Background
Zinc is a vital trace element(Reference Bailey, West and Black1), required for the activity of more than 300 enzymes for multiple aspects of metabolism and physiological process(Reference Roohani, Hurrell and Kelishadi2). It has three very fundamental functions: catalytic, structural and regulatory(Reference Cousins, Bowman and Russell3). It plays roles in cellular proliferation and differentiation(Reference Hotz and Brown4), gene expression(Reference Prasad5), reproductive function and neurobehavioral development, anti-inflammatory and antioxidant function(Reference Mistry, Kurlak and Young6), sensory activities like vision, smell, appetite and taste(Reference Taghizadeh, Samimi and Tabassi7,Reference Lewicka, Kocylowski and Grzesiak8) .
Pregnancy is the most nutritionally demanding period in a women's life(Reference Bhutta, Das and Rizvi9). Adequate level of zinc in the serum during this physiologically demanding period is vital for the optimal health and well-being of both the mother and the fetus(Reference Brown, Rivera and Bhutta10). It is required for normal growth and development from in utero until puberty(Reference Bailey, West and Black1), and maternal tissue accretion(Reference Brown, Rivera and Bhutta10). The Zinc Deficiency is associated with adverse maternal and pregnancy outcomes such as pregnancy complications, intrauterine growth retardation, low birth weight, congenital anomalies(Reference Tamura and Goldenberg11–Reference Castillo-Durán and Weisstaub13), increased neonatal morbidity, and poor neurobehavioral development(Reference Shah and Sachdev14,Reference Fischer Walker, Ezzati and Black15) , fetal death, impaired immune function, and impairment of learning and memory function(Reference Fischer Walker, Ezzati and Black15,Reference Caulfield, Zavaleta and Shankar16) . For a normal metabolic and physiological function, serum zinc concentration should not be lower than 56 μg/dl during the first trimester or 50 μg/dl during the second or third trimester(Reference Christine and Kenneth17).
Zinc deficiency (ZD) remained as public health concern affecting 17 % of the global population with the highest prevalence in Sub-Saharan Africa and South Asia(Reference Ryan Wessells and Brown18). Ethiopia is one of the developing countries where ZD among pregnant women is a public health problem(Reference Berhe, Gebrearegay and Gebremariam19). To address this and other micronutrient deficiencies, the government has been taking multiple national initiatives such as launching a national nutrition programme, transforming the health system, and implementing food and nutrition policy since 2018(20–22). In spite of the many efforts, the problem is persisting. For instance, surveys and meta-analysis reported that 55⋅3–72 % of pregnant women in Ethiopia are zinc-deficient(Reference Berhe, Gebrearegay and Gebremariam19,Reference Mekonnen, Terefe and Belachew23–Reference Gebremedhin, Enquselassie and Umeta26) .
Moreover, there is a shortage of information on zinc status of pregnant women who resided in metropolitan cities in Ethiopia like Hawassa. Therefore, the present study was designed to assess the prevalence and associated factors of ZD among pregnant women who resided around Lake Awassa in Hawassa City living around Lake Awasa, Ethiopia.
Materials and Methods
Study area
The study was conducted in Hawassa City, Sidama Region, Ethiopia. The city is located 275 km south of Addis Ababa. It consists of eight subcities. According to the 2007 population projection, the total number of people living in the city is estimated to be 258 808 (133 123 males and 125 685 females)(27). There are two public hospitals (a comprehensive specialised hospital, and a general hospital), twelve health centres and four private hospitals in the city.
Lake Awasa is located in the Central Main Ethiopia Rift valley and lies between latitude 6°48′45″–7°14′49″N and longitude 38°16′34″–38°43′26″E. The lake watershed covers a total area of 1407⋅23 km2 of which 112⋅78 km2 (8 %) is the Lake's surface. It is a closed-watershed Lake with no surface water outflow.
Study design and period
This is a health facility-based cross-sectional study. The data collection was carried out from 08 April to 08 May 2021.
Source and study population
The source population was all pregnant women living around Lake Awasa in Hawassa City. The study populations were randomly selected pregnant women attending antenatal care (ANC) during the survey period.
Inclusion and exclusion criteria
All pregnant women who lived for at least 6 months around Lake Awasa were eligible for the present study. Severely sick women, and women who were on dietary restriction, were excluded from the study.
Sample size determination
A sample size of 376 was computed using a single population proportion formula(Reference Lemeshew, Hoamer and Klar28). In the calculation, the prevalence of ZD among pregnant women in Gondar town, P = 57⋅4 %(Reference Kumera, Awoke and Melese24), margin of error, d = 0⋅05, critical value at 95 % confidence interval, Z 1–α/2 = 1⋅96, and non-response rate of 10 % was considered. Since the number of the source population was <10 000, the sample size was adjusted to 345, employing a finite population correction formula. However, the final analysis considered 333 (96⋅5 %) participants as the blood samples from 12 women were discarded due to haemolysation during serum extraction.
Sampling technique
The participants were selected by a systematic random sampling technique from four health facilities nearest to Lake Awasa. Based on the performance report, the expected number of ANC attendants in a month is 1383. Having this in mind, the sampling interval of 4 was computed by dividing the expected number of 1-month ANC attendants by the calculated sample size (K = 1383/345). Then, a random number was generated between a number 1 and 4 in order to select the first respondent. Finally, every fourth pregnant woman attending ANC was included until the required sample size was achieved.
Variables
The outcome variable was serum zinc status. And, the infections and dietary intakes, household wealth status, socio-demographic variables, obstetrics and clinical characteristics, water, sanitation and hygiene (WASH) practices and facilities, and fasting status were considered as factors.
Data collection
The socio-demographic data was collected using a structured questionnaire. Besides, the household wealth status was assessed by adapting the questionnaire from the 2016 Ethiopian demographic and health survey tool(29).
The animal source foods (ASFs) (meat, fish, egg milk and milk products, chicken and egg) consumption a month prior to the survey period was assessed using the food frequency questionnaire(Reference Gibson30). A minimum woman dietary diversity score (MDDS) was studied following the Food and Agricultural Organization (FAO) and Nutrition Technical Assistant III project (FANTA III) guideline. In this, the pregnant women were asked about the types of foods eaten from breakfast to bed in the past 24 h. The average number of food groups consumed by the wealthiest quintiles was calculated. Then, the dietary diversity score was categorised as ‘adequate’ if the number of food groups consumed by the women was ≥ the mean food groups consumed by the wealthiest quintiles, and ‘poor’ otherwise(31).
The blood sample was collected by an experienced laboratory technician following standard procedures. About 5 ml of blood was collected from the antecubital vein using a plain and closed SARSTEDT Monovette® blood collection system and SARSTEDT® butterfly stainless steel needles. After collection, the blood samples were allowed to clot for 20 min in a closed ice box and centrifuged at 3000 revolutions per minute (RPM) for 10 min. Then, serum was extracted and transferred into labelled screw-top vials; and a few obviously haemolysed samples (n = 12) were identified and discarded. On the same day, they were stored frozen at −20°C and kept frozen at −80°C until analysis. The biochemical analysis was carried out in the Ethiopian Public Health Institute, Addis Ababa. The analysis was done using ShimatznSpectrAA® Flame Atomic Absorption Spectrometers (AAS)(Reference Williams32). The samples were analysed at a wavelength of 213⋅9 nm, with a lamp current of 5 mA and a slit width of 1 nm. Initially, standards of 0, 0⋅1, 0⋅2, 0⋅3 and 0⋅4 PPM was prepared by diluting 1000 PPM zinc standard in 2 ml of 6 % butanol. Then, the standards were used for calibration. The samples were prepared with 200 μl serum samples in 2 ml of 6 % butanol. The samples were analysed in a batch of 25. The control sample with a known concentration and multiple blank samples were analysed with every batch. At times when an unexpected concentration reported either for the control or blank samples, the sample preparation and analyses was repeated for the whole batch. The AAS system was flushed with 6 % butanol after every analysis. Finally, ZD was defined as a serum zinc level <56 μg/dl during the first trimester, or <50 μg/dl in the second or third trimester(33).
The pregnant women were asked whether they took any food or beverage in the preceding 8 h of the blood sample collection to assess their fasting status(34). The C-reactive protein (CRP) was determined qualitatively using CRP-Latex following the procedure provided with the kit(35).
The stool specimens were collected on the spot during the ANC visit, and examination was conducted following the WHO (1994) bench aids for the diagnosis of intestinal parasites procedures. The intestinal parasitic infection was tested in health centres where the women were attending. About 2 g of stool sample was collected from each respondent using a clean, dry and leak-proof cupped plastic container. About 50 mg of stool was mixed with normal saline (0⋅85 % NaCl), and processed using direct saline wet mount to diagnose the intestinal parasites. In the case of the formol-ether concentration technique, 1 g of stool was preserved by 10 % formalin, and diagnosed for intestinal parasites(36).
Data quality control
The questionnaires were firstly prepared in English and translated into the local language (Amharic). Then, it was back-translated into English to check the consistency. The survey tool was pre-tested by 5 % of the sample size on the pregnant women attending ANC in a health facility other than the ones sampled for the present study. The test re-test reliability method was used to conduct a reliability test. The reliability coefficient was calculated using Cronbach's alpha, and the questionnaire out of the acceptable values range, α < 0⋅7, was revised. The validity of the questionnaire was checked by the Pearson's correlation for each domain of questions. The questionnaires with a correlation coefficient of >0⋅05 were declared valid. Eight data collectors were trained for 3 d on the overall questionnaire, stool and blood sample collection. The data collection team consisted of medical laboratory experts, nurses and human nutrition profession. All interviews and specimen collection was carried out at health centres where the women were attending the ANC. The data collection, completeness and consistency were daily supervised by the principal investigator.
Data analysis
The data were coded and entered into the statistical package for social sciences (SPSS) software program for version 20 Armonk, NY: IBM Corporation(37). Data were checked for missing values, and outliers. The normality of data was checked using Kolmogorov–Smirnov test. Multicollinearity was checked by the variance inflation factors (VIFs) test. For wealth index construction, the household's durable assets, productive assets, and water and hygiene service facilities were dichotomised. The wealth index was constructed using a principal component analysis (PCA). Finally, the households were classified as poorest, poor, medium, rich and richest. The serum zinc status was dichotomised as ‘deficient’ and ‘normal’. Binary logistic regression analysis was conducted to assess the association of independent variables with the outcome variable. In order to control confounders, variables with a P-value <0⋅20 in the binary logistic regression were entered into the final multivariable logistic regression model Statistical significance was declared at P-value ≤ 0⋅05. The strength of association was presented using adjusted odds ratio (AOR).
Ethical considerations
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Institutional Review Board of Hawassa University (IRB/279/12). Informed written consent was obtained from all participants. Information provided by the participants was held confidential using pseudonymous code.
Results
Socio-demographics
The present study was completed with a 96⋅5 % response rate. Nearly two-thirds (63⋅8 %) of the pregnant women were in the age range of 22–35 years, and 14⋅4 % were above 35 years. Almost 79 % of them were married and 8⋅7 % did not attend formal education. Concerning the economic status, 18⋅6 and 19⋅8 % of respondents were from poor and poorest households, respectively. The mean family size was 4⋅35 ± 1⋅8 (Table 1).
Table 1. Socio-demographics of the study participating pregnant women around Lake Awasa, Hawassa City, Sidama Region, Ethiopia, 08 April–08 May 2021 (n 333)

WASH
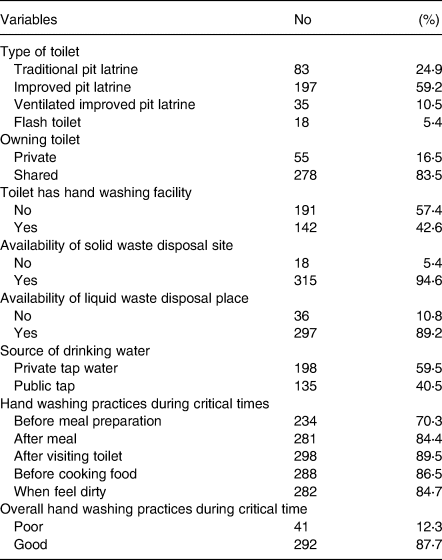
Of the total, 16⋅5 % had private, but 83⋅5 % shared toilet with over half (59⋅2 %) having improved pit latrine. Nearly 60 % had private tape water supply. A majority (87⋅7 %) of the women reported that they practice hand washing during critical times. Nearly 74 and 84⋅9 % of the women wash their hands after visiting the toilet, and when they felt dirty, respectively (Table 2).
Table 2. WASH practices among pregnant women around Lake Awasa, Hawassa City, Sidama Region, Ethiopia, 08 April–08 May 2021 (n 333)
Obstetrics
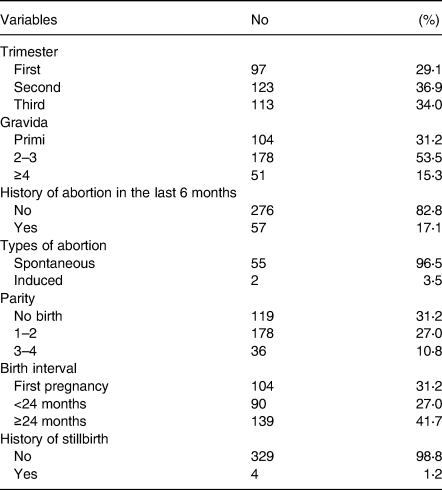
About 29⋅1, 36⋅9 and 33⋅9 % of women were in the first, second and third trimester, respectively. Nearly half (53⋅5 %) had 2–3 Gravida with mean (±sd) pregnancies of 2⋅2 (±1⋅1). Of the total, 16⋅9 % had an abortion history in the last 6 months of which 96⋅5 % occurred spontaneously. Nearly 58 % experienced a shorter than 2 years birth interval, while 1⋅2 % history of stillbirth (Table 3).
Table 3. Obstetrics of the study participating pregnant women around Lake Awasa, Hawassa City, Sidama Region, Ethiopia, 08 April–08 May 2021 (n 333)
Dietary intake
Dietary diversity score for about 46% of the pregnant women was poor with a mean (±SD) score of 5.94 (±1.47). Nearly all (97⋅9 %) of the participants consumed starchy staple foods (cereals, roots and tubers), while the majority (86⋅8%) consumed pulses and 76⋅9 % other fruits (Fig. 1). Among ASFs, dairy intake was the highest (71⋅8 %), but meat intake was the lowest (19⋅2 %). Regarding the consumption frequency of ASFs, 48, 27, 3⋅3, 5⋅4, and 72⋅4 % of the respondents never ate poutry, meat, fish, dairy and egg, respectively, in a month before the day of the data collecion. Almost 92 % of the women consumed three meals in a day. However, only 13⋅8 % consumed at least one additional meal. Of nearly 85 % that reported drinking coffee, 26⋅5 % took it with food, but 60⋅1 % an hour after a meal. Likewise, 58⋅6 % of the women drink tea with food, but 30⋅6 % 1 h after a meal. Less than half of the women reported receiving nutrition education and dietary counselling (Table 4).

Fig. 1. Food groups consumed in the last 24 h by the study participating pregnant women around Lake Awasa, Sidama Region, Ethiopia (08 April–08 May 2021) (n 333).
Table 4. Dietary intake of study participating pregnant women around Lake Awasa, Hawassa City, Sidama Region, Ethiopia, 08 April–08 May 2021 (n 333)

ASF, animal source food.
Infections
Nearly 28 % of the pregnant women experienced diarrhoea in the last 2 weeks before the survey. About 2⋅1 % of respondents tested positive for human immunodeficiency virus (HIV), and nearly half (47⋅4 %) tested positive for intestinal parasitic infection. The prevalence of Ascariasis was 16⋅5 %, while Schistosomiasis was 8⋅7 % and Giardiasis 7⋅8 % (Table 5).
Table 5. Infections in pregnant women around Lake Awasa, Hawassa City, Sidama, Ethiopia, 08 April–08 May 2021 (n 333)
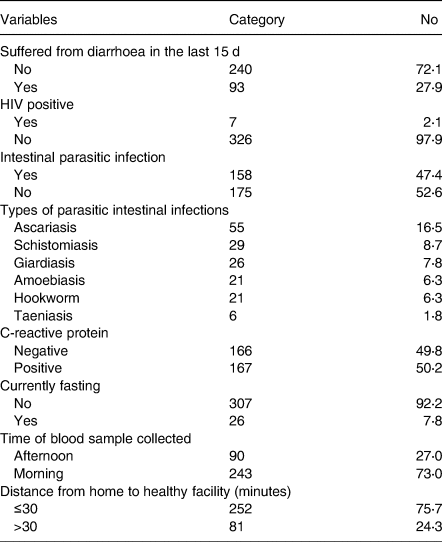
Half, 50⋅2 %, of the respondents tested positive for acute inflammatory marker protein (CRP), and the majority (92⋅2 %) were not fasting at the time of data collection (Table 6). The mean (±sd) serum zinc concentration of samples collected in the afternoon vs. morning was 43⋅06 (±14⋅17) μg/dl and 48⋅12 (±15⋅12) μg/dl, respectively. Similarly, the mean (±sd) of fasting and non-fasting women were 43⋅69 (±15⋅27) μg/dl and 47⋅01 (±14⋅98) μg/dl; whereas the CRP positive and CRP negative women were 57⋅74 (±8⋅86) μg/dl and 35⋅69 (±11⋅36) μg/dl.
Table 6. Factors associated with zinc deficiency among study participating pregnant women around Lake Awasa, Hawassa City, Sidama Region, Ethiopia, 08 April–08 May 2021 (n 333)
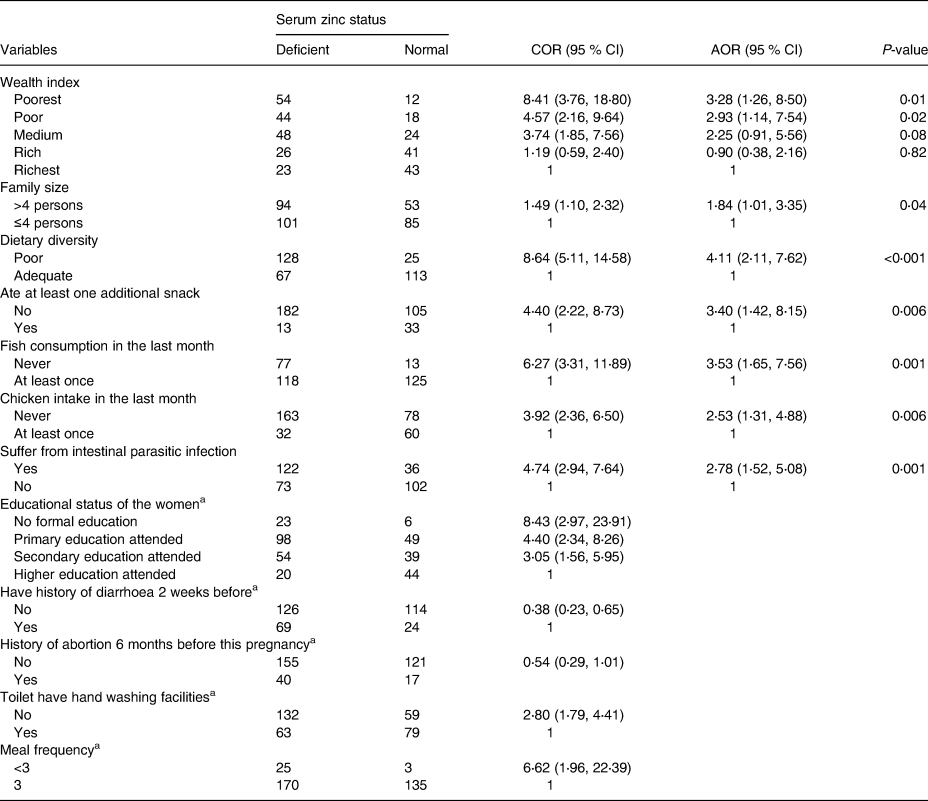
AOR, adjusted odds ratio; OR, crude odds ratio; CI, confidence interval; 1 = reference category.
a Variables not show significant association in multiple logistic regression.
Prevalence of ZD
The mean (±sd) serum zinc concentration was 46 (±15⋅00) μg/dl. Nearly 48 % of the respondents had serum zinc concentration lower than the mean. The mean (±sd) of serum zinc concentration was 48⋅27 (±14⋅43) μg/dl, 48⋅11 (±15⋅67) μg/dl and 43⋅97 (±14⋅49) μg/dl in the first, second and third trimester, respectively. The highest prevalence of ZD was observed in the third trimester (65⋅5 %), and the lowest in the second, whereas 61⋅9 % in the first. Overall, the prevalence of ZD was 58⋅60 % (95 % CI 53⋅31, 63⋅89).
Factors associated with ZD
Table 6 shows the factors associated with ZD among pregnant women. After adjusting for confounders (infections, dietary intakes, household wealth status, socio-demographic variables, obstetrics and clinical characteristics, WASH practices and facilities, and fasting status), women from poorest (AOR = 3.28) and poor (AOR = 2.93) wealth quintiles were almost three times more likely to be zinc-deficient compared to those from richest quintiles, respectively. The odds of ZD was about two times (AOR = 1.84) higher among women from households with more than 4 members. Besides, poor dietary diversity (AOR = 4⋅11; 95 % CI 2⋅11, 7⋅62), not having snack (AOR = 3⋅40; 95 % CI 1⋅42, 8⋅15), not eating fish (AOR = 3⋅53; 95 % CI 1⋅65, 7⋅56) or chicken (AOR = 2⋅53; 95 % CI 1⋅31, 4⋅88) at least once within a month independently predicted the ZD. Moreover, pregnant women tested positive for intestinal parasites were 2⋅78 times more likely to be zinc-deficient as compared with their normal counterparts. An addition of one food groups in the diet decreases women ZD by 0⋅34 times. A unit increase of number gravidity increases the deficiency by 1⋅41 times.
Discussion
The present study revealed that the prevalence of ZD among pregnant women is 58⋅6 %. The International Zinc Consultative Group (IZiNCG) declares that ZD is public health concern when the prevalence of low serum zinc concentration is below 20 %(Reference Brown, Rivera and Bhutta10). Accordingly, the present figure is nearly threefold compared with this value suggesting that ZD is an overwhelming public health problem in the study area. This finding is consistent with the prior research study report from the Sidama zone of Southern Ethiopia, 53⋅0 %(Reference Gebremedhin, Enquselassie and Umeta38). This finding is also in agreement with another study report (55⋅3 %) from Gambella, Western Ethiopia(Reference Ataguadil, Wondwossen and Abate39), (57⋅4 %) Gondar, Northern Ethiopia(Reference Kumera, Awoke and Melese24), and systematic review and meta-analysis reports of 59⋅9 %(Reference Berhe, Gebrearegay and Gebremariam19) and 56 % in Ethiopia(Reference Harika, Faber and Samuel40). However, the current percentage is lower than a previous study report (72⋅0 %) from the former Sidama zone of Southern Ethiopia(Reference Abebe, Bogale and Hambidge25). The possible explanation for the observed discrepancy may be because of an improvement in the quality of health service, and increased ANC service utilisation (34⋅5 % in 2011 to 62⋅9 % in 2016)(29,41) in Ethiopia. The seasonal variation in the data collection period which has implications for food availability could be another possible reason. However, the present percentage is lower than the 66⋅7 % from Gondar, Northern Ethiopia(Reference Kassu, Yabutani and Mulu42), 87 % from a province, Indonesia(Reference Nahrisah, Plianbangchang and Somrongthong43) and 67 % in Australia(Reference Costa, Belo and Souteiro44). On the contrary, the result of the present study is higher than the report from Sunsari district of Nepal (22⋅6 %)(Reference Tamang, Yadav and Acharya45), Asesewa, Ghana (13 %)(Reference Gernand, Aguree and Pobee46) and Zinder, Niger (40⋅7 %)(Reference Wessells, Ouedraogo and Young47). This variation could be explained by the difference in livelihood, dietary practices, and access to health services, and socio-demographics. The high prevalence of the ZD in the present study area could be attributable to the over-dependency (97⋅9 %) on plant-based staple foods like cereals, roots and tubers; and the low intake of ASFs; intake of coffee together with food and intestinal parasitic infection.
In the present study, the serum zinc level was affected by household wealth status such that respondents from the poorest and poor wealth quintiles had a higher risk of ZD. This could be explained by the fact that there might have a reduction in the number of meals, and amount of food consumed, which in turn leads to a decreased dietary zinc intake in pregnant women from poor families(Reference Thompson48). Also, poor families are less likely to access hygiene and sanitation facilities, and health service, and so increased parasitic infection which is an immediate cause of nutrient deficiencies(49).
It was observed that the higher number of household members the greater odds of ZD. This could be because of reduced food intake by the pregnant women due to increased sharing by the larger number of the family members. Besides, the household expense in non-food commodities would also be increased with larger family sizes concomitantly affecting access and affordability to quality foods. Evidence also suggests that larger families reduce the amount of resources available for each individual care(Reference Owoo50). The finding of the present study is consistent with another mixed study from Tanzania such that when the number of family members increases, there is an increased challenge to meet dietary diversity(Reference Powell, Kerr and Young51). However, this contradicts one report from Ethiopia where a family having greater than four members was associated with better dietary qualities and nutritional scores(Reference Workicho, Belachew and Feyissa52).
The zinc status was positively associated with women's inadequate dietary diversity. The possible explanation for this could be that when the number of food groups consumed increases, the probability of nutrient intake and adequacy increase. Existing evidence also suggests that higher dietary diversity is associated with an increased nutrient intake and better nutritional status(Reference Haddad53). The present finding is in line with earlier ones from different parts of Ethiopia(Reference Berhe, Gebrearegay and Gebremariam19,Reference Kumera, Awoke and Melese24,Reference Gebremedhin, Enquselassie and Umeta26) . Eating at least one snack is positively associated with maternal serum zinc concentration. The possible explanation for this could be the additional nutrient obtained from an extra meal.
Consumption of ASFs, especially chicken and fish in the present study, showed a significant positive association with serum zinc status. This could be explained by the fact that ASFs are rich in protein and micronutrients which are highly bioavailable(Reference Darapheak54). On the contrary, limited intake of ASFs coupled with the poor bioavailability of micronutrients in high fibre and phytate plant-based staple foods could result in ZD since plant-based foods are predisposing factors for the deficiency(Reference Gebremedhin, Enquselassie and Umeta26). The finding of the present study is in agreement with others from Ethiopia(Reference Berhe, Gebrearegay and Gebremariam19,Reference Kumera, Awoke and Melese24,Reference Gebremedhin, Enquselassie and Umeta26) .
A negative association was seen between the presence of intestinal parasitic infection and the women's ZD. This could be because the intestinal parasitic infection causes laceration, and enzymatic damage to the mucosa of the small intestine leading to gastrointestinal blood loss(Reference MacLeod and MacLeod55), decreased nutrient absorption and poor appetite(Reference Cline, Little and Bartholomew56), and increased blockage of absorption surface of mucosa by adult worms(Reference Gibson57) and further exuberating ZD. The present finding is in agreement with prior studies from Ethiopia(Reference Kumera, Awoke and Melese24).
The IZiNCG recognises that serum zinc concentration is influenced by fasting, time of blood sample collected and acute inflammation(33). However, we did not find an association despite the high prevalence of acute inflammation markers. The reason might be that, even though the higher prevalence of CRP was determined by the qualitative assessment in the present study, there might not have underlying inflammation to the level that significantly decreases serum zinc concentration. This needs a quantitative assessment whether the CRP is greater than the normal threshold level or not. Moreover, serum zinc status could be influenced by the recent dietary intake even though the acute inflammation is present provided that the infection does not affect the intestinal absorption of zinc. Similarly, fasting did not show a significant association with ZD. This might be explained by the lower number of women, 7⋅8 %, that were fasting during data collection as compared with the National Health and Nutrition Examination Survey II (NHANES II)(Reference Hotz, Peerson and Brown58). The time of blood sampling was also not associated with ZD in the present study.
Strength and limitations
The present study used primary data. However, there might be a memory lapse, and recall bias on the dietary intake data. Moreover, potential social desirability may bias the wealth assessment responses. The pregnancy-related complications were not assessed.
Conclusion
The present study demonstrated that ZD is a public health problem among pregnant women around Lake Hawassa, Sidama Region. It also showed that poor livelihood as measured by low socio-economic status, more family size, poor nutritional practices as determined by poor dietary diversity of women, not having snack, irregular ASFs (chicken and fish) consumption pattern in and intestinal parasitic infection are independent predictors of ZD among pregnant women who resided around Lake Awasa in Hawassa city,, Ethiopia. The prevalent ZD needs further longitudinal analysis to deepen the understanding. It also alarms the need forputting interventions in place with consideration strategies to improve livelihood, dietary diversity, andenvironmental cleanliness so that ZD is prevented and controlled in Hawassa city. Overall, the results indicated the need for consideration of ZD in food and nutrition programmes and policies targeting pregnant women.
Acknowledgements
The authors gratefully thank all respondents, data collectors and supervisors. We would also like to acknowledge Hawassa University for funding this research work.
Hawassa University was the funding organisation for this research. The funding organisation had no role in the design of the study, the collection, analysis and interpretation of the data, the writing of this manuscript and the decision to submit it for publication.
G. A., T. Z. D., T. B., H. H., F. R., A. K. D. and M. G. research design, G. A., T. Z. and T. B. data acquisition, analysis and interpretation. G. A., T. Z., T. B. and T. A. preparing draft manuscript. H. H., F. R., D. G., A. K. D. and M. G. critically reviewed and edited the draft manuscript. All authors read and approved the final manuscript.
There is no conflict of interest among the authors of this research work.
The datasets used for this study is available from the corresponding author upon a reasonable request.









