Introduction
Pharmacotherapy with prescription medications is widely used for migraine prophylaxis and treatment. The complexity and associated adverse effects of most of the available medications pose a significant challenge to physicians, patients, and their families. The efficacy of many of the available prophylactic migraine medications is also questionable and the evidence base supporting them seems incomplete.Reference Pringsheim, Davenport and Mackie1 While structured education and careful attention to lifestyle and varied dietary, physiological, and environmental migraine triggers are highly important and advocated, there is growing evidence to suggest that some vitamins and minerals may be effective as prophylaxis against developing migraine attacks.Reference Sun-Edelstein and Mauskop2–Reference Tepper6 Magnesium, for example, is essential for the activity of many enzymes in the body and plays an important role in neurotransmission and muscular excitability.Reference Mauskop and Varughese7 Vitamin B2 (riboflavin) is an important component of mitochondrial energy production and membrane stability believed to reduce the frequency of some migraine attacks; thus, it can reduce the incidence of migraine attacks and help control migraine symptoms when an attack does occur.Reference Colombo, Saraceno and Comi8
Vitamins and minerals can be sourced naturally or commercially manufactured from artificial sources. In most cases, they have little or no adverse effects, except in large, non-therapeutic doses (e.g. hypervitaminosis).9 Given the adverse event profile and cost of prescription migraine medications, vitamins and minerals may prove to be an appealing alternative option for migraine patients.
The objective of this review is to identify, critically appraise, and meta-analyze data on the efficacy and safety of available over-the-counter vitamins and minerals in reducing the incidence and severity of migraines.
Methods
We undertook a systematic review and meta-analysis in accordance with the Methodological Expectations of the Cochrane Intervention Reviews (MECIR) guidelinesReference Higgins, Lasserson, Chandler, Tovey and Churchill10 and reported as per the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines.Reference Moher, Liberati, Tetzlaff and Altman11 We registered the systematic review protocol with the University of York Centre for Reviews and Dissemination international prospective register of systematic reviews (PROSPERO) prior to executing our literature search (Registration number: CRD42017071440).
Population, Interventions, Comparators, Outcomes, Study Designs (PICOS)
We considered all parallel and crossover randomized controlled trial (RCT) designs reporting efficacy and/or safety of commercially available vitamins and/or minerals (excluding herbs/herbal supplements) for migraine prophylaxis in adult (≥18 years) and pediatric (<18 years) patients. We defined our study population as average-risk individuals (no history of head trauma or neurological disease), irrespective of health status, with a history of migraines. We scoped the literature to identify evidence on vitamins and minerals that have been shown to be effective, and developed the following list of interest to this review: vitamin A (retinol), vitamin B1 (thiamine), vitamin B2 (riboflavin), vitamin B3 (niacin), vitamin B6 (pyridoxine), vitamin B12 (cobalamin), vitamin C (ascorbic acid), vitamin D (cholecalciferol), vitamin E (tocopherol), calcium, iron, magnesium, phosphate, selenium, zinc, and coenzyme Q10 (CoQ10 or Ubiquinone).Reference Sun-Edelstein and Mauskop2, Reference Mottaghi, Khorvash and Askari4–Reference Tepper6 We considered RCTs in which the intervention was administered in the form of a tablet, capsule, suspension, or injection (irrespective of dose and frequency of administration), and the comparator was a placebo or no treatment (active agents were excluded). Our primary outcome measures were migraine frequency (number of attacks) and duration of migraine episodes (hours). Secondary outcomes were migraine severity (intensity), days with migraine, and migraine-associated adverse events.
Trial Identification
We utilized a search strategy developed by a knowledge synthesis librarian (CN) and peer-reviewed by an independent professional librarian using the Peer Review of Electronic Search Strategies (PRESS) checklist.Reference McGowan, Sampson, Salzwedel, Cogo, Foerster and Lefebvre12 Search results were limited to humans and RCTs using a modified version of the Scottish Intercollegiate Guidelines Network (SIGN) filter for RCTs.13 We applied no other limits to the search results. The initial search was designed using MEDLINE (Ovid) (Supplementary Table 1). After peer review, it was adapted for the following bibliographic databases: Embase (Ovid), Cochrane Central Register of Controlled Trials (Wiley), PsycINFO (ProQuest), and CINAHL with Full Text (EBSCO). The search was last updated in June 2017. In order to identify ongoing or unpublished trials, we supplemented the bibliographic database search with a search of clincialtrials.gov, the World Health Organization International Clinical Trials Registry Platform (ICTRP), and the International Federation of Pharmaceutical Manufacturers Associations (IFPMA) website. The reference lists of included trial publications were mined for additional citations. We also used Scopus (Elsevier) to conduct a forward citation search of the included trial publications and to identify additional citations for potential inclusion in the review.
Table 1: Characteristics of included trials

RCT = randomized controlled trial; * = trials considered in meta-analysis; vs. = versus.
Identified citations were imported into a specially designed Microsoft (MS) Access 2016 database (Microsoft Corporation, Redmond, WA, USA) for screening. Two reviewers (GNO, AKW or HHK) screened the citations against the eligibility criteria using a two-stage sifting approach (screening of the titles/abstracts and full-text articles). Disagreements during the screening stages were resolved by discussion between the two reviewers or by involving a third reviewer. We recorded the number of ineligible citations at the title/abstract screening stage and both the number and reason for ineligibility at the full-text article screening stage.
Two reviewers (GNO, AKW or HHK) independently extracted data from the included trials using data extraction forms developed in MS Access 2016 database (Microsoft Corporation, Redmond, WA, USA) and piloted on a small selection of trials prior to use for data extraction. Disagreements were resolved by discussion between the two reviewers or by involving a third reviewer. For crossover trials, we only included data before the crossover. We extracted trial information, trial population characteristics, information regarding interventions and comparators, outcomes assessed and results, and details relevant to the risk of bias assessment.
Risk of Bias Assessment
Two reviewers (GNO, AKW or HHK) independently assessed the risk of bias in each of the included trials at both the trial and outcome levels in compliance with the MECIR guidelines, using the Cochrane Risk of Bias tool.Reference Higgins and Green14 Disagreements were resolved by discussion between the two reviewers or by involving a third reviewer.
Data Synthesis and Analysis
Where possible, we conducted meta-analyses using inverse variance, random effects models implemented in RevMan (version 5.3.5),15 and we used STATA (version 13; StatCorp LP, Texas, USA) to assess publication bias with Egger’s regression test. Meta-analysis was conducted when analyzable data were available from at least two trials. Pooled estimates of effects were calculated using mean differences (MD) and odds ratio (OR); 95% confidence intervals (95% CI) were reported as well for all analyses. In cases where continuous measures of the same outcome were reported with different scales, we used the ratio of means (RoM). If data were presented as medians, we contacted study authors for the mean and standard deviation (SD). If we did not receive a response, then we estimated the mean and SD from the median, range, and the sample size.Reference Hozo, Djulbegovic and Hozo16 We also assessed and quantified statistical heterogeneity between included trials using the I-squared statistic (I 2).Reference Higgins and Thompson17 Publication bias was assessed visually using funnel plots, and using Egger’s regression test.Reference Sterne, Egger and Smith18
We conducted subgroup analyses for migraine severity, frequency, duration, and days with migraine to describe differences between trials according to risk of bias (low, unclear, or high), vitamin or mineral types, age groups ≥18 years versus <18 years, industry- versus non-industry-funded trials, and different outcome measurement tools. Other subgroup analyses that were not possible are available in our PROSPERO protocol.Reference Okoli, Rabbani and Neilson19
Quality of Evidence
Two reviewers (GNO and AMAS) assessed the quality of evidence for the main outcomes (migraine frequency and duration of migraine) and migraine severity using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group methodology.Reference Guyatt, Oxman and Sultan20 Disagreements were resolved by discussion between the two reviewers.
Results
From 5343 citations obtained from all searched sources, we included 18 placebo-controlled trials (17 parallel-design RCTs and 1 crossover design RCT) (Figure 1).Reference Khorvash, Bagheri, Ghasemi, Ghaed-Amini and Maracy21–Reference Gazerani38 One of the included trials is still ongoing.Reference Gazerani38
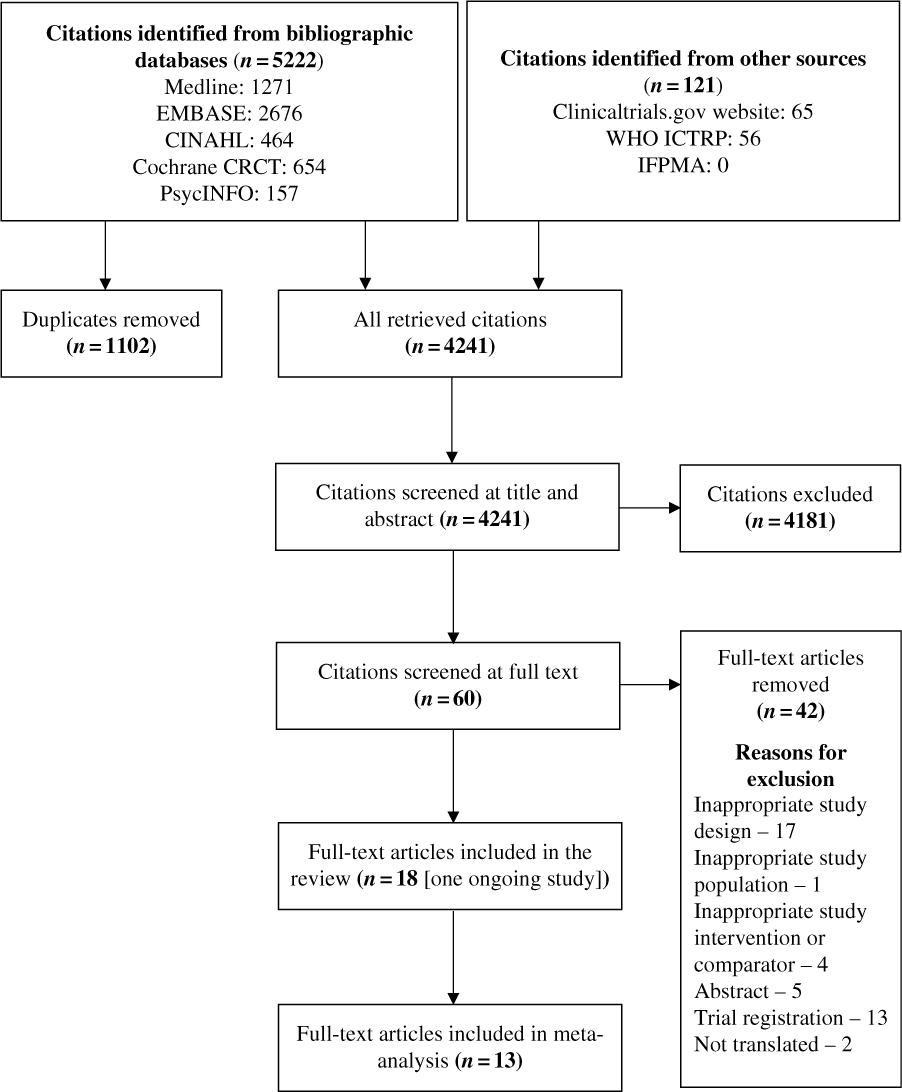
Figure 1: Summary of literature search and screening process (PRISMA flow diagram). Cochrane CRCT = Cochrane Register of Controlled Trials; WHO ICTRP = World Health Organization International Clinical Trials Registry Platform; IFPMA = International Federation of Pharmaceutical Manufacturers Associations.
The 17 completed trials were published from 1996 to 2016. Five of the trials were conducted in Iran,Reference Khorvash, Bagheri, Ghasemi, Ghaed-Amini and Maracy21, Reference Mottaghi, Askari, Khorvash and Maracy23, Reference Sadeghi, Nasiri, Maghsoudi, Pahlavani, Rezaie and Askari24, Reference Tarighat Esfanjani, Mahdavi, Ebrahimi Mameghani, Talebi, Nikniaz and Safaiyan27, Reference Mahdavi, Tarighat Esfanjani, Ebrahimi, Talebi and Ghaem Maghami30 of which two of these were reported in Persian. Four trials were conducted in Australia,Reference Menon, Nasir and Avgan22, Reference Menon, Lea and Roy26, Reference Lea, Colson, Quinlan, Macmillan and Griffiths29, Reference MacLennan, Wade, Forrest, Ratanayake, Fagan and Antony31 six trials in Europe,Reference Gaul, Diener and Danesch25, Reference Bruijn, Duivenvoorden, Passchier, Locher, Dijkstra and Arts28, Reference Sandor, Di Clemente and Coppola33, Reference Schoenen, Jacquy and Lenaerts35–Reference Pfaffenrath, Wessely and Meyer37 and one trial each in the USA,Reference Wang, Van Den Eeden, Ackerson, Salk, Reince and Elin34 and Turkey.Reference Koseoglu, Talaslioglu, Gonul and Kula32
The number of participants ranged from 40 to 300 (Table 1). Twelve trials involved only adults,Reference Menon, Nasir and Avgan22, Reference Sadeghi, Nasiri, Maghsoudi, Pahlavani, Rezaie and Askari24–Reference Tarighat Esfanjani, Mahdavi, Ebrahimi Mameghani, Talebi, Nikniaz and Safaiyan27, Reference Lea, Colson, Quinlan, Macmillan and Griffiths29, Reference Mahdavi, Tarighat Esfanjani, Ebrahimi, Talebi and Ghaem Maghami30, Reference Koseoglu, Talaslioglu, Gonul and Kula32, Reference Sandor, Di Clemente and Coppola33, Reference Schoenen, Jacquy and Lenaerts35–Reference Pfaffenrath, Wessely and Meyer37 three trials involved only children,Reference Bruijn, Duivenvoorden, Passchier, Locher, Dijkstra and Arts28, Reference MacLennan, Wade, Forrest, Ratanayake, Fagan and Antony31, Reference Wang, Van Den Eeden, Ackerson, Salk, Reince and Elin34 and two trials involved both children and adults.Reference Khorvash, Bagheri, Ghasemi, Ghaed-Amini and Maracy21, Reference Mottaghi, Askari, Khorvash and Maracy23 Two trials involved only femalesReference Menon, Nasir and Avgan22, Reference Menon, Lea and Roy26 and the rest of the 15 involved both males and females. All the trials compared vitamins or minerals (magnesium,Reference Tarighat Esfanjani, Mahdavi, Ebrahimi Mameghani, Talebi, Nikniaz and Safaiyan27, Reference Mahdavi, Tarighat Esfanjani, Ebrahimi, Talebi and Ghaem Maghami30, Reference Koseoglu, Talaslioglu, Gonul and Kula32, Reference Wang, Van Den Eeden, Ackerson, Salk, Reince and Elin34, Reference Peikert, Wilimzig and Kohne-Volland36, Reference Pfaffenrath, Wessely and Meyer37 vitamin B2,Reference Bruijn, Duivenvoorden, Passchier, Locher, Dijkstra and Arts28, Reference MacLennan, Wade, Forrest, Ratanayake, Fagan and Antony31, Reference Schoenen, Jacquy and Lenaerts35 and coenzyme Q10 (vitamin-like supplement),Reference Khorvash, Bagheri, Ghasemi, Ghaed-Amini and Maracy21, Reference Sandor, Di Clemente and Coppola33 multivitamins containing folic acid, vitamins B6 and B12,Reference Menon, Nasir and Avgan22, Reference Menon, Lea and Roy26, Reference Lea, Colson, Quinlan, Macmillan and Griffiths29 vitamin B6,Reference Sadeghi, Nasiri, Maghsoudi, Pahlavani, Rezaie and Askari24 vitamin D,Reference Mottaghi, Askari, Khorvash and Maracy23 and a multivitamin containing vitamin B2, magnesium and coenzyme Q10)Reference Gaul, Diener and Danesch25 against placebo. Utilized doses, dosing frequency, and duration of treatment were comparable across trials for magnesium but varied considerably for vitamins. For example, three trials utilized 500 mg of oral magnesium once daily or 250 mg twice daily (same as 500 mg daily)Reference Tarighat Esfanjani, Mahdavi, Ebrahimi Mameghani, Talebi, Nikniaz and Safaiyan27, Reference Mahdavi, Tarighat Esfanjani, Ebrahimi, Talebi and Ghaem Maghami30, Reference Pfaffenrath, Wessely and Meyer37 and two other trials utilized 600 mg of magnesium once daily or 300 mg twice daily,Reference Koseoglu, Talaslioglu, Gonul and Kula32, Reference Peikert, Wilimzig and Kohne-Volland36 with almost all the trials lasting for 12 weeks. On the other hand, trials on vitamins involving vitamins B2, B6, D, or coenzyme Q10Reference Khorvash, Bagheri, Ghasemi, Ghaed-Amini and Maracy21, Reference Mottaghi, Askari, Khorvash and Maracy23, Reference Sadeghi, Nasiri, Maghsoudi, Pahlavani, Rezaie and Askari24, Reference Bruijn, Duivenvoorden, Passchier, Locher, Dijkstra and Arts28, Reference MacLennan, Wade, Forrest, Ratanayake, Fagan and Antony31, Reference Sandor, Di Clemente and Coppola33, Reference Schoenen, Jacquy and Lenaerts35 used considerably different daily doses, dosing frequency, and duration of treatment, even for trials using the same vitamin.
Measurement of migraine severity was carried out using visual analog scale in six trials,Reference Khorvash, Bagheri, Ghasemi, Ghaed-Amini and Maracy21, Reference Mottaghi, Askari, Khorvash and Maracy23, Reference Sadeghi, Nasiri, Maghsoudi, Pahlavani, Rezaie and Askari24, Reference Koseoglu, Talaslioglu, Gonul and Kula32, Reference Peikert, Wilimzig and Kohne-Volland36, Reference Slater, Nelson and Kabbouche39 4-point scale in two trials,Reference Bruijn, Duivenvoorden, Passchier, Locher, Dijkstra and Arts28, Reference Schoenen, Jacquy and Lenaerts35 3-point scale in two trials,Reference Gaul, Diener and Danesch25, Reference Tarighat Esfanjani, Mahdavi, Ebrahimi Mameghani, Talebi, Nikniaz and Safaiyan27 a pain score (1–10) scale in one trial,Reference Lea, Colson, Quinlan, Macmillan and Griffiths29 migraine diary in two trials,Reference Menon, Nasir and Avgan22, Reference Pfaffenrath, Wessely and Meyer37 and not reported in three trials.Reference Menon, Lea and Roy26, Reference Mahdavi, Tarighat Esfanjani, Ebrahimi, Talebi and Ghaem Maghami30, Reference Sandor, Di Clemente and Coppola33 Two trials were funded by industry,Reference Gaul, Diener and Danesch25, Reference Sandor, Di Clemente and Coppola33 four trials did not report on funding,Reference MacLennan, Wade, Forrest, Ratanayake, Fagan and Antony31, Reference Koseoglu, Talaslioglu, Gonul and Kula32, Reference Peikert, Wilimzig and Kohne-Volland36, Reference Pfaffenrath, Wessely and Meyer37 and the rest reported non-industry funding.Reference Khorvash, Bagheri, Ghasemi, Ghaed-Amini and Maracy21–Reference Sadeghi, Nasiri, Maghsoudi, Pahlavani, Rezaie and Askari24, Reference Menon, Lea and Roy26–Reference Mahdavi, Tarighat Esfanjani, Ebrahimi, Talebi and Ghaem Maghami30, Reference Wang, Van Den Eeden, Ackerson, Salk, Reince and Elin34, Reference Schoenen, Jacquy and Lenaerts35 Thirteen trials provided data that were considered in the meta-analyses.Reference Khorvash, Bagheri, Ghasemi, Ghaed-Amini and Maracy21, Reference Mottaghi, Askari, Khorvash and Maracy23–Reference Gaul, Diener and Danesch25, Reference Tarighat Esfanjani, Mahdavi, Ebrahimi Mameghani, Talebi, Nikniaz and Safaiyan27, Reference Bruijn, Duivenvoorden, Passchier, Locher, Dijkstra and Arts28, Reference Mahdavi, Tarighat Esfanjani, Ebrahimi, Talebi and Ghaem Maghami30, Reference Koseoglu, Talaslioglu, Gonul and Kula32–Reference Pfaffenrath, Wessely and Meyer37 Results from trials involving adult patients were meta-analyzed separately from those involving pediatric patients. Meta-analysis of results from trials involving pediatric patients was not feasible due to a lack of sufficiently reported data.
Risk of Bias
We judged a high proportion of the trials to have unclear risk of bias for sequence generation (52.9%), and unclear or high risk of bias for allocation concealment (76.5%), incomplete outcome reporting (58.8%), blinding of participants and personnel (41.2%), and outcome assessment (52.9%) (Table 2). Overall, 1 trial was judged to be of low risk,Reference Lea, Colson, Quinlan, Macmillan and Griffiths29 12 trials of unclear risk,Reference Khorvash, Bagheri, Ghasemi, Ghaed-Amini and Maracy21–Reference Mottaghi, Askari, Khorvash and Maracy23, Reference Gaul, Diener and Danesch25, Reference Menon, Lea and Roy26, Reference Bruijn, Duivenvoorden, Passchier, Locher, Dijkstra and Arts28, Reference MacLennan, Wade, Forrest, Ratanayake, Fagan and Antony31, Reference Sandor, Di Clemente and Coppola33–Reference Schoenen, Jacquy and Lenaerts35, Reference Pfaffenrath, Wessely and Meyer37 and 5 trials of high risk of bias.Reference Sadeghi, Nasiri, Maghsoudi, Pahlavani, Rezaie and Askari24, Reference Tarighat Esfanjani, Mahdavi, Ebrahimi Mameghani, Talebi, Nikniaz and Safaiyan27, Reference Mahdavi, Tarighat Esfanjani, Ebrahimi, Talebi and Ghaem Maghami30, Reference Koseoglu, Talaslioglu, Gonul and Kula32, Reference Peikert, Wilimzig and Kohne-Volland36 Supplementary Table 2 summarizes the reasons for the risk of bias judgment decisions.
Table 2: Risk of bias judgment for included trials
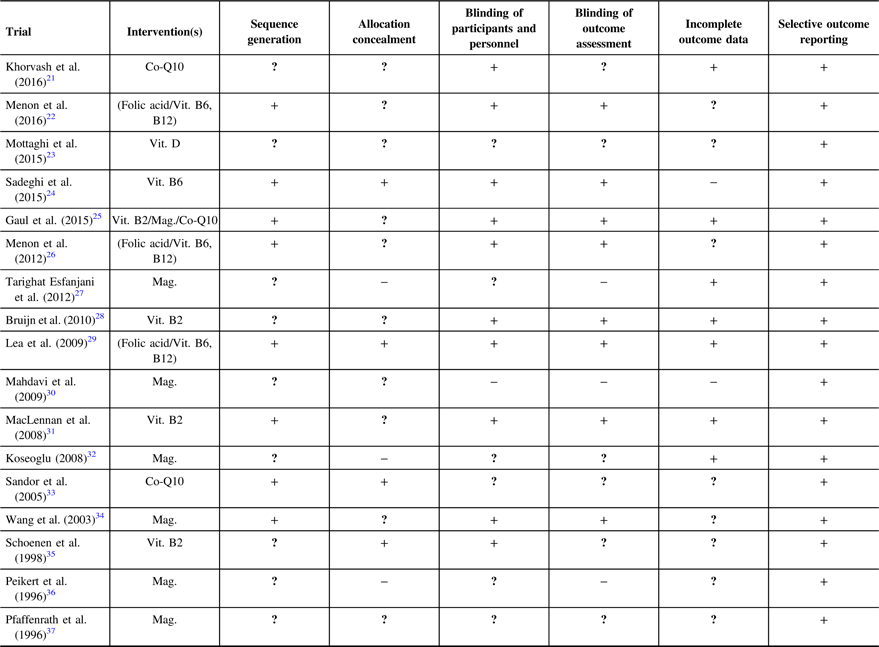
Co-Q10 = Coenzyme Q10; Vit. = Vitamin; Mag = Magnesium; vs = Versus
Primary Outcomes
Migraine Frequency (per Month)
Compared with placebo, coenzyme Q10 was found to be associated with a non-statistically significant reduction in migraine frequency (MD −0.44 (95% CI −2.14 to 1.26); I 2 53%; 2 trials; 97 participants; moderate strength of the evidence; Figure 2) among adults. Due to marked heterogeneity between the included trials for assessment of magnesium (I 2 88%; 4 trials, 266 participants), we decided not to present the result of the pooled analysis although a statistically significantly associated reduction in migraine frequency was found among adults. The effect estimates ranged from −2.57 to −0.93. Our sensitivity analyses and examination of the trial characteristics did not resolve potential sources of heterogeneity. There was only one trial on vitamin B2 and it reported a statistically significant reduction in migraine frequency for vitamin B2 among adults (Table 3). Assessment of other vitamins and minerals was not feasible due to a lack of sufficiently reported data. There were two trials in pediatric patients and both examined vitamin B2. Only one trial reported analyzable data and the result showed no association between vitamin B2 and migraine frequency (Table 3).
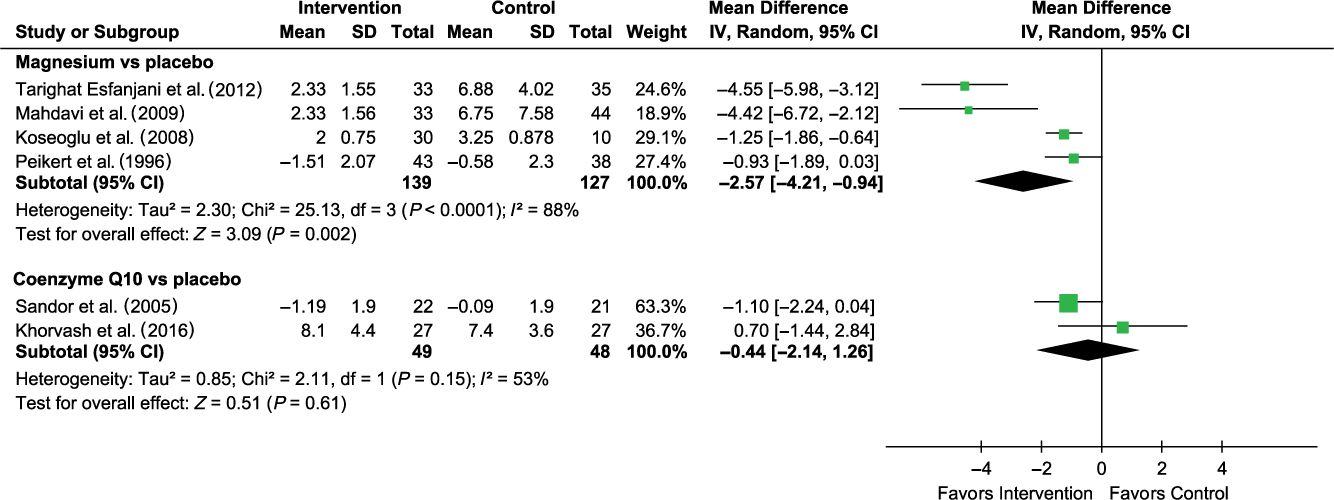
Figure 2: Forest plot for migraine frequency. Only one trial on vitamin B2 (MD −2.00 (−2.60 to −1.40)) – not included in the forest plot.
Table 3: Subgroup meta-analysis for migraine frequency, duration, and severity
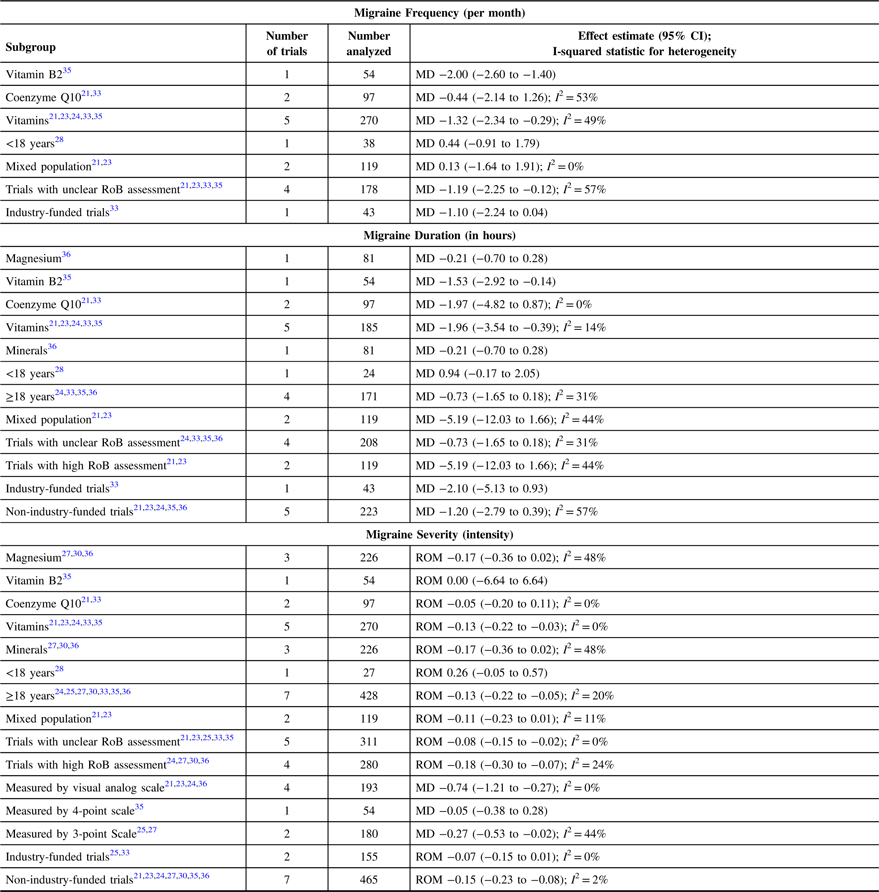
CI = Confidence Interval; ROM = Ratio of Means; MD = Mean Difference; I 2 = I-squared statistic; RoB = Risk of Bias
Migraine Duration (Hours)
Compared with placebo, coenzyme Q10 was associated with a non-statistically significant reduction in migraine duration (MD −1.97 (95% CI −4.82 to 0.87); I 2 0%; 2 trials; 97 participants; moderate strength of the evidence; Figure 3) among adults. There was only one trial each for magnesium and vitamin B2 assessments, and only the latter had an association with a statistically significant reduction in migraine duration among adults (Table 3). Assessment of other vitamins and minerals was not feasible due to a lack of sufficiently reported data. There was only one trial in pediatric patients and the trial reported no association between vitamin B2 and migraine duration (Table 3).
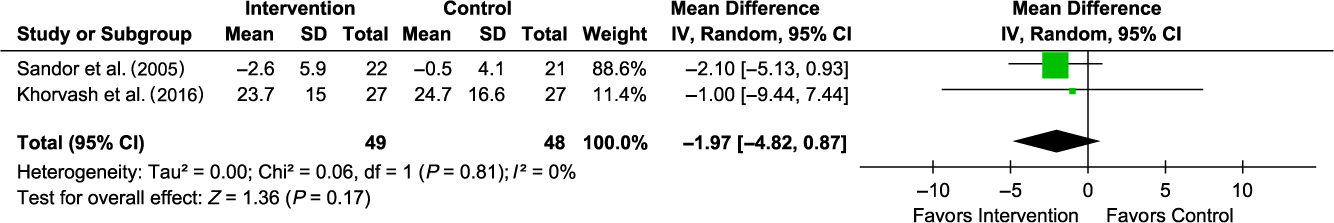
Figure 3: Forest plot for migraine duration. Only one trial each on vitamin B2 (MD −1.53 (−2.92 to −0.14)) and magnesium (MD −0.21 (−0.70 to 0.28)) – not included in the forest plot.
Secondary Outcomes
Migraine Severity
Compared with placebo, magnesium and coenzyme Q10 were each non-statistically significantly associated with a reduction in migraine severity among adults (RoM −0.17 (95% CI −0.36 to 0.02); I 2 48%; 3 trials; 226 participants; low strength of the evidence) and (RoM −0.05 (95% CI −0.20 to 0.11); I 2 0%; 2 trials; 97 participants; moderate strength of the evidence), respectively (Supplementary Figure 1). We found only one trial on vitamin B2 and migraine severity, and the trial reported no association for adults. Assessment of other vitamins and minerals was not feasible due to a lack of sufficiently reported data. Irrespective of intervention type, there was no difference in pooled analysis between trials among adults with high and unclear risk of bias; both were associated with statistically significant reductions in migraine severity (Table 3). Measurements of migraine severity among adults using the visual analog scale or the 3-point scale were associated with statistically significant reductions in migraine severity irrespective of intervention type (Table 3). There was only one trial in pediatric patients and the trial reported no association between vitamin B2 and migraine severity (Table 3).
Days with Migraine
Compared with placebo, magnesium was found to be statistically significantly associated with a reduction in days with migraine among adults but with marked unexplained heterogeneity (I 2 87%; Supplementary Figure 2). There was only one trial each on vitamin B2 and coenzyme Q10; only the former was found to be statistically significantly associated with a reduction in days with migraine and the latter showed a non-statistically significant association. Assessment of vitamins and other minerals was not feasible due to a lack of sufficiently reported data. Results of subgroup analysis are shown in Supplementary Table 3. There were no trials in pediatric patients reporting on the number of days with migraine.
Adverse Events
Adverse events examined in included trials among adults were mainly gastrointestinal side effects, for example, diarrhea, nausea, gastric irritation, vomiting, and soft stool. Assessment of individual vitamins and minerals was not feasible due to a lack of data. Two trials provided data appropriate for meta-analysis but these trials assessed different intervention; therefore, their data were not pooled. There were two trials in pediatric patients reporting on adverse events. One trial examined vitamin B2 and the other examined magnesium. Only one trial reported analyzable data and the result showed no association between vitamin B2 and migraine adverse events.
Discussion
In this systematic review and meta-analysis examining the use of vitamins and minerals for migraine prophylaxis, coenzyme Q10 was found to be non-statistically significantly associated with a reduction in migraine frequency, duration, and severity among adults. Magnesium was also found to be non-statistically significantly associated with a reduction in migraine severity and days with migraine among adults, and although a statistically significantly associated reduction was found with migraine frequency, there was a high level of heterogeneity precluding pooling. However, the larger two trials in the analysis for migraine frequency reported statistically significant associations, whereas the third trial reported no association. Meta-analysis of other vitamins and minerals, and adverse events were not feasible due to a lack of sufficiently reported data.
Potentially insufficient statistical power in some of the analyses as a result of inadequacy of the evidence, variations in the studied populations’ age, sex, comorbidity status and type of migraine, study geographical differences, and quality are likely contributors to the observed heterogeneity and the inability to conclusively determine the efficacy of the interventions. Utilized doses, dosing frequency, and duration of treatment with magnesium were comparable across trials. On the other hand, trials on the same vitamin or coenzyme Q10 had considerably differing daily doses, dosing frequency, and duration of treatment. Overall, the majority of the included trials were small (ranging from 40 to 300 patients) and there were underlying differences between the studied populations. For example, they differed in the length of time patients had suffered migraine before enrolment in the trials, migraine type (whether with or without associated aura, and whether patients with menstrual migraine were allowed to be included or not), and proportion of patients with family history of migraine. In addition, there were potential differences in baseline frequency of attacks, average age of study population, patients’ comorbidities, socioeconomic status, and social habits such as smoking and alcohol consumption, all of which can influence how well a patient will respond to an intervention. The washout period, if any, was not clearly stated in some of the trials and, in some trials, it was also not clear whether patients were allowed to use pain/acute headache medications (e.g. non-steroidal anti-inflammatory drugs) if they needed to do so. Another potential consideration is the different physiologic mechanisms of action of different vitamins and minerals against different migraine outcomes in different migraine patient populations. Internal validity of trials is also an important consideration especially as it relates to risk of bias in key assessment domains, for example, blinding of patients and outcome assessors. All of these may influence the reported efficacy of the interventions and could possibly account for the observed heterogeneity in some of the pooled analyses.
To the best of our knowledge, our review is the first to conduct a comprehensive meta-analysis of the available evidence from RCTs on vitamins and minerals for migraine prophylaxis. A previous review compared oral magnesium with either placebo or an active intervention and reported a significant reduction in the frequency and severity of migraine with oral magnesium but with very high heterogeneity, which the study authors ignored and failed to explore.Reference Chiu, Yeh, Huang and Chen40 In comparison, our review did not include active interventions as comparators. A recent systematic review concluded that vitamin B2 is efficacious in reducing adult patients’ migraine frequency but had a mixed effect in pediatric and adolescent patient populations.Reference Thompson and Saluja41 However, the conclusions were not based on meta-analysis but rather on narrative summary of the results of 11 trials, some of which did not compare vitamin B2 against placebo or no treatment but against prescription medications. A narrative review of 30 observational studies and RCTs involving vitamin B2, magnesium, coenzyme Q10, and butterbur for pediatric and adult migraine prophylaxis reported no specific conclusions on efficacy; although the authors made weak recommendations in favor of magnesium, coenzyme Q10, and butterbur for pediatric migraine.Reference Orr and Venkateswaran42 There have also been other reviews (without meta-analysis) of vitamins and minerals for migraine prophylaxis and all suggested that these interventions are effective.Reference Sun-Edelstein and Mauskop2, Reference Prousky and Seely43, Reference Sangermani and Boncimino44
Our systematic review has its strengths and weaknesses. We registered the review protocol a priori in PROSPERO, conducted the review in accordance with the MECIR guidelines,Reference Higgins, Lasserson, Chandler, Tovey and Churchill10 and reported as per the PRISMA guidelines.Reference Moher, Liberati, Tetzlaff and Altman11 We employed a unique approach (the RoM statistic) to pool the results from all trials that utilized different measurement scales for migraine severity. Pooling the results together using MD or standardized MD statistics would not have been appropriate because the statistics do not account for variations in measurement scales, and combination of data from post-treatment means and change scores, respectively. Our inabilities to examine all or most of the a priori determined vitamins/minerals, to conduct subgroup analyses, and to explore the reason(s) for the observed heterogeneity in some of the meta-analyses are limitations to this review. This was, however, due to the unavailability or insufficiency of reporting of trials. Additionally, almost all of the available trials were judged to be of unclear to high risk of bias.
Our findings may not be applicable to all migraine adult patient populations but it is evidence that the efficacy of vitamins and minerals for migraine prophylaxis in adults is not yet established. As such, physicians and migraine patients should be cautious in using and relying on these supplements for migraine prophylaxis. Public health messages should also emphasize the fact that available evidence is poor and inconclusive. Given the low number of RCTs and the low quality of the available evidence, larger and higher quality trials are required in order to draw conclusions on efficacy of vitamins and minerals for migraine prophylaxis.
Conclusions
Based on the available but insufficient evidence, it is unknown if coenzyme Q10 and magnesium are effective for migraine prophylaxis in adults. It is important to note that the available evidence is of low to moderate strength and from trials with substantial risk of bias. High-quality, adequately powered RCTs are needed to fully evaluate the efficacy and safety of vitamins and minerals to be able to make clinical recommendations on their use for migraine prophylaxis.
Statement of Authorship
GNO and AMAS conceived and designed the study. CN designed and carried out search strategy for literature. GNO, AMAS, RR, and CN developed study protocol. GNO, HHK, and AKW carried out literature sifting, data extraction, and risk of bias assessment. GNO AMAS, RR, RZ, and BM analyzed the data and interpreted the results. GNO drafted initial manuscript. GNO, AMAS, RZ, and BM provided scientific critique. All authors reviewed and contributed to the final version of the manuscript. GNO coordinated the study.
Acknowledgements
We thank Professor Lyn Griffiths and Drs. Parisa Gazerani, Shirley Wee, and Bridget Maher for responding kindly to our request for additional information regarding their trials. We also thank Dr. David Lightfoot (Health Sciences Library, St. Michael’s Hospital, Toronto, Canada) for peer reviewing the MEDLINE search strategy.
Disclosures
Dr. Zarychanski is a recipient of the new investigator salary award from the Canadian Institutes of Health Research. Dr. Mansouri has received previous research funding from Allergan Canada Ltd. All other authors declare that they have no competing interests. The primary and corresponding author had full access to data presented in this systematic review and all the authors had final responsibility for the decision to submit a manuscript for publication.
Funding
No funding was attained for this project.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2018.394.








