaponocapsa spp.
Remarks
Various elongated forms of Striatojaponcapsa have been found in our samples. The small number of specimens does not allow the description of new species. They differ from other Striatojaponocapsa by the elongated outline and small size.
Striatojaponocapsa? spp.
Remarks
The genus is queried because the distal part and the classical appendage on these forms are not preserved, but the surface ornamentation fits well with the general pore patterns of Striatojaponocapsa.
Genus Japonocapsa Kozur, Reference Kozur1984
Type species
Tricolocapsa? fusiformis Yao, Reference Yao1979.
Remarks
This form is close to Japonocapsa fusiformis (Yao, Reference Yao1979), but the basal dish-like appendage seems broken off in this specimen.
Family Unumidae Kozur, Reference Kozur1984
Included genera.—
Guttacapsa O’Dogherty, Reference O’Dogherty1994; Helvetocapsa O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006; Protunuma Ichikawa and Yao, Reference Ichikawa and Yao1976; Quarticella Takemura, Reference Takemura1986; Spinunuma Ichikawa and Yao, Reference Ichikawa and Yao1976 (syn. Unuma); Turbocapsula O’Dogherty, Reference O’Dogherty1994; Unuma Ichikawa and Yao, Reference Ichikawa and Yao1976; and Yamatoum Takemura, Reference Takemura1986.
Remarks.—
According to De Wever et al., Reference De Wever, Dumitrica, Caulet and Caridroit2001 (p. 265) and O’Dogherty et al., Reference O’Dogherty, De Wever, Goričan, Carter and Dumitrica2011 (p. 112) this family was placed under the superfamily Archaeodictyomitroidea. However, the family Unumidae as stated by Takemura, Reference Takemura1986 (p. 36) does not share the same cephalic structure. In this paper, we prefer to consider this family as more closely related to the Japonocapsidae. We also tentatively reassign the genera Quarticella and Yamatoum under this family because the cephalic structure (Yamatoum-type) is the same as in Unuma. Genus Protunuma Ichikawa and Yao, Reference Ichikawa and Yao1976
Type species
Protunuma fusiformis Ichikawa and Yao, Reference Ichikawa and Yao1976
Occurrence
Middle Toarcian to upper Tithonian.
Protunuma europeus O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006
2006 Protunuma europeus Reference O’Dogherty, Goričan and DumitricaO’Dogherty, Goričan, and Dumitrica in Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 433, pl. 10, figs. 6–8.
Protunuma japonicus Matsuoka and Yao, Reference Matsuoka and Yao1985
1985 Protunuma japonicus Reference Matsuoka and YaoMatsuoka and Yao, p. 130, pl. 1, figs. 11–15; pl. 3, figs. 6–9.
1995b Protunuma japonicus; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 434, pl. 3292, fig. 1–8.
2003 Protunuma multicostatus (Heitzer); Reference Suzuki and GawlickSuzuki and Gawlick, p. 197, fig. 5.43. [See for complete synonymy]
Protunuma ochiensis Matsuoka, Reference Matsuoka1983
1983 Protunuma (?) ochiensis Reference MatsuokaMatsuoka p. 26, pl. 4, figs. 8–11; pl. 9, figs. 3–7.
2003 Protunuma ochiensis; Reference Suzuki and GawlickSuzuki and Gawlick, p. 197, fig. 6.90.
2006 Protunuma ochiensis; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 433, pl. 7, figs. 11–13. [See for complete synonymy]
Protunuma turbo Matsuoka, Reference Matsuoka1983
1983 Protunuma turbo Reference MatsuokaMatsuoka, p. 24, pl. 4, figs. 4–7; pl. 8, figs. 16–18; pl. 9, figs. 1–2.
1995b Protunuma turbo; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 436, pl. 4034, figs. 1–3. [See for complete synonymy]
2008 Protunuma turbo; Reference Baumgartner, Flores, Bandini, Girault and CruzBaumgartner et al., pl. 4, figs. 2–3.
2012 Protunuma turbo; Reference Goričan, Pavšič and RožičGoričan et al., pl. 1, fig. 28.
Genus Unuma Ichikawa and Yao, Reference Ichikawa and Yao1976
1976 Spinunuma Ichikawa and Yao.
Type species
Unuma (Unuma) typicus Ichikawa and Yao, Reference Ichikawa and Yao1976.
Occurrence
Lower Toarcian to upper Bathonian.
Unuma gordus Hull, Reference Hull1997
1997 Unuma gorda Reference HullHull, p. 172, pl. 43, figs. 9, 11–12.
2003 Unuma gorda; Reference Suzuki and GawlickSuzuki and Gawlick, p. 98; figs. 5.36, 6.68.
2006 Unuma gordus; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 434, pl. 7, figs. 15–18. [See for complete synonymy]
2007 Unuma gorda; Gawlick et al., fig. 7.21; fig. 8.44; figs. 17.30, 18.13.
2009 Unuma gordus; Reference Suzuki and GawlickSuzuki and Gawlick, p. 177, figs. 6.2A–B.
2013 Unuma gordus; Reference Chiari, Baumgartner, Bernoulli, Bortolotti, Marcucci, Photiades and PrincipiChiari et al., fig. 14n.
Unuma latusicostatus (Aita, Reference Aita1987)
1987 Tricolocapsa latusicostata Reference AitaAita, p. 76, pl. 4, figs. 7a–8b; pl. 10, figs. 8–9.
1995b Unuma latusicostatus; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 622, pl. 4058, figs. 1–4.
2012 Unuma latusicostatus; Reference Goričan, Pavšič and RožičGoričan et al., pl. 1, fig. 30.
Genus Quarticella Takemura, Reference Takemura1986
2009 Minutusolla Reference YehYeh, p. 72.
Type species
Quarticella ovalis Takemura, Reference Takemura1986
Other species
Minutusolla yaoi Yeh, Reference Yeh2009; Quarticella dura Takemura, Reference Takemura1986; Stichocapsa cicciona Chiari, Marcucci, and Prela, Reference Chiari, Marcucci and Prela2002.
Emended diagnosis
We consider under this genus only those species possessing a latticed fourth inflated segment with somewhat spiny surface, but never with long and stout spines.
Occurrence
Upper Aalenian−upper Bathonian.
Quarticella cicciona (Chiari, Marcucci, and Prela, Reference Chiari, Marcucci and Prela2002)
2002 Stichocapsa cicciona Reference Chiari, Marcucci and PrelaChiari, Marcucci, and Prela, p. 76, pl. 3, figs. 8−12.
2006 Stichocapsa cicciona; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 441, pl. 6, fig. 36.
Quarticella ovalis Takemura, Reference Takemura1986
1986 Quarticella ovalis Reference TakemuraTakemura, p. 58, pl. 8, figs. 17−21.
1995b Quarticella ovalis; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 466, pl. 4078, figs. 1−3.
2003 Quarticella ovalis; Reference Suzuki and GawlickSuzuki and Gawlick, p. 199, fig. 5.40.
2005a Quarticella ovalis; Reference Nishihara and YaoNishihara and Yao, fig. 2.24.
2008 Quarticella ovalis; Reference Auer, Gawlick, Suzuki and SchlagintweiAuer et al., fig. 9.57.
2009 Quarticella ovalis; Reference Suzuki and GawlickSuzuki and Gawlick, figs. 5.23 A–B.
Quarticella sp. A
Remarks
This specimen differs from Q. ovalis by having a smooth surface and more regular arrangement of pores. Circular pores are surrounded by polygonal pore frames. Very small pyramidal spines occur at vertices.
Quarticella sp. B
?1985 Stichocapsa sp. B Reference Yamamoto, Mizutani and KagamiYamamoto et al., pl. 7, fig. 6.
Remarks
These specimens differ from Q. ovalis by having meshwork of larger and equally sized pores. Distal segment has a wide aperture with characteristic flat rim.
Quarticella sp. C
1997 Sethocapsa? sp. D Reference YaoYao, fig. 542.
1982 Stichocapsa (?) sp. α Reference Mizutani and KoikeMizutani and Koike, pl. 2, figs. 1, 2a–b.
1982 Stichocapsa (?) sp. A Reference WakitaWakita, pl. 3, fig. 8.
Remarks
This species is very similar to Quarticella sp. D, but bears a row of tubercles in the equatorial part of the inflated postabdominal segment.
Quarticella sp. D
Remarks
This species differs from Q. ovalis by having a smooth surface without spines and regular arrangement of circular pores.
Genus Yamatoum Takemura, Reference Takemura1986
Type species
Yamatoum elegans Takemura, Reference Takemura1986.
Other species
Quarticella conica Takemura, Reference Takemura1986; Quarticella levis Takemura, Reference Takemura1986; Quarticella? quinaria Takemura, Reference Takemura1986; Quarticella spinosa Takemura, Reference Takemura1986; Yamatoum atlanticum O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006; Yamatoum caudatum Takemura, Reference Takemura1986; Yamatoum connicinum Takemura, Reference Takemura1986; Yamatoum komamiensis Takemura, Reference Takemura1986; Yamatoum spinosum Takemura, Reference Takemura1986.
Emended diagnosis
Yamatoum is emended in order to include spindle-shaped forms with a large abdomen armed with numerous (three or more) radiate equatorial spines and, when preserved, a strong (frequently tapered to branched) terminal spines. The last inverted conical segment may be missing in many specimens due to its delicate nature.
Occurrence
Upper Aalenian to upper Bathonian.
Yamatoum spinosum (Takemura, Reference Takemura1986)
1986 Quarticella spinosa Reference TakemuraTakemura, p. 59, pl. 9, figs. 1−6.
Remarks
This species has been transferred into Yamatoum because it bears strong lateral spines on its abdomen. Our specimens have shorter spines than the type-material.
Yamatoum sp. A
Remarks
This species differs from Y. spinosum by having a narrower final segment and shorter spines.
Superfamily Archaeodictyomitroidea Pessagno, Reference Pessagno1976
Family Hsuuidae Pessagno and Whalen, Reference Pessagno and Whalen1982
Genus Hsuum Pessagno, Reference Pessagno1977a
1992 Ogivus Reference El KadiriEl Kadiri.
Type species
Hsuum cuestaense Pessagno, Reference Pessagno1977a.
Occurrence
Lower Pliensbachian to lower Cenomanian.
Hsuum arabicum Dumitrica in Dumitrica et al., Reference Dumitrica, Immenhauser and Dumitrica-Jud1997
1997 Hsuum arabicum Reference DumitricaDumitrica in Reference Dumitrica, Immenhauser and Dumitrica-JudDumitrica et al., p. 46, pl. 10, figs. 2–3.
1999 Hsuum arabicum; Reference HoriHori, p. 82, fig. 7.14.
Hsuum obispoense Pessagno, Reference Pessagno1977a
1977a Hsuum obispoensis Reference PessagnoPessagno, p. 82, pl. 8, figs. 3–4.
1977b Hsuum obispoensis; Reference PessagnoPessagno, p. 44, pl. 6, fig. 7.
Genus Transhsuum Takemura, Reference Takemura1986
Type species
Transhsuum medium Takemura, Reference Takemura1986
Occurrence
Lower Pliensbachian to upper Kimmeridgian.
Transhsuum brevicostatum (Ožvoldová, Reference Ožvoldová1975)
1975 Lithostrobus brevicostatus Reference OžvoldováOžvoldová, p. 84, pl. 102, fig. 1.
1995b Transhsuum brevicostatus gr.; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 578, pl. 3181, figs. 1–5.
2009 Hsuum brevicostatus gr.; Reference Suzuki and GawlickSuzuki and Gawlick, p. 168, fig. 5.6. [See for complete synonymy]
Transhsuum maxwelli (Pessagno, Reference Pessagno1977a)
1977a Hsuum maxwelli Reference PessagnoPessagno, p. 81, pl. 7, figs. 14−16.
2006 Transhsuum maxwelli gr.; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 433, pl. 2, figs. 14, 18−23, 25. [See for complete synonymy]
2009 Hsuum maxwelli; Reference Suzuki and GawlickSuzuki and Gawlick, p. 168, fig. 5.7. [See for complete synonymy]
Transhsuum sp. E sensu (Yao, Reference Yao1997)
1997 Hsuum sp. E sensu Reference YaoYao, pl. 15, fig. 197.
Remarks
The main characteristic of this morphotype is its short skeleton consisting of only five segments.
Genus Parahsuum Yao, Reference Yao1982
Type species
Parahsuum simplum Yao, Reference Yao1982.
Occurrence
Lower Hettangian to upper Kimmeridgian.
Parahsuum carpathicum Widz and De Wever, Reference Widz and De Wever1993
1993 Parahsuum carpathicum Reference Widz and De WeverWidz and De Wever, p. 85, pl. 1, figs. 14–16.
1995b Parahsuum sp. S Baumgartner et al., p. 384, pl. 3240, figs. 1–5.
1999 Parahsuum officerense (Pessagno and Whalen); Reference HoriHori, p. 82 fig. 7.15.
2003 Parahsuum carpathicum; Reference Suzuki and GawlickSuzuki and Gawlick, p. 182, fig. 5.30.
2006 Parahsuum carpathicum; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 432, pl. 2, figs. 6–13. [See for complete synonymy]
2009 Parahsuum sp. S; Reference Suzuki and GawlickSuzuki and Gawlick, p. 167, fig. 5.5.
Parahsuum sp. aff. P. probosum (Pessagno and Whalen, Reference Pessagno and Whalen1982)
1982 Droltus (?) probosus Reference Pessagno and WhalenPessagno and Whalen, p. 122, pl. 6, figs. 12, 16−17; pl. 12, fig. 12.
Remarks
This species differs from the typical P. probosum by having a longer shell with broadened last segment.
Parahsuum snowshoense (Pessagno and Whalen, Reference Pessagno and Whalen1982)
1982 Lupherium snowshoense Reference Pessagno and WhalenPessagno and Whalen, p. 136, pl. 6, figs. 6, 20; pl. 12, fig. 6.
2006 Parahsuum snowshoense; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 432, pl. 1, figs. 1−3.
2007b Parahsuum snowshoense; Reference Gawlick, Schlagintweit and SuzukiGawlick et al., fig. 18.6.
Remarks
We also include forms with an apical horn (e.g., Takemura, Reference Takemura1986, pl. 5, fig. 16) in this species.
Parahsuum sp. aff. P. snowshoense (Pessagno and Whalen, Reference Pessagno and Whalen1982)
Remarks
This species differs from P. snowshoense by having less-pronounced strictures and partly thickened costae.
Parahsuum sp. 1 sensu O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006
Remarks
Our specimen is closely related to P. snowshoense (Pessagno and Whalen), but differs by having a slender shell not constricted distally. Segments are trapezoidal in outline and possess four longitudinal rows of pores.
Parahsuum sp. 2 sensu O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006
Remarks
This species shows an irregular arrangement of costae over most of the shell. The linear arrangement of costae, characteristic of Parahsuum, is only visible in the distalmost part.
Parahsuum sp. 3 sensu O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006
Remarks
Shell conical proximally, cylindrical distally. Costae sharp, high in relief, faintly disappearing proximally.
Genus Semihsuum Pessagno, Blome, and Hull in Pessagno et al., Reference Pessagno, Blome, Hull and Six1993
Type species
Hsuum? inexploratum Blome, Reference Blome1984.
Occurrence
Lower Bajocian to upper Callovian.
Semihsuum amabile (Aita, Reference Aita1987)
1985 Archaeodictyomitra (?) amabilis Reference AitaAita, fig. 6.6.
2006 Hsuum (?) amabile; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 431, pl. 1, figs. 21−22. [See for complete synonymy]
2009 Archaeodictyomitra amabilis; Reference Suzuki and GawlickSuzuki and Gawlick, p. 169, figs. 5.10, 6.20 A–B.
2012 Semihsuum amabile; Reference Djerić, Schmid and GerzinaDjeric et al., pl. 1, fig. 28.
2013 Archaeodictyomitra (?) amabilis; Reference Chiari, Baumgartner, Bernoulli, Bortolotti, Marcucci, Photiades and PrincipiChiari et al., Fig. 12b.
Family Archaeodictyomitridae Pessagno, Reference Pessagno1976
Genus Belleza Hull, Reference Hull1997
Type species
Stichocapsa decora Rüst, Reference Rüst1885.
Occurrence
Upper Bajocian to upper Callovian.
Belleza decora (Rüst, Reference Rüst1885)
1885 Stichocapsa decora Reference RüstRüst, p. 319, pl. 17, fig. 3.
1990 Sethocapsa (?) lineaplena Reference Yang and WangYang and Wang, p. 210, pl. 4, figs. 7?, 12–13.
1995bStichocapsa decora; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 520, pl. 3269, figs. 1−2, not figs. 3−4.
1997 Belleza decora (Rüst); Reference HullHull, p. 142, pl. 47, figs. 5−6, 14, 22−23
2006 Mictyoditra decora; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 431, pl. 1, fig. 23 [See for complete synonymy]
Genus Archaeodictyomitra Pessagno, Reference Pessagno1976
Type species
Archaeodictyomitra squinaboli Pessagno, Reference Pessagno1976.
Occurrence
Lower Pliensbachian to upper Campanian.
Archaeodictyomitra sp. cf. A. annulata Kozur and Mostler in Grill and Kozur, Reference Grill and Kozur1986
1986 Archaeodictyomitra annulata Reference Kozur and MostlerKozur and Mostler in Reference Grill and KozurGrill and Kozur, p. 257, pl. 10, fig. 2.
Remarks
As stated by the original authors, this species is very distinct by its hoop-like second-last segment.
Archaeodictyomitra minoensis (Mizutani, Reference Mizutani1981)
1981 Pseudodictyomitra minoensis Reference MizutaniMizutani, p. 178, pl. 58, fig. 4; pl. 63, figs. 9–10.
1995b Archaeodictyomitra minoensis; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 104, pl. 3305, figs. 1–5. [See for complete synonymy]
1999 Archaeodictyomitra minoensis; Reference HoriHori, p. 81, fig. 7.12.
2008 Archaeodictyomitra minoensis; Reference Auer, Gawlick, Suzuki and SchlagintweiAuer et al., fig. 9.4.
Archaeodictyomitra praeapiarium Cordey, Reference Cordey1998
1982 Archaeodictyomitra apiara (Rüst); Reference Nishizono, Ohishi, Sato and MurataNishizono et al., pl. 3, fig. 4.
1998 Archaeodictyomitra praeapiarium Reference CordeyCordey, p. 99, pl. 28, figs. 9−10.
Remarks
According to Cordey Reference Cordey1998, this species differs from A. apiarium by less-pronounced constrictions and continuous costae, also in the most proximal part.
Archaeodictyomitra prisca Kozur and Mostler in Grill and Kozur, Reference Grill and Kozur1986
1986 Archaeodictyomitra prisca Reference Kozur and MostlerKozur and Mostler in Reference Grill and KozurGrill and Kozur, p. 258, pl. 8, figs. 3−6; pl. 9, fig. 1.
2006 Archaeodictyomitra prisca; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 430, pl. 1, figs. 4−5. [See for complete synonymy]
Archaeodictyomitra publica (Hull, Reference Hull1997)
1997 Combusta (?) publica Reference HullHull, p. 84, pl. 34, figs 3, 13, 20−21.
2006 Combusta (?) publica; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 431, pl. 1, fig. 26. [See for complete synonymy]
2011 Parahsuum publicum; Reference YehYeh, p. 11, pl. 2, fig. 18.
Archaeodictyomitra rigida Pessagno, Reference Pessagno1977a
1977a Archaeodictyomitra rigida Reference PessagnoPessagno, p. 81, pl. 7, figs. 10–11.
2003 Archaeodictyomitra rigida; Reference Suzuki and GawlickSuzuki and Gawlick, p. 179, fig. 5.18; fig. 6.20.
2008 Archaeodictyomitra rigida; Reference Auer, Gawlick, Suzuki and SchlagintweiAuer et al., fig. 9.7.
2009 Archaeodictyomitra rigida; Reference Suzuki and GawlickSuzuki and Gawlick, p. 168, fig. 5.9. [See for complete synonymy]
Archaeodictyomitra spelae Chiari, Cortese, and Marcucci in Chiari et al., Reference Chiari, Cortese and Marcucci1997
1997 Archaeodictyomitra spelae Reference Chiari, Cortese and MarcucciChiari, Cortese, and Marcucci in Reference Chiari, Cortese, Marcucci and NozzoliChiari et al., p. 64, pl. 1, figs. 13−15.
2006 Archaeodictyomitra spelae; Reference Danelian, Lahsini and De RafélisDanelian et al., pl. 1, fig. 11.
Remarks
This species is distinguished from other Middle Jurassic Archaeodictyomitra species by its long slender shell and the marked strictures between adjacent segments.
Archaeodictyomitra tyaughtonensis Cordey, Reference Cordey1998
Archaeodictyomitra tyaughtonensis Cordey, Reference Cordey1998, p. 98, pl. 28, figs. 5−6.
Remarks
According to Cordey (Reference Cordey1998) this species is characterized by the well-visible segmentation and the fusiform shape.
Archaeodictyomitra sp. aff. A. exigua Blome, Reference Blome1984
aff. 1984 Archaeodictyomitra exigua Blome, p. 356, pl. 8, figs. 4, 7–8, 10, 12–13; pl. 15, figs. 14–15.
Remarks
This species resembles Archaeodictyomitra exigua in its conical shape, but differs by lacking the constricted final segment.
Archaeodictyomitra whalenae Kozur and Mostler in Grill and Kozur, Reference Grill and Kozur1986
1986 Archaeodictyomitra whalenae Reference Kozur and MostlerKozur and Mostler in Reference Grill and KozurGrill and Kozur, p. 260, pl. 9, figs. 2–5.
2006 Archaeodictyomitra whalenae; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 430, pl. 1, figs. 8–9. [See for complete synonymy]
Genus Thanarla Pessagno, Reference Pessagno1977b
Type species
Phormocyrtis veneta Squinabol, Reference Squinabol1903.
Occurrence
Lower Bathonian to upper Cenomanian.
Thanarla patricki gr. (Kocher, Reference Kocher1981)
1981 Archaeodictyomitra patricki Reference KocherKocher, p. 57, pl. 12, figs. 14–17.
2003 Archaeodictyomitra patricki; Reference Suzuki and GawlickSuzuki and Gawlick, p. 178, fig. 5.19.
2006 Archaeodictyomitra patricki; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 430, pl. 1, figs. 15–17. [See for complete synonymy]
2007b Archaeodictyomitra patricki; Reference Gawlick, Schlagintweit and SuzukiGawlick et al., fig. 19.13.
2013 Archaeodictyomitra sp. cf. A. patricki; Reference Chiari, Baumgartner, Bernoulli, Bortolotti, Marcucci, Photiades and PrincipiChiari et al., Fig. 12c.
Superfamily Amphipyndacoidea Riedel, Reference Riedel1967
Family Amphipyndacidae Riedel, Reference Riedel1967
Genus Eoxitus Kozur, Reference Kozur1985
Type species
Eoxitus hungaricus Kozur, Reference Kozur1985.
Other species
Eoxitus baloghi Kozur, Reference Kozur1985 (syn. Eoxitus nodosus); ?Eoxitus brevis Kozur, Reference Kozur1985; Eoxitus elongatus Kozur, Reference Kozur1985; Eoxitus nodosus Kozur, Reference Kozur1985; Parvicingula dhimenaensis Baumgartner, Reference Baumgartner1984, Triversus kasinzovae Vishnevskaya, Reference Vishnevskaya1991; ?Triversus strobilatus Vishnevskaya, Reference Vishnevskaya1991;?Triversus triquetrum Vishnevskaya, Reference Vishnevskaya1991.
Occurrence
The stratigraphic occurrence of Eoxitus should be considered as lower Bajocian to Tithonian (see discussion above). The last representative of Eoxitus is E. dhimenaensis, which goes extinct in the Tithonian.
Remarks
Eoxitus is very similar to its allied Tethysetta, but is much more elongated and does not have the characteristic broad spindle shape frequently observed throughout the Cretaceous forms assigned in Tethysetta. By definition, Tethysetta species have well-developed circumferential ridges and do not have spines on tubercles, as is the case of older species of Eoxitus.
The stratigraphic range of both genera in O’Dogherty et al. (Reference O’Dogherty, Carter, Dumitrica, Goričan, De Wever, Bandini, Baumgartner and Matsuoka2009) was lower Bajocian−lower Aptian. These two genera are clearly distinguishable both morphologically and stratigraphically. At that time, we included the only species bearing faint spines in the Cretaceous—Parvicingula usotanensis Tumanda, Reference Tumanda1989. Nonetheless, we currently believe that this species should be assigned to Tethysetta because it displays the characteristic circumferential ridges, although it also possesses somewhat spiny tubercles. This morphotype is stratigraphically disconnected from other Jurassic spiny parvicingulids.
The genus Tethysetta includes at least the following species: Dictyomitra boesii Parona, Reference Parona1890; Lithocampe fasciata Rüst, Reference Rüst1898; Parvicingula mashitaensis Mizutani, Reference Mizutani1981; Parvicingula usotanensis Tumanda, Reference Tumanda1989; Tethysetta cingulifera Dumitrica in Dumitrica et al., Reference Dumitrica, Immenhauser and Dumitrica-Jud1997; Tethysetta hullae Dumitrica in Dumitrica et al., Reference Dumitrica, Immenhauser and Dumitrica-Jud1997; Tethysetta ovoidala Dumitrica in Dumitrica et al., Reference Dumitrica, Immenhauser and Dumitrica-Jud1997; Tethysetta pygmaea Dumitrica in Dumitrica et al., Reference Dumitrica, Immenhauser and Dumitrica-Jud1997.
Eoxitus baloghi Kozur, Reference Kozur1985
1985 Eoxitus baloghi Reference KozurKozur, p. 216, fig. 2c.
1985 Eoxitus nodosus Reference KozurKozur, p. 218, figs. 2a−b, not fig. 2d.
1997 Parvicingula dhimenaensis ssp. A Baumgartner et al.; Reference ArakawaArakawa, pl. 4, fig. 8, not fig. 4 (= E. hungaricus).
1987 Parvicingula sp. B Reference AitaAita, p. 66, pl. 5, figs. 9a−10b; pl. 11, fig. 1.
1987 Parvicingula sp. C Reference AitaAita, p. 66, pl. 5, figs. 12a−13b?; pl. 11, fig. 2.
1989 Triversus spinifer Reference Hattori and SakamotoHattori and Sakamoto, pl. 12, fig. D.
2006 Tethysetta baloghi; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 436, pl. 3, figs. 20−21.
Eoxitus? brevis Kozur, Reference Kozur1985
1985 Eoxitus brevis Reference KozurKozur, p. 217, figs. 2e−f.
Remarks
This species is questionably assigned to Eoxitus because it has more than three rows of pores per segment.
Eoxitus dhimenaensis (Baumgartner, Reference Baumgartner1984)
1976 Amphipyndax sp. Reference Baumgartner and BernoulliBaumgartner and Bernoulli, p. 611, figs. 12 e, i, m.
1981 Parvicingula boesii (Parona); Reference De Wever and CabyDe Wever and Caby, pl. 2, fig. C.
1981Parvicingula boesii; Reference KocherKocher, p. 81, pl. 15, fig. 11 only.
1982 Parvicingula sp. C Reference AitaAita, pl. 1, figs. 13, 14.
1982 Amphipyndax ? sp. Reference Nishizono, Ohishi, Sato and MurataNishizono et al., pl. 3, fig. 16.
1984 Parvicingula dhimenaensis Reference BaumgartnerBaumgartner, p. 778, pl. 7, figs. 2−3, not fig. 4.
1985 Parvicingula dhimenaensis; Reference De Wever and Miconnet.De Wever and Miconnet, p. 389, pl. 4, figs. 4, 6−8.
1985 Parvicingula dhimenaensis; Reference Yamamoto, Mizutani and KagamiYamamoto et al., p. 36, pl. 6, fig. 1.
1986 Parvicingula dhimenaensis; Reference ContiConti, pl. 1, fig. 1.
1986 Parvicingula dhimenaensis; Reference Kishida and HisadaKishida and Hisada, fig. 2.4; fig. 8.2.
1986b Parvicingula dhimenaensis; Reference MatsuokaMatsuoka, pl. 2, fig. 12.
1987 Parvicingula dhimenaensis; Reference AitaAita, p. 66, ?pl. 2, figs. 3a−b, 5a−b; pl. 9, figs. 12−13.
1987 Parvicingula dhimenaensis; Reference GoričanGoričan, p. 185, pl. 3, figs. 13−14.
1988 Parvicingula dhimenaensis; Reference WakitaWakita, pl. 4, fig. 10; pl. 5, fig. 7.
1991b Parvicingula dhimenaensis; Reference MatsuokaMatsuoka, pl. 1, fig. 7.
1995bParvicingula dhimenaensis dhimenaensis; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 406, pl. 4072, only fig. 1.
1998 Parvicingula dhimenaensis dhimenaensis; Reference ArakawaArakawa, pl. 9, fig. 416.
1998 Parvicingula ? sp. B0 Reference ArakawaArakawa, pl. 9, fig. 448.
2009 Parvicingula dhimenaensis; Reference Suzuki and GawlickSuzuki and Gawlick, p. 169, figs. 6.9A–B.
2012 Eoxitus dhimenaensis; Reference Goričan, Pavšič and RožičGoričan et al., pl. 1, figs. 12–13.
Eoxitus hungaricus Kozur, Reference Kozur1985
1982 Amphipyndax (?) sp. A Reference Kido, Kawaguchi, Adachi and Mizutani.Kido et al., pl. 4, figs. 1−2.
1982 Parvicingula sp. J Reference Kishida and SuganoKishida and Sugano, pl. 12, figs. 6−7.
1982 Parvicingula (?) sp. Reference Imoto, Tamaki, Tanabe and IshigaImoto et al., pl. 3, 6−7.
1985 Eoxitus elongatus Reference KozurKozur, p. 217, fig. 1h.
1985 Eoxitus hungaricus Reference KozurKozur, p. 216, figs. 1a–b, d–e.
1985 Parvicingula spinosa Reference AitaAita, figs. 6.12−6.13.
1992 Eoxitus hungaricus; Reference OžvoldováOžvoldová, pl. 1, fig. 14; pl. 4, fig. 8.
1994 Parvicingula dhimenaensis Baumgartner; Reference GoričanGoričan, p. 80, pl. 24, figs. 12−13.
1995 Parvicingula dhimenaensis; Reference Takahashi and IshiiTakahashi and Ishii, pl. 1, fig. 27.
1995bParvicingula dhimenaensis ssp. A Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 406, pl. 4071, only fig. 1−3.
1997 Parvicingula dhimenaensis; Reference ArakawaArakawa, pl. 4, fig. 4, not fig. 8 (= E. baloghi).
1998 Parvicingula dhimenaensis; Reference ArakawaArakawa, pl. 9, fig. 417.
2003 Triversus hungaricus; Reference Suzuki and GawlickSuzuki and Gawlick, p. 195, fig. 6.58–6.60.
2009 Triversus hungaricus; Reference Suzuki and GawlickSuzuki and Gawlick, p. 170, fig. 5.14; figs. 6.6A–B, 6.7–6.8.
Eoxitus? sp. A
1982 Parvicingula decora Reference Pessagno and WhalenPessagno and Whalen; Aubrecht and Ožvoldová, Reference Aubrecht and Ožvoldová1994, pl. 4, fig. 1.
1995b Parvicingula(?) spinata; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 412, pl. 3187, fig. 1 only, fig. 2?
1998 Parvicingula sp. E0 Reference ArakawaArakawa, pl. 10, fig. 430.
1998 Parvicingula sp. E1 Reference ArakawaArakawa, pl. 10, fig. 431.
Remarks
The apical horn and pointed nodes enable differentiation from Praeparvicingula Pessagno, Blome, and Hull in Pessagno et al., Reference Pessagno, Blome, Hull and Six1993.
Eoxitus? sp. B
Remarks
This morphotype is easily distinguished from other species of Eoxitus by is enormous apical horn.
Family Canoptidae Pessagno in Pessagno et al., Reference Pessagno, Finch and Abbott1979
Type species
Canoptum poissoni Pessagno in Pessagno et al., Reference Pessagno, Finch and Abbott1979.
Occurrence
Ladinian to upper Bajocian.
Genus Canoptum Pessagno, Reference Pessagno, Finch and Abbott1979
Remarks
Includes generic concepts of Paracanoptum Yeh, Reference Yeh1987b and Pseudocanoptum Suzuki, Reference Suzuki1997.
Canoptum krahsteinense (Suzuki and Gawlick in Gawlick et al., Reference Gawlick, Schlanginweit, Ebli and Suzuki2004)
1985 Canoptum sp. Reference Yamamoto, Mizutani and KagamiYamamoto et al., p. 34, pl. 3, fig. 10.
2003 Spongocapsula krahsteinensis Reference Suzuki and GawlickSuzuki and Gawlick, p. 189, fig. 6.95. (Premature name)
2004 Spongocapsula krahsteinensis Reference Gawlick and SuzukiSuzuki and Gawlick in Gawlick et al., p. 313, figs. 4.7−4.10.
2007b Spongocapsula krahsteinensis; Reference Gawlick, Schlagintweit and SuzukiGawlick et al., fig. 7.14.
2009 Spongocapsula krahsteinensis; Reference IshidaIshida, figs. 10.18, 10.35.
2011 Spongocapsula cf. krahsteinensis; Reference IshidaIshida, fig. 6.23.
2014 Canoptum krahsteinense; Reference Šegvić, Kukoć, Dragičević, Vranjković, Brčić, Goričan, Babajić and HrvatovićŠegvić et al., pl. 1, figs. 8−9.
Genus Cinguloturris Dumitrica in Dumitrica and Mello, Reference Dumitrica and Mello1982
Type species
Cinguloturris carpatica Dumitrica in Dumitrica and Mello, Reference Dumitrica and Mello1982.
Occurrence
Upper Bathonian to lower Valanginian.
Cinguloturris carpatica Dumitrica in Dumitrica and Mello, Reference Dumitrica and Mello1982
1982 Cinguloturris carpatica Reference DumitricaDumitrica in Reference Dumitrica and MelloDumitrica and Mello, p. 23, pl. 4, figs. 7−11.
2006 Cinguloturris carpatica; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 435, pl. 3, figs. 8−9. [See for complete synonymy]
2009 Cinguloturris carpatica; Reference Suzuki and GawlickSuzuki and Gawlick, p. 167, figs. 5.2, 6.1A–B.
Cinguloturris getsensis O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006
2006 Cinguloturris getsensis Reference O’Dogherty, Goričan and DumitricaO’Dogherty, Goričan, and Dumitrica in Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 435, pl. 3, figs. 10−12. [See for complete synonymy]
Cinguloturris latiannulata (Grill and Kozur, Reference Grill and Kozur1986)
1986 Canoptum latiannulatum Reference Grill and KozurGrill and Kozur, p. 250, pl. 7, figs. 4−5.
Remarks
This species is closely related morphologically to C. carpatica, but differs by having no pores in the intersegmental depressions at strictures.
Family Obeliscoitidae O’Dogherty, Reference O’Dogherty1994
Genus Olanda Hull, Reference Hull1997
Type species
Olanda olorina Hull, Reference Hull1997.
Remarks
The single specimen found in sample BMW-28 from the lower Bathonian is broken and cannot be determined at species level, but the proximal part is in good agreement with the genus Olanda.
Family Parvicingulidae Pessagno, Reference Pessagno1977a
Genus Caneta Pessagno, Blome, and Hull in Pessagno et al., Reference Pessagno, Blome, Hull and Six1993
Type species
Parvicingula hsui Pessagno, Reference Pessagno1977a.
Occurrence
Lower Kimmeridgian to upper Tithonian.
Caneta hsui (Pessagno, Reference Pessagno1977a)
1977a Ristola hsui Reference PessagnoPessagno, p. 85, pl. 8, figs. 15−16; pl. 9, figs. 1−5.
1984 Ristola hsui; Reference Pessagno, Blome and LongoriaPessagno et al., p. 29, pl. 4, figs. 2−3.
1993 Parvicingula sp. cf. hsui; Reference YangYang, p. 119, pl. 19, figs. 4−5, 14, 17, 21.
1995 Caneta hsui; Hull, p. 16, pl. 1, figs. 6, 10, 18, 22; pl. 6, fig. 10.
1996 Caneta hsui; Reference Kiessling and ScassoKiessling and Scasso, pl. 1, fig. 17.
1999 Caneta hsui s.l.; Reference KiesslingKiessling, p. 48, pl. 10, figs. 12−13.
2003 Wrangellium hsuei; Reference Suzuki and GawlickSuzuki and Gawlick, p. 194, fig. 6.99.
2007b Wrangellium hsuei; Reference Gawlick, Schlagintweit and SuzukiGawlick et al., fig. 8.48.
Remarks
Our specimen does not have a constricted last segment as illustrated in the holotype.
Genus Takemuraella new name
1986 Triversus Reference TakemuraTakemura, preoccupied name (non Triversus Sher Reference Sher1973, Nematoidea).
Type species
Triversus japonicus Takemura, Reference Takemura1986.
Other species
Praeparvicingula tlellensis Carter in Goričan et al., Reference Goričan, Carter, Dumitrica, Whalen, Hori, De Wever, O’Dogherty, Matsuoka and Guex2006; Triversus fastigatus Hull, Reference Hull1997; Triversus schardti O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006; Triversus spinifer Takemura, Reference Takemura1986; Triversus preconicus Vishnevskaya, Reference Vishnevskaya1991.
Diagnosis
Multicyrtid having three rows of circular pores by segment, which are externally weakly defined by ridges or strictures, and usually without apical horn or occasionally a very faint apical horn is present (e.g., Triversus fastigatus Hull Reference Hull1997, pl. 51, fig. 5).
Occurrence
Upper Pliensbachian to upper Bathonian.
Etymology
In honor of our friend and colleague Prof. Atsushi Takemura, for his great contribution of the taxonomy and stratigraphy of Paleozoic and Mesozoic radiolarians.
Remarks
Takemura (Reference Takemura1986) emphasized the Amphipyndax-type cephalic skeletal structure of this genus as a distinction from Parvicingula. In addition, we note that Takemuraella shares with other parvicingulids the three rows of pores per segment. but it lacks the typical circumferential ridges always visible in Praeparvicingula and Parvicingula.
Takemuraella japonica (Takemura, Reference Takemura1986)
1986 Triversus japonicus Reference TakemuraTakemura, p. 62, pl. 16−20.
notTriversus japonicus; Reference Hattori and SakamotoHattori and Sakamoto, pl. 12, 1989 figs. B−C.
2006 Triversus japonicus; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., 434, pl. 4, fig. 3.
2013 Triversus japonicus; Reference Chiari, Baumgartner, Bernoulli, Bortolotti, Marcucci, Photiades and PrincipiChiari et al., p. 418, fig. 14k.
Takemuraella schardti (O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006)
1997 Ristola (?) turpicula Pessagno and Whalen; Reference ArakawaArakawa, pl. 4, fig. 18.
2006 Stichocapsa tuscanica Chiari, Cortese, and Marcucci; Reference Danelian, Lahsini and De RafélisDanelian et al., pl. 2, fig. 8.
2006 Triversus schardti Reference O’Dogherty, Goričan and DumitricaO’Dogherty, Goričan, and Dumitrica in Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 434, pl. 4, figs. 4−10. [See for complete synonymy]
2008 Parvicingula spinata (Vinassa de Regny); Reference Auer, Gawlick, Suzuki and SchlagintweiAuer et al., fig. 9.47.
2009 Triversus hexagonatus (Heitzer); Reference Suzuki and GawlickSuzuki and Gawlick, p. 170, figs. 5.15; 6.11A–B.
Takemuraella sp. cf. T. schardti (O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006)
Remarks
This species differs from T. schardti by having fewer segments, a wider conical shell, and larger pores.
Takemuraella spinifera (Takemura, Reference Takemura1986)
1982 Amphipyndax sp. A Reference Kido, Kawaguchi, Adachi and Mizutani.Kido et al., pl. 4, figs. 1, 2.
1982 Amphipyndax (?) sp. Reference KojimaKojima, p. 88, pl. 1, fig. 4.
1986 Triversus spinifer Reference TakemuraTakemura, p. 63, pl. 10, figs. 21−23; pl. 11, figs. 1−2.
1987 Ristola sp. E Reference HattoriHattori, pl. 19, fig. 6.
1987 Triversus aff. T. spinifer Reference HattoriHattori, pl. 20, fig. 7.
1989 Parvicingula (?) sp. Reference Hattori and SakamotoHattori and Sakamoto, pl. 12, fig. A.
1989 Ristola spp. Reference HattoriHattori, pl. 14, fig. H.
1997 Parvicingula aff. spinifer; Reference YaoYao, pl. 13, fig. 609.
1998 Parvicingula sp. H0 Reference ArakawaArakawa, pl. 9, fig. 434.
2003 Parvicingula spinifer; Reference Goričan, Šmuc and BaumgartnerGoričan et al., p. 297, pl. 5, fig. 5.
2004 Triversus spinifer; Reference MatsuokaMatsuoka, fig. 239.
2006 Praeparvicingula? spinifera; Reference Goričan, Carter, Dumitrica, Whalen, Hori, De Wever, O’Dogherty, Matsuoka and GuexGoričan et al., p. 338, pl. TVS01, figs. 1−5.
2012 Eoxitus spinifer (Takemura); Reference Goričan, Pavšič and RožičGoričan et al., pl. 1, fig. 40.
2013 Triversus sp. cf. T. spinifer Takemura; Reference Chiari, Baumgartner, Bernoulli, Bortolotti, Marcucci, Photiades and PrincipiChiari et al., fig. 14l.
Remarks
As in Takemuraella schardti, the presence or absence of a delicate and narrower last segment (even broken, see Fig. 6.17−18) is not considered a significant characteristic to separate two morphotypes belonging to different subspecies.
Family Pseudodictyomitridae Pessagno, Reference Pessagno1977b
Genus Loopus Yang, Reference Yang1993
Type species
Pseudodictyomitra primitiva Matsuoka and Yao, Reference Matsuoka and Yao1985.
Other species
Candissa mexicana Hull, Reference Hull1997; Cinguloturris? venusta Chiari, Cortese, and Marcucci in Chiari et al., Reference Chiari, Cortese and Marcucci1997; Loopus doliolum Dumitrica in Dumitrica et al., Reference Dumitrica, Immenhauser and Dumitrica-Jud1997; Loopus doliolum martae Beccaro, Reference Beccaro2004; Loopus yangi Dumitrica in Dumitrica et al., Reference Dumitrica, Immenhauser and Dumitrica-Jud1997; Pseudodictyomitra blabla Schaaf, Reference Schaaf1981.
Occurrence
Lower Bathonian to lower Aptian.
Remarks
Includes generic concepts of Candissa Hull, Reference Hull1997.
Loopus martae Beccaro, Reference Beccaro2004
2004 Loopus doliolum martae Reference BeccaroBeccaro, p. 13, pl. 1, figs. 3−5.
2008 Loopus doliolum martae; Reference Beccaro, Diserens, Goričan and MartireBeccaro et al., pl. 3, fig. 17.
Remarks
In this paper the record of the taxon is raised to species level. This new taxonomic assignation is justified by the clear morphological distinction and different stratigraphic ranges between Loopus doliolum Dumitrica in Dumitrica et al., Reference Dumitrica, Immenhauser and Dumitrica-Jud1997 and Loopus martae Beccaro.
Loopus mexicanus (Hull, Reference Hull1997)
1997 Candissa mexicana Reference HullHull, p. 144, pl. 47, figs. 11−12, 17, 21, 24.
Remarks
Loopus mexicanus is distinguished from other Loopus included by having a lobate outline with well-pronounced constrictions.
Loopus venustus (Chiari, Cortese, and Marcucci in Chiari et al., Reference Chiari, Cortese and Marcucci1997)
1982? Unnamed multicyrtoid nassellaria Reference AdachiAdachi, pl. 2, fig. 7.
1986a Pseudodictyomitra (?) sp. D Reference MatsuokaMatsuoka, pl. 4, only figs. 2−6.
1986b Pseudodictyomitra (?) sp. D Reference MatsuokaMatsuoka, pl. 2, fig. 11; pl. 3, figs. 12a–b.
1988 Pseudodictyomitra sp. D Reference WakitaWakita, pl. 1, fig. 12.
1988 Pseudodictyomitra (?) sp. D Reference WakitaWakita, pl. 4, fig. 12.
1990 Pseudodictyomitra (?) sp. D Reference Matsuoka and OjiMatsuoka and Oji, pl. 1, fig. 8.
1997 Cinguloturris (?) venusta Reference Chiari, Cortese and MarcucciChiari, Cortese, and Marcucci in Reference Chiari, Cortese, Marcucci and NozzoliChiari et al., p. 66, pl. 2, figs. 4−5.
1997 Cinguloturris (?) sp. Reference Chiari, Cortese and MarcucciChiari, Cortese, and Marcucci in Reference Chiari, Cortese, Marcucci and NozzoliChiari et al., pl. 2, fig. 6.
2006 Dictyomitrella (?) sp. 3 Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 436, pl. 3, fig. 16.
2006 Loopus venustus; Reference Danelian, Lahsini and De RafélisDanelian et al., p. S40, pl. 1, figs. 21–23.
Remarks
We group a large variety of Loopus with complex irregular ornamentation of disconnected short costae and small polygonal depressions in L. venustus. This species clearly differs from L. primitivus, which has regularly distributted vertical costae, no polygonal depressions, and stronger strictures.
Genus Mizukidella new genus
Type species
Dictyomitrella? kamoensis Mizutani and Kido, Reference Mizutani and Kido1983.
Other species
Canoptum hungaricum Grill and Kozur, Reference Grill and Kozur1986; ?Canoptum rudabanyaense Grill and Kozur, Reference Grill and Kozur1986 ; Mizukidella mokaensis new species.
Diagnosis
Multicyrtid conical to subcylindrical shell. Cephalis dome-shaped without horn. Abdomen and post-abdominal cylindrical segments separated by nodose circumferential ridges, with paired pores just below and above the ridges. Abdomen and post-abdominal chambers have more or less regularly arranged rows of circular pits.
Etymology
Achronym of parts of Mizutani, Kido, and Dictyomitrella; feminine gender.
Occurrence
Bajocian to upper Berriasian.
Remarks
Mizukidella n. gen. is distinguished from other parvicingulids by having a characteristic surface with pits (very small depressions) on the medial part of post-abdominal segments, which are bounded by circumferential ridges and limited by one row of pores below and above. A possible origin from Canoptum is suspected. Through the latest Jurassic, this genus may have given rise to Svinitzium by the regularization of the size and arrangement of pores and pits around the circumferential ridges.
Mizukidella kamoensis (Mizutani and Kido, Reference Mizutani and Kido1983)
1983 Dictyomitrella (?) kamoensis Reference Mizutani and KidoMizutani and Kido, p. 258, pl. 53, figs. 2−4b.
2006 Dictyomitrella (?) kamoensis; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 435, pl. 3, fig. 15. [See for complete synonymy]
2009 Wrangellium oregonense Yeh; Reference YehYeh, p. 59, pl. 17, figs. 13, 22.
2013 Svinitzium kamoense; Reference Chiari, Baumgartner, Bernoulli, Bortolotti, Marcucci, Photiades and PrincipiChiari et al., fig. 14d.
Mizukidella mokaensis new species
1998 Praecaneta ? sp. 6 Reference MatsuokaMatsuoka, pl. 9, fig. 129.
Holotype
The specimen illustrated on figure 6.27 from sample BMW-35, upper Tithonian of Sillenkopf Formation (Northern Calcareous Alps, Austria).
Diagnosis
Parvicingulid with very small depressions (pits) arranged linearly on the medial part of post-abdominal segments. A middle row of pores is always present in distal segments.
Occurrence
Middle Oxfordian to upper Berriasian.
Description
Shell as with genus having commonly a conical shell with eight segments. Cephalis and thorax poreless, conical, and separated by rows of small pores. Distal segments increase gradually in width. Circumferential ridges strong and bearing vertical bars displaying a typical “H-linked” structure. Each segment has two rows of pits at the ridges and a row of small pores in the middle part. Two rows of bigger pores are always present just above and below the ridges. In well-preserved specimens (e.g., Mariana trench, Matsuoka, Reference Matsuoka1998, pl. 9, fig. 129), a velum on the distalmost segment may be preserved.
Etymology
Mokaensis is an anagram of kamoensis, which is the other species included into this genus (see above).
Measurements
(in micrometers; µm) maximum diameter of conical shell 226−263, mean 240; maximum length 113−119, mean 116, based on three specimens.
Remarks
Mizukidella mokaensis differs from M. kamoensis by having a more regular shell structure. The pits are linearly arranged, pores are larger, ridges are stronger, and a middle row of pores is always present in distal segments.
Family Xitidae Pessagno, Reference Pessagno1977b
Genus Xitus Pessagno, Reference Pessagno1977b
Type species
Xitus plenus Pessagno, Reference Pessagno1977b.
Occurrence
Upper Bajocian to upper Campanian.
Remarks
Includes generic concepts of Antexitus Yeh, Reference Yeh2009.
Xitus skenderbegi (Chiari, Marcucci, and Prela, Reference Chiari, Marcucci and Prela2002)
1993 Xitus? sp. A Reference CorteseCortese, p. 181, pl. 7, figs. 6−7.
1997 Xitus sp. Reference Matsuoka and BaumgartnerMatsuoka and Baumgartner, pl. 3, fig. 16.
1999 Xitus sp. A Reference Halamić, Goričan, Slovenec and Kolar-JurkovsekHalamić et al., pl. 3, figs. 1−4.
2002 Neorelumbra skenderbegi Reference Chiari, Marcucci and PrelaChiari, Marcucci, and Prela, p. 68, pl. 1, figs. 14−21.
2003 Neorelumbra skenderbegi; Reference Suzuki and GawlickSuzuki and Gawlick, p. 190, fig. 6.32.
2004 Neorelumbra skenderbegi; Reference Chiari, Marcucci and PrelaChiari et al., pl. 2, fig. 2.
2005 Xitus skenderbegi; Reference Šmuc and GoričanŠmuc and Goričan, p. 62, pl. 4, figs. 15−16.
2008 Xitus skenderbegi; Reference Auer, Gawlick, Suzuki and SchlagintweiAuer et al., fig. 9.42
2009 Xitus skenderbegi; Reference Suzuki and GawlickSuzuki and Gawlick, fig. 5.11.
Xitus sp. A
Remarks
This species differs from X. skenderbegi by having a more slender shape; it differs from X. magnus Baumgartner in Baumgartner et al., 1995 in being smaller and having fewer segments.
Family Xitomitridae new family
Type genus
Xitomitra new genus.
Included genera
Campanomitra new genus; Parvimitrella new genus; Pseudodictyomitrella Grill and Kozur, Reference Grill and Kozur1986; Xitomitra new genus.
Diagnosis
Multicyrtid nassellarians with small poreless cephalis and all other segments covered by a latticed meshwork of uniform size polygonal pore frames. Shell largely open distally and lacking any kind of distal projections or appendages. The genera are distinguished on the basis of presence or absence of apical horn and presence or absence of stricture at intersegmental constrictions. Only one genus, Xitomitra n. gen., may develop a secondary layer of very faint nodes on distal segments.
Occurrence
Aalenian to Maastrichtian.
Remarks
The Xitomitridae n. fam. is related to the Canoptidae, Parvicingulidae, and Xitidae. It differs from the Canoptidae by having a latticed shell with larger pores. It differs from Parvicingulidae by lacking the typical arrangement of horizontal rows of pores per segment. Xitomitridae n. fam. is also distinguished from Xitidae by lacking the typical double layer latticed meshwork bearing large tubercles on the entire surface.
Genus Campanomitra new genus
Type species
Stichocapsa praepulchella Hori, Reference Hori1999.
Other species
Amphipyndax awaensis Nakaseko and Nishimura in Nakaseko et al., Reference Nakaseko, Nishimura and Sugano1979; Amphipyndax conicus Nakaseko and Nishimura, Reference Nakaseko and Nishimura1981; Amphipyndax ellipticus Nakaseko and Nishimura, Reference Nakaseko and Nishimura1981; Archicorys pulchella Rüst, Reference Rüst1885; Parvicingula cappa Cortese, Reference Cortese1993; ?Pseudoeucyrtis buekkensis Grill and Kozur, Reference Grill and Kozur1986, Quarticella hunzikeri O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006; ?Stichocapsa labyrinthica Dumitrica in Dumitrica et al., Reference Dumitrica, Immenhauser and Dumitrica-Jud1997; Stichocapsa tuscanica Chiari, Cortese, and Marcucci in Chiari et al., Reference Chiari, Cortese and Marcucci1997; Stichocapsa ulivii Chiari, Cortese, and Marcucci in Chiari et al., Reference Chiari, Cortese and Marcucci1997; Stichocapsa devorata arctica Vishnevskaya and Murchey, Reference Vishnevskaya and Murchey2002; forms included as Stichocapsa spp. by Matsuoka (Reference Matsuoka1998, figs. 35, 37−40).
Diagnosis
Campanulate shell composed of four to ten segments. Largely open distally. Small, poreless cephalis without apical horn. Other segments covered by a latticed meshwork consisting of relatively large polygonal pore frames. The strictures between junctions of the segments are never present.
Etymology
Genus name derived from campano (bell) and mitra (ecclesiastical headgear: miter). Feminine gender.
Occurrence
Bajocian to Cenomanian.
Remarks
Campanomitra n. gen. differs from Pseudodictyomitrella by lacking an apical horn. It differs from Parvimitrella and Xitomitra in having larger pores and no intersegmental constrictions. Campanomitra n. gen. differs from Takemuraella by lacking the arrangement of three rows of pores per segment and having no circumferential ridges.
A common character of the genus in Cretaceous species is the presence of a large sutural pore on the surface of the shell. Stichocapsa labyrinthica Dumitrica in Dumitrica et al., Reference Dumitrica, Immenhauser and Dumitrica-Jud1997 is tentatively assigned in this genus because it lacks the distinctive sutural pore present in all other Cretaceous representatives of the genus.
Campanomitra sp. aff. C. buekkensis (Grill and Kozur, Reference Grill and Kozur1986)
1986 Pseudoeucyrtis buekkensis Reference Grill and KozurGrill and Kozur, p. 251, pl. 6, fig. 4.
Remarks
This specimen is characterized by an irregular arrangement of circular pores throughout the entire shell. It differs from typical C. buekkensis in which the pores are arranged in horizontal rows distally.
Campanomitra tuscanica (Chiari, Cortese, and Marcucci in Chiari et al., Reference Chiari, Cortese and Marcucci1997)
1986 Pseudodictyomitrella hexagonata; Reference Grill and KozurGrill and Kozur, pl. 4, fig. 2.
1997 Stichocapsa tuscanica Reference Chiari, Cortese and MarcucciChiari, Cortese, and Marcucci in Reference Chiari, Cortese, Marcucci and NozzoliChiari et al., p. 70, pl. 4, figs. 8−9.
2003 Triversus hexagonatus; Reference Suzuki and GawlickSuzuki and Gawlick, p. 194, figs. 5.48, 6.61.
2006 Pseudodictyomitrella tuscanica; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 440, pl. 5, figs. 7−9.
2006 Triversus hexagonatus; Reference Gawlik, Suzuki and SchlagintweitGawlick et al., fig. 8c.40; fig. 9b.20.
2006 Triversus hexagonatus; Reference Auer, Gawlick and SuzukiAuer et al., fig. 6.48.
2007b Triversus hexagonatus; Reference Gawlick, Schlagintweit and SuzukiGawlick et al., fig. 17.29.
2013 Pseudodictyomitrella tuscanica; Reference Chiari, Baumgartner, Bernoulli, Bortolotti, Marcucci, Photiades and PrincipiChiari et al., p. 415, fig. 13t.
Campanomitra sp. aff. C. tuscanica (Chiari, Cortese, and Marcucci in Chiari et al., Reference Chiari, Cortese and Marcucci1997)
Remarks
This species differs from C. tuscanica by having cylindrical instead of conical shell distally.
Campanomitra sp. aff. C. ulivii (Chiari, Cortese, and Marcucci in Chiari et al., Reference Chiari, Cortese and Marcucci1997)
1997 Stichocapsa ulivii Reference Chiari, Cortese and MarcucciChiari, Cortese, and Marcucci in Reference Chiari, Cortese, Marcucci and NozzoliChiari et al., p. 70, pl. 4, figs. 10−11.
?1997 Stichocapsa sp. aff. S. ulivii Reference Chiari, Cortese and MarcucciChiari, Cortese, and Marcucci in Reference Chiari, Cortese, Marcucci and NozzoliChiari et al., p. 72, pl. 4, fig. 12.
Remarks
This species resembles C. ulivii in shape and pore size, but differs by having a smoother surface without raised pore frames.
Campanomitra? sp. A
Remarks
The specimens included in this species have a barrel-shape outline (fusiform) and consist of six or more segments.
Campanomitra? spp.
1982 Stichocapsa sp. A Reference Sashida, Igo, Igo, Takizawa, Hisada, Shibata, Tsukada and NishimuraSashida et al., pl. 2, fig. 2.
1989 Bagotum sp. A Reference Hattori and SakamotoHattori and Sakamoto, pl. 13, fig. J.
Remarks
Various species of Campanomitra n. gen. with fewer than five segments commonly occur in our material. Similar forms have been previously illustrated (see synonymy). However, more in depth taxonomical research is needed to describe new species. This morphotype vaguely resembles the Cretaceous species Trimulus parmatus O’Dogherty, Reference O’Dogherty1994.
Genus Parvimitrella new genus
Type species
Pseudodictyomitrella wallacheri Grill and Kozur Reference Grill and Kozur1986 (syn. Parvifavus irregularis Takemura, Reference Takemura1986).
Included species
Amphipyndax plousios Foreman, Reference Foreman1968; Parvifavus irregularis Takemura, Reference Takemura1986; Pseudodictyomitrella wallacheri Grill and Kozur, Reference Grill and Kozur1986; Stichomitra? angulata Bragin and Tekin in Bragin et al., Reference Bragin, Tekin and Özçelik2002; Stichomitra cathara Foreman, Reference Foreman1968; Stichomitra? cechena Foreman, Reference Foreman1968; Stichomitra communis Squinabol, Reference Squinabol1903; Stichomitra compsa Foreman, Reference Foreman1968; Stichomitra magna Squinabol, Reference Squinabol1904; ?Stichomitra navalis O’Dogherty, Reference O’Dogherty1994.
Diagnosis
Thick-walled shell, multisegmented, having a single hexagonal closely packed layer of pores per segment. Strictures between segments usually well marked.
Etymology
The generic name comes from a combination of Pseudodictyomitrella and Parvifavus, to which the type species was originally assigned.
Occurrence
Aalenian to Maastrichtian.
Remarks
Parvimitrella n. gen. is erected to replace the genus Stichomitra Cayeux, Reference Cayeux1897, sensu O’Dogherty, Reference O’Dogherty1994, due to problems related to the poor definition of the type species (see discussion regarding this subject in O’Dogherty, Reference O’Dogherty1994; O’Dogherty et al., Reference O’Dogherty, Carter, Dumitrica, Goričan, De Wever, Bandini, Baumgartner and Matsuoka2009).
Parvimitrella n. gen. (ex. Stichomitra) is easily distinguished from other Jurassic and Cretaceous multi-segmented forms by its typical pattern of hexagonal close-packed layer of pores per chamber and its frequently dome-shaped cephalis, divided into two chambers by a transverse internal ledge. Parvimitrella n. gen. is distinguished from Amphipyndax in lacking two structurally distinct layers of test material and if circumferential ridges develop, they never occur at segmental divisions.
Parvimitrella n. gen. differs from Pseudodictyomitrella by having a rounded cephalis and strictures at segmental divisions. As mentioned above, many species originally included under the genus Stichomitra (especially in the Cretaceous) belong at least to three distinct described genera: Amphipternis Foreman, Reference Foreman1973a; Schaafella Vishnevskaya in Basov and Vishnevskaya, Reference Basov and Vishnevskaya1991; and Eostichomitra Empson-Morin, Reference Empson-Morin1981.
Species frequently ascribed to Stichomitra, but now considered as belonging to other genera: Amphipternis: Dictyomitra mediocris Tan, Reference Tan1927; Stichocapsa? stocki Campbell and Clark, Reference Campbell and Clark1944; and Stichomitra tosaensis Nakaseko and Nishimura, Reference Nakaseko and Nishimura1981.
Schaafella: Schaafella deweveri Vishnevskaya in Basov and Vishnevskaya, Reference Basov and Vishnevskaya1991; Schaafella nodosa Vishnevskaya in Basov and Vishnevskaya, Reference Basov and Vishnevskaya1991; Schaafella tochilinae Vishnevskaya in Basov and Vishnevskaya, Reference Basov and Vishnevskaya1991; Stichomitra tosaensis Nakaseko and Nishimura in Nakaseko et al., Reference Nakaseko, Nishimura and Sugano1979; and Stichopilium bonum Kozlova in Kozlova and Gorbovetz, Reference Kozlova1966.
Eostichomitra: Eostichomitra warzigita Empson-Morin, Reference Empson-Morin1981; Stichomitra asymbatos Foreman, Reference Foreman1968; Vistularia magna Gorka, Reference Górka1989; and ?Cyrtocapsa pseudacerra Tan, Reference Tan1927.
Parvimitrella wallacheri (Grill and Kozur, Reference Grill and Kozur1986)
1986 Pseudodictyomitrella wallacheri Reference Grill and KozurGrill and Kozur, p. 253, pl. 4, figs. 5−7.
1986 Parvifavus irregularis Reference TakemuraTakemura, p. 9, pl. 10, figs. 10−13.
2008 Parvifavus wallacheri; Reference Auer, Gawlick, Suzuki and SchlagintweiAuer et al., fig. 9.48.
2011 Parvifavus irregularis; Reference YehYeh, p. 8, pl. 2, figs. 1−2.
Remarks
Because the paper by Grill and Kozur was published in January 1986 but the paper by Takemura in December 1986, the species name of P. wallacheri has priority. It is also considered the type species of this genus.
Genus Pseudodictyomitrella Grill and Kozur, Reference Grill and Kozur1986
Type species
Pseudodictyomitrella spinosa Grill and Kozur, Reference Grill and Kozur1986.
Other species
Parvicingula limana Cortese, Reference Cortese1993 ; Pseudodictyomitrella badouxi O’Dogherty, Goričan and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006; Pseudodictyomitrella escheri O’Dogherty, Goričan and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006; Pseudodictyomitrella renevieri O’Dogherty, Goričan and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006.
Remarks
All species here included under Pseudodictyomitrella have a conical shell with an acute cephalis tapering in an apical horn. For a detailed description of all these species the reader is referred to O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006.
Occurrence
Lower Bajocian to upper Bathonian.
Pseudodictyomitrella limana (Cortese, Reference Cortese1993)
1985 Parvicingula ? sp. Reference Yamamoto, Mizutani and KagamiYamamoto et al., p. 36, pl. 6, fig. 5.
1993 Parvicingula limana Reference CorteseCortese; p. 177; pl. 4, figs. 5–7.
2003 Parvicingula cappa Cortese; Reference Suzuki and GawlickSuzuki and Gawlick, p. 187, fig. 6.84 only.
2006 Pseudodictyomitrella limana; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 440, pl. 5, fig. 18.
Pseudodictyomitrella renevieri O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006
1998 Parvicingula ? sp. A1 Reference ArakawaArakawa, pl. 10, fig. 454.
1998 Parvicingula ? sp. J0 Reference ArakawaArakawa, pl. 10, fig. 462.
2006 Pseudodictyomitrella renevieri Reference O’Dogherty, Goričan and DumitricaO’Dogherty, Goričan, and Dumitrica in Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 440, pl. 5, figs. 19−22.
Pseudodictyomitrella sp. cf. P. renevieri O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., Reference O’Dogherty, Goričan and Dumitrica2006
Remarks
The specimen has a pointed cephalis but supposedly due to preservation it lacks the apical horn.
Genus Xitomitra new genus
Type species
Stichomitra tairai Aita, Reference Aita1987.
Other species
Stichomitra annibill Kocher, Reference Kocher1981 (syn. Stichomitra? matsuokai Hull, Reference Hull1997).
Diagnosis
Shell conical with four or more segments and a large distal aperture. Very large cephalis, imperforate and usually bearing a small apical horn. Subsequent segments increasing gradually in height, with well-defined external strictures. All segments covered by a regular pattern of polygonal (mostly hexagonal) pore frames. Distalmost segments may bear thick nodes.
Etymology
Arbitrary combination of letters (ICZN, Art. 11.3) plus mitra (from Latin, meaning cap); feminine gender.
Remarks
Xitomitra n. gen. differs from other genera included in this family by having a more lobate outline with well-marked strictures. It differs from Parvimitrella n. gen. by having a thick, wider and pointed cephalic region (see pl. 3, fig. 8a of Aita, Reference Aita1987).
Occurrence
Middle Bathonian to lower Callovian.
Xitomitra annibill (Kocher, Reference Kocher1981)
1981 Stichomitra annibill Reference KocherKocher, p. 96, pl. 16, figs. 24−26.
1989 Gen 2 sp. B Reference Hattori and SakamotoHattori and Sakamoto, ? pl. 14, fig. E.
1996 Stichomitra tairai Aita; Reference Marcucci and PrelaMarcucci and Prela, pl. 2, fig. 14.
1997 Stichomitra (?) matsuokai Reference HullHull, p. 164, pl. 49, figs. 2−3, 13−14, 19−20.
2003 non Stichomitra annibill; Reference Suzuki and GawlickSuzuki and Gawlick, figs. 6.35−6.36. [= X. tairai]
2005 Stichomitra annibill; Reference Šmuc and GoričanŠmuc and Goričan, pl. 4, fig. 24.
2006 Stichomitra annibill; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 442, pl. 5, figs. 1−3.
2006 non Stichomitra annibill; Reference Auer, Gawlick and SuzukiAuer et al., fig. 6.37. [= X. tairai]
2008 non Stichomitra annibill; Reference Auer, Gawlick, Suzuki and SchlagintweiAuer et al., fig. 9.66. [= X. tairai]
2009 non Stichomitra annibill; Reference Suzuki and GawlickSuzuki and Gawlick, fig. 5.16. [= X. tairai]
Remarks
Xitomitra annibill differs from Xitomitra tairai by having a smaller cephalis and a four-segmented shell. Furthermore, the shell does not have the outer characteristic layer of ornametation as developed in the Japanese species.
Xitomitra tairai (Aita, Reference Aita1987)
1987 Stichomitra (?) tairai Reference AitaAita, p. 72, pl. 3, figs. 7a−9; pl. 10, figs. 3−4.
1989 Stichomitra (?) sp. cf. S. tairai; Reference Okada, Tarduno, Nakaseko, Nishimura, Sliter and OkadaOkada et al., pl. 32, fig. 12.
1996 non Stichomitra tairai; Reference Marcucci and PrelaMarcucci and Prela, pl. 2, fig. 14. [= X. annibill]
2002 Stichomitra (?) tairai; Reference Bragin, Tekin and ÖzçelikBragin et al., fig. 6.19.
2002 Stichomitra (?) sp. aff. S. (?) takanoensis Aita; Reference Bragin, Tekin and ÖzçelikBragin et al., fig. 6.20.
2003 Stichomitra annibill Kocher; Reference Suzuki and GawlickSuzuki and Gawlick, figs. 6.35−6.36.
2005 Stichomitra (?) tairai; Reference Šmuc and GoričanŠmuc and Goričan, pl. 4, fig. 23.
2006 Stichomitra (?) tairai; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 442, pl. 5, fig. 4−5.
2006 Stichomitra annibill; Reference Auer, Gawlick and SuzukiAuer et al., fig. 6.37.
2008 Stichomitra annibill; Reference Auer, Gawlick, Suzuki and SchlagintweiAuer et al., fig. 9.66.
2009 Stichomitra annibill; Reference Suzuki and GawlickSuzuki and Gawlick, fig. 5.16.
Remarks
Xitomitra tairai is distinguished from its allied X. annibill by having a characteristic and delicate double layer of rhomboidal pore frames covering the post-thoracic segments. This ornamentation is specially marked on proximal segments, which fade out distally where very faint nodes tend to regularly cover the surface.
Xitomitra? sp. A
Remarks
This very small species is characterized by proximally conical to distally subcylidrical shell covered by a double-layered pore pattern similar to that of X. tairai. The specimen is questionably assigned to Xitomitra because its lacks the well-defined strictures and the thick large cephalis.
Order Spumellaria Ehrenberg, Reference Ehrenberg1876
‘Actinommids with single medulary shell’
Superfamily Actinommoidea Haeckel, Reference Haeckel1862
Family Pantanelliidae Pessagno, Reference Pessagno1977b
Subfamily Pantanelliinae Pessagno, Reference Pessagno1977b
Genus Pantanellium Pessagno, Reference Pessagno1977a
Type species
Pantanellium riedeli Pessagno, Reference Pessagno1977a.
Occurrence
Upper Carnian to upper Aptian.
Pantanellium riedeli Pessagno, Reference Pessagno1977a
1977a Pantanellium riedeli Reference PessagnoPessagno, p. 78, pl. 6, figs. 5–11.
2006 Pantanellium riedeli; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 460, pl. 11, figs. 9–14. [See for complete synonymy]
Genus Gorgansium Pessagno and Blome, Reference Pessagno and Blome1980
Type species
Gorgansium silviesense Pessagno and Blome, Reference Pessagno and Blome1980.
Occurrence
Upper Norian to upper Valanginian.
Gorgansium silviesense Pessagno and Blome, Reference Pessagno and Blome1980
1980 Gorgansium silviesense Reference Pessagno and BlomePessagno and Blome, p. 235, pl. 11; figs. 2−3, 11, 24.
1982 Gorgansium silviesense; Reference Mizutani and KoikeMizutani and Koike, pl. 1, fig. 3.
1987 Gorgansium silviesense; Reference GoričanGoričan, p. 182, pl. 3, fig. 4.
2006 Gorgansium silviesense; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 460, pl. 11, fig. 22.
‘Actinommids with multiple medulary shell’
Superfamily Pylonoidea Haeckel, Reference Haeckel1881 emend Dumitrica, Reference Dumitrica1989
Family Dactyliosphaeridae Squinabol, Reference Squinabol1904
Subamily Hagiastrinae Riedel, Reference Riedel1971
Genus Crucella Pessagno, Reference Pessagno1971
Type species
Crucella messinae Pessagno, Reference Pessagno1971.
Occurrence
Lower Carnian to upper Campanian.
Crucella theokaftensis Baumgartner, Reference Baumgartner1980
1980 Crucella theokaftensis Reference BaumgartnerBaumgartner, p. 308, pl. 8, figs. 19–22; pl. 12, fig. 1.
2003 Crucella theokaftensis; Reference Suzuki and GawlickSuzuki and Gawlick, p. 174, fig. 6.17.
2006 Crucella theokaftensis; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 464, pl. 12, fig. 16. [See for complete synonymy]
Subfamily Emiluviinae Dumitrica, Reference Dumitrica1995
Genus Emiluvia Foreman, Reference Foreman1973b
Neosophia Özdikmen Reference Özdikmen2009 pro Sophia Whalen and Carter in Carter et al., Reference Carter, Whalen and Guex1998 (preoccupied name).
Type species
Emiluvia chica Foreman, Reference Foreman1973b.
Occurrence
Lower Sinemurian to upper Valanginian.
Emiluvia salensis Pessagno, Reference Pessagno1977a
1977a Emiluvia salensis Reference PessagnoPessagno, p. 77, pl. 5, figs. 9–11.
1995b Emiluvia salensis; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 210, pl. 3215, figs. 1–3.
2006 Emiluvia salensis; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 468, pl. 12, figs. 12–15. [See for complete synonymy]
Emiluvia sedecimporata (Rüst, Reference Rüst1885)
1885 Staurosphaera sedecimporata Reference RüstRüst, p. 288, pl. 28(3), fig. 1.
1994 Emiluvia sedecimporata; Reference GoričanGoričan, p. 67, pl. 4, fig. 4.
1995b Emiluvia sedecimporata; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 210, pl. 3216, figs. 1–3. [See for complete synonymy]
1997 Emiluvia sedecimporata; Reference Chiari, Cortese, Marcucci and NozzoliChiari et al., pl.2, fig. 9.
2004 Emiluvia peteri Reference BeccaroBeccaro, p. 16, fig. 4a; pl. 2, figs. 6–8.
Emiluvia? sp.
Remarks
This form is questionably assigned to Emiluvia. Similar forms have been assigned to Staurolonche Rüst, Reference Rüst1885 (e.g,. Staurolonche robusta), but this genus must be considered as nomen dubium (O’Dogherty et al., Reference O’Dogherty, Carter, Dumitrica, Goričan, De Wever, Bandini, Baumgartner and Matsuoka2009). Further taxonomic studies are required in order to fix the taxonomic position of this species.
Family Patulibracchiidae Pessagno, Reference Pessagno1971
Subamily Angulobracchinae Baumgartner, Reference Baumgartner1980
Genus Angulobracchia Baumgartner, Reference Baumgartner1980
Type species
Paronaella? purisimaensis Pessagno, Reference Pessagno1977a.
Occurrence
Upper Aalenian to lower Albian.
Remarks
Includes generic concept of Cavabracchia Kito and De Wever, Reference Kito and De Wever1992.
Angulobracchia spp.
Remarks
These forms are very rare and badly preserved in our material; hence they have not been studied in depth in this publication.
Genus Paronaella Pessagno, Reference Pessagno1971
Type species
Paronaella solanoensis Pessagno, Reference Pessagno1971.
Occurrence
Lower Rhaetian to upper Coniacian.
Paronaella kotura Baumgartner, Reference Baumgartner1980
1980 Paronaella kotura Reference BaumgartnerBaumgartner, p. 302, pl. 9, figs. 15–19; pl. 12, fig. 8.
2006 Paronaella kotura; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 468, pl. 12, figs. 12–15. [See for complete synonymy]
Paronaella pristidentata Baumgartner, Reference Baumgartner1980
1980 Paronaella pristidentata Reference BaumgartnerBaumgartner, p. 304, pl. 9, fig. 7; pl. 12, fig. 3.
1992 Paronaella pristidentata; Reference SteigerSteiger, p. 44, pl. 10, figs. 8−9.
1995b Paronaella pristidentata; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 396, pl. 3138, figs. 1−2.
1993 Paronaella cleopatrensis Reference Pessagno, Blome and HullPessagno, Blome, and Hull in Pesagno et al., p. 122, pl. 2, figs. 3, 25.
1997 Paronaella pristidentata; Reference YaoYao, pl. 7, fig. 309.
2001 Paronaella pristidentata; Reference VishnevskayaVishnevskaya, pl. 46, figs. 3−4; pl. 91, figs. 4−5.
2009 Paronaella pristidentata; Reference NishiharaNishihara, pl. 8, fig. 186.
Subfamily Patulibracchiinae Pessagno, Reference Pessagno1971
Genus Homoeoparonaella Baumgartner, Reference Baumgartner1980
Type species
Paronaella elegans Pessagno, Reference Pessagno1977a.
Occurrence
Upper Sinemurian to upper Cenomanian.
Homoeoparonaella elegans (Pessagno, Reference Pessagno1977a)
1977a Paronaella elegans Pessagno, p. 70, pl. 1, figs. 10−11.
1995b Homoeoparonaella elegans; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 272, pl. 3104, figs. 1–5. [See for complete synonymy]
1999 Homoeoparonaella elegans yangi Reference KiesslingKiessling, p. 32, pl. 6, figs. 13−14, 16.
2008 Homoeoparonaella elegans; Reference Beccaro, Diserens, Goričan and MartireBeccaro et al., pl. 1, fig. 22.
Homoeoparonaella spp.
Remarks
Our specimens are badly preserved, with ray tips broken off, therefore determination at species level is not possible.
Family Pseudoaulophacinae Riedel, Reference Riedel1967
Genus Alievium Pessagno, Reference Pessagno1972
Type species
Theodiscus superbus Squinabol, Reference Squinabol1914.
Occurrence
Upper Bajocian to upper Maastrichtian.
Alievium? sp. aff. A. crassum (Kiessling, Reference Kiessling1999)
1999 Tripocyclia crassa Reference KiesslingKiessling, p. 40, pl. 8, figs. 14, 22.
Remarks
Our specimen has shorter spines and somewhat larger nodes on the surface than the Antarctic specimens. The genus is queried because the name Tripocyclia is not valid (nomen dubium see O’Dogherty et al., Reference O’Dogherty, Carter, Dumitrica, Goričan, De Wever, Bandini, Baumgartner and Matsuoka2009, p. 288) and these forms belong to a new genus not described yet.
Alievium? longispineum Yang and Wang, Reference Yang and Wang1990
1990 Alievium longispineum Reference Yang and WangYang and Wang, p. 204, pl. 2, figs. 2, 4.
Remarks
This form has high generic affinity with the previous species and the genus is queried for the same reasons.
Family Tritrabidae Baumgartner, Reference Baumgartner1980
Genus Tritrabs Baumgartner, Reference Baumgartner1980
Type species
Paronaella? casmaliaensis Pessagno, Reference Pessagno1977a.
Occurrence
Lower Aalenian to lower Aptian.
Tritrabs ewingi (Pessagno, Reference Pessagno1971)
1971 Paronaella (?) ewingi Reference PessagnoPessagno, p. 47, pl. 19, figs. 2–5.
2006 Tritrabs ewingi; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 472, pl. 11, figs. 37, 43. [See for complete synonymy]
Superfamily Sponguroidea Haeckel, Reference Haeckel1862
Family Archaeospongoprunidae Pessagno, Reference Pessagno1973
Genus Archaeospongoprunum Pessagno, Reference Pessagno1973
Type species
Archaeospongoprunum venadoensis Pessagno, Reference Pessagno1973.
Occurrence
Upper Permian to upper Campanian.
Archaeospongoprunum elegans Wu, Reference Wu1993
1993 Archaeospongoprunum elegans, p 118, pl. 1, figs. 5, 7, 23.
1997 Archaeospongoprunum aff. elegans Wu; Reference HullHull, p. 27, pl. 8, figs. 4, 9, 18−19.
2001 Archaeospongoprunum sp. Reference NishizonoNishizono, pl. 1, fig. 3.
2002 Archaeospongoprunum elegans; Reference Beccaro, Baumgartner and MartireBeccaro et al., pl. 1, fig. 22.
2006 Archaeospongoprunum elegans; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 472, pl. 11, figs. 24−25.
Remarks
Archaeospongoprunum elegans differs from A. imlayi Pessagno, by its subrectangular shape and massive spines with prominent subsidiary grooves. We considered specimens showing slightly torsioned spines distally to be included in this species (see Fig. 12.11–12 and Hull’s Reference Hull1997 material).
Family Bernoulliidae Pessagno, Blome, and Hull in Pessagno et al., Reference Pessagno, Blome, Hull and Six1993
Genus Bernoullius Baumgartner, Reference Baumgartner1984
Type species
Eucyrtis ? dicera Baumgartner in Baumgartner et al., Reference Baumgartner, De Wever and Kocher1980.
Occurrence
Lower Toarcian to lower Aptian.
Bernoullius cristatus Baumgartner, Reference Baumgartner1984
1984 Bernoullius cristatus Reference BaumgartnerBaumgartner, p. 760, pl. 2, figs. 14−15.
1993 Bernoullius cristatus; Reference Pessagno, Blome and HullPessagno et al., p. 119, pl. 1, fig. 14.
1995b Bernoullius cristatus; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p.122, pl. 3221, figs. 1–3.
2003 Bernoullius cristatus; Reference Suzuki and GawlickSuzuki and Gawlick, p. 172, fig. 5.10. [See for complete synonymy]
Superfamily Saturnaloidea Deflandre, Reference Deflandre1953
Family Saturnalidae Deflandre, Reference Deflandre1953
Subfamily Hexasaturnalinae Kozur and Mostler, Reference Kozur and Mostler1983
Genus Hexasaturnalis Kozur and Mostler, Reference Kozur and Mostler1983
Type species
Spongosaturnalis ? hexagonus Yao Reference Yao1972.
Occurrence
Middle Toarcian to upper Valanginian.
Remarks
Includes generic concepts of Kozurastrum De Wever, Reference De Wever1984 and Yaosaturnalis Kozur and Mostler, Reference Kozur and Mostler1983.
Hexasaturnalis minor (Baumgartner in Baumgartner et al., Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and Steiger1995b)
1995b Acanthocircus suboblongus minor Baumgartner in Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 66, pl. 3085, only fig. 1–3, not fig. 4 (H. nakasekoi)
2005 Hexasaturnalis minor; Reference Dumitrica and Dumitrica-JudDumitrica and Dumitrica-Jud, pl. 2, figs. 6, 9–13.
2008 Hexasaturnalis minor; Reference Beccaro, Diserens, Goričan and MartireBeccaro et al., pl. 1, fig. 18.
Hexasaturnalis nakasekoi Dumitrica and Dumitrica-Jud Reference Dumitrica and Dumitrica-Jud2005
2005 Hexasaturnalis nakasekoi Reference Dumitrica and Dumitrica-JudDumitrica and Dumitrica-Jud, p. 161, pl. 1, figs. 3–13; pl. 2, figs. 1−4, 7−8. [See for complete synonymy]
2006 Hexasaturnalis nakasekoi; Reference O’Dogherty, Bill, Goričan, Dumitrica and MassonO’Dogherty et al., p. 473, pl. 12, fig. 31. [See for complete synonymy]
2008 Hexasaturnalis minor; Reference Beccaro, Diserens, Goričan and MartireBeccaro et al., pl. 1, fig. 19.
Hexasaturnalis suboblongus (Yao, Reference Yao1972)
1972 Spongosaturnalis (?) suboblongus Reference YaoYao, p. 29, pl. 3, figs. 1–6; pl. 10, figs. 3a–c.
1995bAcanthocircus suboblongus suboblongus; Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 68, pl. 3088, fig. 2–4, non fig 1 [= H. nakasekoi].
2005 Hexasaturnalis suboblongus; Reference Dumitrica and Dumitrica-JudDumitrica and Dumitrica-Jud, pl. 1, figs. 1−2.
2013 Hexasaturnalis suboblongus; Reference Chiari, Baumgartner, Bernoulli, Bortolotti, Marcucci, Photiades and PrincipiChiari et al., p. 411, fig. 12r.
Hexasaturnalis tetraspinus (Yao, Reference Yao1972)
1972 Spongosaturnalis? tetraspinus Reference YaoYao, p. 29, pl. 4, figs. 1–6; pl. 11, figs. 1−2.
1995b Hexasaturnalis tetraspinus (Yao); Reference Baumgartner, O’Dogherty, Goričan, Dumitrica-Jud, Dumitrica, Pillevuit, Urquhart, Matsuoka, Danelian, Bartolini, Carter, De Wever, Kito, Marcucci and SteigerBaumgartner et al., p. 254, pl. 3089, figs. 1–3.
2006 Hexasaturnalis tetraspinus; Reference Goričan, Carter, Dumitrica, Whalen, Hori, De Wever, O’Dogherty, Matsuoka and GuexGoričan et al., p. 190, pl. 3089, figs. 1–5. [See for complete synonymy]
2013 Hexasaturnalis tetraspinus; Reference Chiari, Baumgartner, Bernoulli, Bortolotti, Marcucci, Photiades and PrincipiChiari et al., fig. 12s.
Age of radiolarian samples
In this section, we present the radiolarian age and results obtained for the Hallstatt Mélange in four localities at Bad Mittendorf area (Table 2). Only the stratigraphically most important species are discussed. For the complete inventory of all samples, see Table 1.
Table 2 Assigned ages and zones of studied samples.
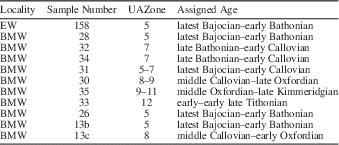
Kumitzberg
In this area, the massive dark-gray radiolarite beds (sample EW-158) are intercalated by thin layers of cherty shales. The co-occurrence of Unuma latusicostatus (Aita) with Bernoullius cristatus Baumgartner (Table 1) suggests assignment to UAZ 5 (latest Bajocian–early Bathonian) of Baumgartner et al. (Reference Baumgartner, Bartolini, Carter, Conti, Cortese, Danelian, De Wever, Dumitrica, Dumitrica-Jud, Goričan, Guex, Hull, Kito, Marcucci, Matsuoka, Murchey, O’Dogherty, Savary, Vishnevskaya, Widz and Yao1995a). Pantanellium riedeli Pessagno, which first appears in UAZ 7, was also found. This species has a very large variability that overlaps with other Pantanellium species. Its range is thus ignored in the age determination.
Steinwand north
In this area two sections accurately determine the age of the radiolarite succession. The most complete section, in the northeastern part of the syncline structure (Fig. 4), records continuous radiolarite deposition from Bathonian to the Oxfordian. The lowermost part of the radiolarite succession (near the entrance of the valley, sample BMW-28, Fig. 4) yielded a radiolarian assemblage of latest Bajocian–early Bathonian age (UAZ 5 of Baumgartner et al., Reference Baumgartner, Bartolini, Carter, Conti, Cortese, Danelian, De Wever, Dumitrica, Dumitrica-Jud, Goričan, Guex, Hull, Kito, Marcucci, Matsuoka, Murchey, O’Dogherty, Savary, Vishnevskaya, Widz and Yao1995a), defined on the co-occurrence of Protunuma ochiensis Matsuoka with Unuma latusicostatus (Aita). The conflicting range of Pantanellium riedeli Pessagno (UAZ 7–12) was ignored for the same reason as in the aforementioned sample EW-158 from Kumitzberg.
The youngest part of the succession is preserved in the core of the syncline (sample BMW-35, Fig. 4) and yielded a radiolarian assemblage of middle–late Oxfordian to late Kimmeridgian–early Tithonian age (UAZs 9–11 of Baumgartner et al., Reference Baumgartner, Bartolini, Carter, Conti, Cortese, Danelian, De Wever, Dumitrica, Dumitrica-Jud, Goričan, Guex, Hull, Kito, Marcucci, Matsuoka, Murchey, O’Dogherty, Savary, Vishnevskaya, Widz and Yao1995a), based on the occurrence of Archaeodictyomitra minoensis (Mizutani), Zhamoidellum ovum Dumitrica, and Emiluvia sedecimporata (Rüst). The interval between these two ages was also recognized. UAZ 7 (late Bathonian–early Callovian) or UAZ 8 (middle Callovian–early Oxfordian) was determined in samples BMW-32 and BMW-34. Striatojaponocapsa conexa (Matsuoka) in sample BMW-32 and Preawilliriedellum robustum (Matsuoka) in sample BMW-34 suggest that these two samples are not younger than UAZ 7, but Gongylothorax favosus Dumitrica, which first occurs in UAZ 8 is also associated.
At the end of the valley is located the second stratigraphic section outcropping in this area. The radiolarian assemblage in the youngest investigated sample (BMW-33) indicates a Tithonian age. Early–early late Tithonian UAZ 12 is inferred from the co-occurrence of Eucyrtidiellum pyramis (Aita in Aita and Okada) with Protunuma japonicus Matsuoka and Yao.
Area between Krautmoos and Mischenirwiese
The thick succession of mass-flow deposits in this area is dated by the assemblages studied in the intercalated radiolarite matrix. The radiolarite sample BMW-26 collected below the first debris-flow deposit is dated latest Bajocian–early Bathonian age (UAZ 5 of Baumgartner et al., Reference Baumgartner, Bartolini, Carter, Conti, Cortese, Danelian, De Wever, Dumitrica, Dumitrica-Jud, Goričan, Guex, Hull, Kito, Marcucci, Matsuoka, Murchey, O’Dogherty, Savary, Vishnevskaya, Widz and Yao1995a). This age is constrained with Semihsuum amabile (Aita), Saitoum trichylum De Wever, and Mizukidella kamoensis (Mizutani and Kido). The same age (UAZ 5) was obtained from a chert clast (sample BMW-13b) in the upper part of the succession. The age-diagnostic species in this clast are Theocapsomella cordis (Kocher), Eucyrtidiellum pustulatum Baumgartner, and Unuma latusicostatus (Aita). The matrix between the chert clasts (sample BMW-13c) yielded a significantly younger radiolarian fauna of middle Callovian–early Oxfordian age (UAZ 8 of Baumgartner et al., Reference Baumgartner, Bartolini, Carter, Conti, Cortese, Danelian, De Wever, Dumitrica, Dumitrica-Jud, Goričan, Guex, Hull, Kito, Marcucci, Matsuoka, Murchey, O’Dogherty, Savary, Vishnevskaya, Widz and Yao1995a), as indicated by co-occurrence of Spinosicapsa spinosa (Ožvoldová) with Hemicryptocapsa marcucciae (Cortese).
As proven by radiolarian dating, the resedimentation of the Hallstatt Limestone started in the region of Bad Mitterndorf area since the Bathonian and prevailed at least until the Oxfordian. The area of the Hallstatt Limestone mass flows and slide blocks is separated from the coeval radiolarite succession without mass-flow deposits (the Steinwand section) by a younger thrust or fault.
Discussion
The time span of deposition of the radiolarite basin, which contains the far-traveled Hallstatt Limestone blocks, is determined as Bathonian to Oxfordian. Redeposition started in the ?late Bathonian and ended in the Oxfordian, as proven by the radiolarite matrix age and the age of the overlying sediments (O’Dogherty and Gawlick, Reference O’Dogherty and Gawlick2008). Radiolarian ages and component spectrum define this redeposit as part of the Sandlingalm Basin (Sandlingalm Formation: Fig. 2), one of the oldest basins formed in a relative early stage of compression of the Neotethys (see Gawlick et al., Reference Gawlick, Schlagintweit and Missoni2007a, Reference Gawlick, Missoni, Schlagintweit, Suzuki, Frisch, Krystyn, Blau and Lein2009, Reference Gawlick, Missoni, Schlagintweit and Suzuki2012; Missoni and Gawlick, Reference Missoni and Gawlick2011b for details).
The depositional areas of the radiolarites of the section Steinwand-Mischenirwiese (Fig. 4) mass flows are missing. In the more northern areas of the Northern Calcareous Alps (Fig. 3), radiolarite deposition starts relatively early, indicating an early deepening event due to the tectonic load of the advancing Hallstatt nappes. First imbricates started to form in the Bathonian–Callovian (I and II in Fig. 5), as indicated by age dating of the matrix radiolarites. In the Oxfordian (III–IIIa in Fig. 5) the mass flows also contain components of the older radiolarite “mélange” basins and the section Mischenirwiese attained a more basinal position, as indicated by the sedimentological features of the radiolarite succession. In the late Oxfordian the nappe front reached the depositional area of the Mischenirwiese section, as shown in the overlying mélange (IV in Fig. 5). In the Kimmeridgian–Tithonian, nappe propagation stopped and the nappe stack was sealed by a carbonate platform. In the Kimmeridgian, coarse reefal debris was shed into this deep-water basin (O´Dogherty and Gawlick, Reference O’Dogherty and Gawlick2008) with a general fining-upward trend. Due to uplift and demise of the southern Platform in the late Tithonian (Fig. 5; Gawlick and Missoni Reference Missoni and Gawlick2011a), only fine-grained siliceous limestones were deposited in the basin, as proven with radiolarian dating in this study (see the youngest radiolarian sample of the Steinwand locality).

Figure 5 Reconstruction of the geologic evolution from Bathonian to Tithonian times. The radiolarite section Mischenirwiese is a complete radiolarite succession, reworked material from the advancing nappe stack, which consists of different Hallstatt imbricates, is missing. In Oxfordian times the succession was overridden by the Hallstatt mélange, which formed contemporaneously more to the south as indicated by the different radiolarite matrix ages. In Kimmeridgian–Tithonian times this tectonic nappe stack was sealed by the Plassen Carbonate Platform sensu latu (Lärchberg Formation; see Figure 2).

Figure 6 Scale bars (a, b) measure 50 µm; each radiolarian scanning micrograph is designated by sample and specimen number, followed by the corresponding scale. (1−6) Eoxitus hungaricus Kozur, sample BMW-28 (1, 073/a; 2, 072/a; 6, 043/a); sample EW-158 (3, 005/a; 5, 017/a); sample BMW-31 (4, 008/a). (7−11) Eoxitus baloghi Kozur, sample BMW-28 (7, 045/a; 8, 070/a; 11, 061/a); sample BMW-26 (9, 011/a); sample BMW-13b (10, 017/a). (12−14) Mizukidella kamoensis (Mizutani and Kido), sample BMW-28 (12, 064/a); sample EW-158 (13, 009/a; 14, 015/a). (15−16) Eoxitus dhimenaensis (Baumgartner), sample BMW-32 (15, 077/a); sample BMW-13c (16, 052/a). (17) Eoxitus? sp. A, sample BMW-31 (17, 011/a). (18−21) Takemuraella schardti (O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al.) sample BMW-35 (18, 006/a); sample BMW-26 (19, 008/a; 20, 016/a; 21, 055/a). (22−24) Takemuraella spinifera (Takemura), sample BMW-28 (22, 060/a); sample BMW-31 (23, 018/a); sample EW-158 (24, 060/a). (25) Caneta hsui (Pessagno), sample BMW-35 (25, 015/a). (26−27) Mizukidella mokaensis n. sp., sample BMW-33 (26, 004/b, paratype); sample BMW-35 (27, 001/a, holotype). (28) Eoxitus? sp. B, sample BMW-31 (28, 028/b). (29−30) Cinguloturris carpatica Dumitrica in Dumitrica and Mello, sample BMW-13c (29, 026/a; 30, 018/a). (31−32) Cinguloturris getsensis O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., sample BMW-26 (31, 025/a); sample BMW-30 (32, 001/b). (33) Canoptum krahsteinense (Suzuki and Gawlick in Gawlick et al.), sample BMW-13b (33, 19/b). (34) Cinguloturris latiannulata (Grill and Kozur), sample BMW-26 (34, 010/b). (35−38) Xitomitra tairai (Aita), sample BMW-13c (35, 055/a; 37, 043/a; 38, 070/a); sample BMW-33 (36, 001/a). (39) Parvimitrella wallacheri (Grill and Kozur), sample BMW-13c (39, 036/b). (40−43) Campanomitra? sp. A, sample BMW-13c (40, 021/a; 41, 054/a; 42, 010/a; 43, 030/a). (44) Campanomitra sp. aff. C. buekkensis (Grill and Kozur), sample BMW-35 (44, 012/a). (45−48) Pseudodictyomitrella limana (Cortese), sample BMW-13b (45, 032/b); sample BMW-28 (46, 014/b); sample BMW-26 (47, 048/b; 48, 039/b). (49−50) Takemuraella japonica (Takemura), sample BMW-13b (49, 013/b); sample BMW-13c (50, 034/a). (51−54) Xitomitra annibill (Kocher), sample BMW-13c (51, 088/a; 52, 028/a; 53, 044/a; 54, 042/a). (55) Xitomitra? sp. A, sample BMW-35 (55, 020/a). (56−61) Campanomitra? spp., sample BMW-26 (56, 054/b); sample EW-158 (57, 026/b); sample BMW-35 (58, 026/a); sample BMW-13c (59, 045/a; 60, 092/b); sample BMW-13b (61, 050/b). (62−64) Campanomitra sp. aff. C. tuscanica (Chiari, Cortese, and Marcucci in Chiari et al.), sample BMW-32 (62, 041/b); sample BMW-35 (63, 023/b); sample BMW-13b (64, 012/b). (65−69) Campanomitra tuscanica (Chiari, Cortese, and Marcucci in Chiari et al.), sample BMW-32 (65, 030/a); sample BMW-13c (66, 126/a; 67, 077/b); sample BMW-28 (68, 001/a); sample BMW-33 (69, 008/b). (70−73) Campanomitra sp. aff. C. ulivii (Chiari, Cortese, and Marcucci in Chiari et al.), sample BMW-34 (70, 014/b; 71, 012/b; 72, 034/b); sample BMW-32 (73, 102/b).

Figure 7 Scale bars (a, b, c) measure 50 µm; each radiolarian scanning micrograph is designated by sample and specimen number, followed by the corresponding scale. (1−3) Xitus skenderbegi (Chiari, Marcucci, and Prela), sample BMW-26 (1, 009/c; 3 , 066/c); sample EW-158 (2, 014/c). (4) Doliocapsa keni (Kocher), sample BMW-34 (4, 035/c). (5−7) Pseudodictyomitrella renevieri O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., sample EW-158 (5, 055/c); sample BMW-26 (6, 036/c); sample BMW-28 (7, 003/c). (8) Pseudodictyomitrella sp. cf. P. renevieri O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., sample BMW-28 (8, 046/c). (9) Eoxitus? brevis Kozur, sample BMW-26 (9, 038/b). (10–11) Takemuraella sp. cf. T. schardti (O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al.), sample BMW-28 (10, 063/c; 11, 053/c). (12–13) Xitus sp. A, sample BMW-34 (12, 008/c; 13, 005/c). (14) Olanda sp., sample BMW-28 (14, 067/c). (15–16) Semihsuum amabile (Aita), sample BMW-26 (15, 070/c); sample EW-158 (16, 007/c). (17) Archaeodictyomitra publica (Hull), sample BMW-13c (17, 099/c). (18−20) Archaeodictyomitra sp. aff. A. exigua Blome, sample BMW-13b (18, 013/c; 19, 028/c); sample BMW-28 (20, 062/c). (21) Archaeodictyomitra sp. cf. A. annulata Kozur and Mostler in Grill and Kozur, sample BMW-30 (21, 59/c). (22−32) Thanarla patricki gr. (Kocher), sample BMW-33 (22, 022/c); sample BMW-35 (23, 028/c; 24, 024/c; 25, 037/c; 26, 030/c; 29, 007/c; 32, 025/c); sample EW-158 (27, 063/c); sample BMW-13c (28, 129/c); sample BMW-13b (30, 004/c; 31, 022/c). (33−35) Archaeodictyomitra rigida Pessagno, sample BMW-30 (33, 039/c); sample BMW-28 (34, 042/c); sample BMW-35 (35, 021/c). (36−37) Archaeodictyomitra praeapiarium Cordey, sample BMW-26 (36, 024/b); sample EW-158 (37, 059/b). (38−39) Archaeodictyomitra tyaughtonensis Cordey, sample BMW-35 (38, 005/b; sample BMW-33 (39, 005/b). (40−42) Archaeodictyomitra minoensis (Mizutani), sample BMW-30 (40, 057/b); sample BMW-35 (41, 017/b; 42, 010/b). (43−45) Archaeodictyomitra prisca Kozur and Mostler in Grill and Kozur, sample BMW-13c (43, 075/b; 44, 051/b; 45, 017/b). (46–47) Archaeodictyomitra whalenae Kozur and Mostler in Grill and Kozur, BMW-31 (46, 026/b); sample BMW-13c (47, 049/b). (48) Archaeodictyomitra spelae Chiari, Cortese and Marcucci in Chiari et al., sample BMW-26 (48, 043/b). (49) Parahsuum sp. 1 sensu O’Dogherty et al., sample BMW-34 (49, 006/c). (50) Parahsuum snowshoense (Pessagno and Whalen), sample BMW-31 (50, 021/b). (51) Parahsuum sp. aff. P. snowshoense (Pessagno and Whalen), sample BMW-13c (51, 031/b). (52–53) Parahsuum carpathicum Widz and De Wever, sample BMW-13c (52, 050/b; 53, 076/b). (54) Hsuum arabicum Dumitrica in Dumitrica et al., sample BMW-35 (54, 009/a). (55) Hsuum obispoense Pessagno, sample BMW-34 (55, 7/b). (56−60) Parahsuum sp. 2 sensu O’Dogherty et al., sample BMW-13c (56, 020/b; 57, 025/b; 58, 033/b); sample BMW-31 (59, 016/b; 60, 070/b). (61) Parahsuum sp. aff. P. probosum (Pessagno and Whalen), sample BMW-28 (61, 054/c). (62–63) Parahsuum sp. 3 sensu O’Dogherty et al., sample BMW-13c (62, 035/b; 63, 080/b). (64−66) Loopus martae Beccaro, sample BMW-34 (64, 003/b; 65, 009/b); sample BMW-33 (66, 007/b). (67−70) Loopus venustus (Chiari, Cortese, and Marcucci in Chiari et al.), sample BMW-13b (67, 001/b); sample BMW-32 (68, 009/c); sample BMW-13c (69, 082/c); sample BMW-35 (70, 011/c). (71) Loopus mexicanus (Hull), sample BMW-34 (71, 020/c).

Figure 8 Scale bars (a, b) measure 50 µm; each radiolarian scanning micrograph is designated by sample and specimen number, followed by the corresponding scale. (1−3) Transhsuum brevicostatum (Ožvoldová), sample BMW-26 (1, 013/b); sample BMW-28 (2, 068/a); sample BMW-34 (3, 002/a). (4−8, 10−18) Transhsuum maxwelli (Pessagno), BMW-13c (4, 083/a; 6, 021/a; 7, 048/a; 18, 024/a); sample BMW-35 (5, 003/a; 13, 027/a; 15, 004/a; 16, 002/a); sample BMW-26 (8, 015/a; 11, 060/a); sample BMW-28 (10, 071/a); sample EW-158 (12, 062/a; 14, 012/a; 17, 061/a). (9) Transhsuum sp. E sensu (Yao), sample BMW-28 (9, 066/a). (19) Eucyrtidiellum pyramis (Aita in Aita and Okada), sample BMW-33 (19, 019/b). (20) Eucyrtidiellum ptyctum (Riedel and Sanfilippo), BMW-13c (20, 139/b). (21−23) Eucyrtidiellum nodosum Wakita, sample BMW-13c (21, 074/b; 22, 005/b; 23, 134/b). (24−25) Eucyrtidiellum unumaense (Yao), sample BMW-28 (24, 017/b); sample BMW-31 (25, 045/b). (26−27) Eucyrtidiellum pustulatum Baumgartner, sample BMW-33 (26, 028/b); sample BMW-13b (27, 060/b). (28) Spinosicapsa basilica (Hull), sample BMW-13c (28, 064/a). (29) Spinosicapsa sp. cf. S. triacantha (Fischli), sample BMW-13c (29, 046/a). (30) Spinosicapsa lata (Yang), sample BMW-33 (30, 018/a). (31) Spinosicapsa spinosa (Ožvoldová), sample BMW-13c (31, 057/a). (32) Yamatoum sp. A, sample BMW-28 (32, 047/b). (33–34) Doliocapsa matsuokai (Yeh), sample BMW-13b (33, 049/b); sample BMW-31 (34, 048/b). (35−37) Doliocapsa planata (Wu), sample BMW-13c (35, 131/a; 37, 089/a); sample BMW-34 (36, 029/a). (38−42) Yamatoum spinosum (Takemura), sample EW-158 (38, 022/b); sample BMW-13b (39, 054/b); sample BMW-26 (40, 073/b; 41, 049/b); sample BMW-28 (42, 051/b). (43–44) Crococapsa sp. C, sample EW-158 (43, 067/b; 44, 070/b). (45) Crococapsa sp. B, sample BMW-35 (45, 032/b). (46–47), Crococapsa tansinhoki (Hull), sample BMW-33 (46, 023/b); sample BMW-35 (47, 022/b). (48) Crococapsa sp. aff. C. truncata (Wu), sample BMW-28 (48, 057/b). (49−52) Crococapsa sp. A, sample BMW-13c (49, 009/b; 50, 073/b; 51, 007/b; 52, 004/b). (53) Belleza decora (Rüst), sample BMW-13c (53, 012/b).

Figure 9 Scale bar measures 50 µm; each radiolarian scanning micrograph is designated by sample and specimen number. (1−6) Hemicryptocapsa buekkensis (Kozur), sample BMW-28 (1, 029; 2, 028); sample BMW-26 (3, 033); sample BMW-31 (4, 032); sample BMW-32 (5, 018); sample BMW-30 (6, 010). (7–8) Zhamoidellum sp. A, sample BMW-32 (7, 051); sample BMW-33 (8, 031). (9) Kilinora? oblongula (Kocher in Baumgartner et al.), sample BMW-32 (9, 005). (10–11) Zhamoidellum sp. aff. Z. ovum Dumitrica, sample BMW-30 (10, 015); sample BMW-32 (11, 086). (12−16) Hemicryptocapsa marcucciae (Cortese), sample BMW-32 (12, 087); sample BMW-26 (13, 074); sample BMW-13c (14, 141); sample BMW-13b (15, 065); sample BMW-30 (16, 043). (17–18) Crococapsa sp. aff. C. tansinhoki (Hull), sample BMW-13c (17, 140; 18, 011). (19) Hemicryptocapsa nonaginta n. sp., BMW-13b (19, 039, paratype). (20−23) Hemicryptocapsa carpathica (Dumitrica), sample BMW-13c (20, 100; 21, 123; 22, 119; 23, 091). (24) Quarkus madstonensis Pessagno, Blome, and Hull in Pessagno et al., sample BMW-31 (24, 029). (25–26) Quarkus japonicus (Yao), sample BMW-13b (25, 026); sample BMW-31 (26, 067). (27–28) Praewilliriedellum convexum (Yao), sample BMW-13b (27, 027); sample BMW-28 (28, 038). (29–30) Quarticella sp. B, sample BMW-28 (29, 056; 30, 010). (31−37) Hemicryptocapsa yaoi (Kozur), sample BMW-13c (31, 116; 32, 112; 34, 118); sample EW-158 (33, 028); sample BMW-33 (35, 011); sample BMW-13b (36, 073); sample BMW-32 (37, 061). (38) Gongylothorax sp. A, sample BMW-34 (38, 019). (39−42), Gongylothorax favosus Dumitrica, sample BMW-32 (39, 058); sample BMW-30 (40, 045); sample BMW-34 (41, 025); sample BMW-33 (42, 036). (43–44) Williriedellum yahazuense (Aita), sample BMW-34 (43, 032); sample BMW-33 (44, 009). (45−48) Williriedellum sp. cf. W. formosum (Chiari, Marcucci, and Prela), sample BMW-28 (45, 049; 46, 033; 47, 058); sample BMW-13b (48, 067). (49) Williriedellum sp. B, sample BMW-13b (49, 053). (50–51) Williriedellum crystallinum Dumitrica, sample BMW-32 (50, 032); sample BMW-30 (51, 007). (52) Quarticella ovalis Takemura, sample BMW-28 (52, 034). (53) Hiscocapsa kodrai (Chiari, Marcucci, and Prela), sample BMW-13b (53, 030). (54–55) Quarticella sp. C, sample BMW-13b (54, 041); sample BMW-28 (55, 050). (56), Quarticella cicciona (Chiari, Marcucci, and Prela), sample BMW-13b (56, 033). (57) Quarticella sp. D, sample BMW-28 (57, 041). (58) Quarticella sp. A, sample EW-158 (58, 065).

Figure 10 Scale bars (a, b) measure 50 µm; each radiolarian scanning micrograph is designated by sample and specimen number, followed by the corresponding scale. (1–2) Williriedellum? sp. C, sample BMW-13b (1, 066/a); sample BMW-28 (2, 007/a). (3) Arcanicapsa exquisita (Hull), sample BMW-32 (3, 013/a). (4) Arcanicapsa sp. A, sample BMW-13b (4, 029/a). (5−16) Arcanicapsa funatoensis (Aita), sample BMW-26 (5, 035/a; 6, 031/a; 10, 037/a; 13, 035/a; 15, 032/a); sample EW-158 (7, 021/a; 8, 029/a; 9, 019/a); sample BMW-34 (11, 011/a); sample BMW-13b (12, 103/a); sample BMW-13c (14, 106/a; 16, 132/a). (17−19) Zhamoidellum ventricosum Dumitrica, sample BMW-13c (17, 121/a; 18, 114/a; 19, 136/a). (20−22) Arcanicapsa undulata (Heitzer), sample BMW-32 (20, 024/a); sample BMW-33 (21, 032/a); sample BMW-34 (22, 016/a). (23–24) Williriedellum sp. A, sample BMW-26 (23, 059/a); sample BMW-13b (24, 074/b). (25−26) Kilinora sp. aff. K. oblongula (Kocher in Baumgartner et al.), sample EW-158 (25, 027/b); sample BMW-32 (26, 047/b). (27−30) Zhamoidellum sp. B, sample BMW-13c (27, 093/b); sample BMW-35 (28, 033/b); sample BMW-32 (29, 084/b); sample BMW-30 (30, 013/b). (31−40) Zhamoidellum ovum Dumitrica, sample BMW-33 (31, 021/a; 32, 012/a; 33, 016/a; 35, 017/a; 39, 034/a); sample BMW-32 (34, 046/a); sample BMW-13c (36, 130/a; 37, 101/a); sample BMW-35 (38, 034/a); sample BMW-34 (40, 018/a). (41−44) Zhamoidellum sp. C, sample BMW-26 (41, 005/a; 44, 063/a); sample BMW-35 (42, 035/a); sample EW-158 (43, 004/a). (45−47) Praewilliriedellum robustum (Matsuoka), sample EW-158 (?45, 025/b); sample BMW-31 (46, 071/a); sample BMW-34 (47, 017/a).

Figure 11 Scale bars measure 50 µm for a magnification of x200 (a) and x250 (b). Each radiolarian scanning micrograph is designated by the sample and the specimen number followed by the corresponding scale. (1−3) Gongylothorax marmoris Kiessling in Kiessling and Zeiss, sample BMW-32 (1, 097/b; 2, 002/b; 3, 057/b). (4) Japonocapsa sp. A, sample BMW-13b (4, 072/b). (5−7) Striatojaponocapsa conexa (Matsuoka), sample EW-158 (5, 003/a; 6, 001/a); sample BMW-32 (7, 089/a). (8–9) Striatojaponocapsa riri O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., sample BMW-32 (8, 056/b; 9, 008/b). (10−13) Striatojaponocapsa synconexa O’Dogherty, Goričan, and Dumitrica in O’Dogherty et al., sample EW-158 (10, 006/a; 12, 056/b; 13, 071/b); sample BMW-26 (11, 023/b). (14−16) Striatojaponocapsa spp., sample BMW-32 (14, 101/b; 16, 060/b); sample BMW-34 (15, 030/b). (17−20) Striatojaponocapsa? spp., sample BMW-32 (17, 025/a; 19, 059/b); sample BMW-13b (18, 024/b); sample EW-158 (20, 073/b). (21−26) Unuma latusicostatus (Aita), sample BMW-13b (21, 059/a; 25, 069/a); sample EW-158 (22, 023/a); sample BMW-28 (23, 055/a; 24, 005/a; 26, 026/a). (27–28) Arcanicapsa sp. aff. A. funatoensis (Aita), sample BMW-28 (27, 011/a; 28, 031/a). (29−38, 45) Unuma gordus Hull Reference Hull1997, sample BMW-31 (29, 054/b; 37, 031/a); sample BMW-28 (30, 039/a; 35, 048/a); sample EW-158 (31, 024/a; 36, 058/a); sample BMW-13b (32, 025/a; 34, 055/a); sample BMW-26 (33, 061/a; 38, 072/a); sample BMW-32 (45, 066/a). (39–40) Protunuma turbo Matsuoka, sample BMW-13b (39, 058/a; 40, 052/a). (41) Protunuma japonicus Matsuoka and Yao, sample BMW-33 (41, 014/a). (42) Protunuma europeus O’Dogherty, Goričan and Dumitrica in O’Dogherty et al., sample BMW-13b (42, 061/b). (?43–44) Protunuma ochiensis Matsuoka, sample BMW-33 (43, 013/a); sample BMW-28 (44, 052/a). (46), Theocapsomella cordis (Kocher in Baumgartner et al.), sample BMW-13b (46, 020/b). (47) Theocapsomella medvednicensis (Goričan in Halamić et al.), sample BMW-32 (47, 003/b).

Figure 12 Scale bars (a, b, c, d) measure 50 µm; each radiolarian scanning micrograph is designated by sample and specimen number, followed by the corresponding scale. (1) Saitoum pagei Pessagno, sample BMW-28 (1, 015/c). (2–3) Saitoum trichylum De Wever, sample BMW-28 (2, 004/c); sample BMW−26 (3, 051/c). (4) Bernoullius cristatus Baumgartner, sample EW-158 (4, 038/d). (5–6) Gorgansium silviesense Pessagno and Blome, sample BMW-28 (5, 009/c); sample BMW-13b (6, 008/c). (7–8) Alievium? longispineum Yang and Wang, sample BMW-26 (7, 040/c); sample BMW-28 (8, 023/b). (9) Alievium? sp. aff. A. crassum (Kiessling), sample BMW-26 (9, 069/d). (10–12) Archaeospongoprunum elegans Wu, sample EW-158 (10, 051/b); sample BMW-28 (11, 035/b); sample BMW-34 (12, 023/b). (13−15) Pantanellium riedeli Pessagno, sample EW-158 (13, 050/b); sample BMW-28 (14, 036/b); sample BMW-31 (15, 001/b). (16−18) Hexasaturnalis tetraspinus (Yao), sample EW-158 (16, 033/a; 17, 039/a; 18, 035/c). (19–20) Hexasaturnalis nakasekoi Dumitrica and Dumitrica-Jud, sample BMW-28 (19, 021/a); sample EW-158 (20, 041/a). (21–22) Hexasaturnalis suboblongus (Yao), sample BMW-28 (21, 019/a); sample BMW-158 (22, 036/a). (23) Hexasaturnalis minor (Baumgartner in Baumgartner et al.), sample BMW-13c (23, 071/a). (24−27) Tritrabs ewingi (Pessagno), sample BMW-32 (24, 080/b); sample BMW-28 (25, 016/b; 26, 008/b; 27, 006/b). (28−30) Angulobracchia spp., sample BMW-28 (28, 002/c; 30, 018/c); sample BMW-26 (29, 057/c). (31) Paronaella pristidentata Baumgartner, sample EW-158 (31, 044/b). (32) Homoeoparonaella elegans (Pessagno), sample BMW-28 (32, 012/b). (33–34) Paronaella kotura Baumgartner, sample EW-158 (33, 042/a); sample BMW-34 (34, 033/a). (35–36), Homoeoparonaella spp., sample BMW-31 (35, 060/b; 36, 073/b). (37) Crucella theokaftensis Baumgartner, sample BMW-13c (37, 061/b). (38) Emiluvia sedecimporata (Rüst), sample BMW-32 (38, 069/b). (39–40) Emiluvia salensis Pessagno, sample BMW-26 (39, 044/b); sample EW-158 (40, 030/b). (41), Emiluvia? sp., sample BMW-28 (41, 024/c).
Acknowlegments
This work is a contribution of the research projects P14131-TEC, P15060, and P16812 (Austrian Science Foundation FWF projects), research program P1-0008 (Slovenian Research Agency), and CGL2011-23759 (Spanish Ministry of Science and Technology). We are greatly indebted to Elizabeth S. Carter for her positive comments and careful review. Steve Hageman, editor of the Journal, is also gratefully acknowledged for his suggestions and comments improving the final version of this paper.
















