Introduction
Traumatic brain injury (TBI) is a significant cause of morbidity and mortality in trauma-related injuries. Reference Bruns and Hauser1–Reference Thurman and Guerrero3 Many patients with severe TBI will require mechanical ventilation and intensive care unit (ICU) admission. Reference Hammell and Henning4 Mortality from severe TBI has ranged from 13 to 40%. Reference Gerber, Chiu and Carney5–Reference Kuenzler, Braun and Maeder7 Improvements in pre-hospital and hospital care, such as transferring patients directly to level I or level II trauma centres, have been associated with lower mortality. Reference Bekelis, Missios and Mackenzie8–Reference Fuller, Woodford, Lawrence, Coats and Lecky11
In British Columbia (BC), the BC Ambulance Service introduced an autolaunch programme in 2006 to improve outcomes among patients with severe injuries. Reference Schuurman, Bell, L’Heureux and Hameed12 During an emergency medical service (EMS) 9-1-1 call, dispatchers identify patients that could potentially benefit from faster transport. During an autolaunch, they simultaneously dispatch paramedics by helicopter and by ground to the scene. Prior studies in patients with TBI have demonstrated that helicopter transport was associated with improved survival. Reference Bekelis, Missios and Mackenzie8,Reference Missios and Bekelis9,Reference Davis, Peay and Serrano13 Nonetheless, it is possible that these differences may be due to transportation time rather than transportation mode. Prior research in trauma patients has shown that helicopter transport may not be advantageous in all situations, particularly in urban areas where transportation may be quicker by ground. Reference Shatney, Homan, Sherck and Ho14
We aimed to report the impact of transportation time to a neurosurgical trauma centre on in-hospital mortality and discharge disposition in critically ill patients with severe TBI.
Methods
We reported this study in accordance with the strengthening the reporting of observational studies in epidemiology (STROBE) statement. Reference von Elm, Altman and Egger15 We had obtained ethics approval through the University of British Columbia Research Ethics Board (H13-01198).
Study Design
We retrospectively analysed data from the British Columbia Trauma Registry (BCTR), which contains clinical, demographic and administrative data on trauma patients admitted to BC hospitals. We included critically ill adult patients (≥18 years) with severe TBI, who were admitted or transported to a neurosurgical trauma centre ICU in <24 hours of time of injury between January 1, 2000 and March 31, 2013. We defined severe TBI using an abbreviated injury scale (AIS) head score ≥4. We defined a level I or level II neurosurgical trauma centre as a facility that has a neurosurgical service. Reference Hameed, Schuurman and Razek16 We included critically ill patients to ensure we had selected a cohort of patients with severe TBI. We excluded patients that had missing data for the times required to determine transportation time to a neurosurgical centre (N = 710). We also excluded non-BC residents. Patients who died in transport prior to arrival to hospital could not be included, as they were not captured in the registry.
Our primary outcome was in-hospital mortality by the time of hospital discharge. Our exposure of interest was transportation time to a neurosurgical centre. We defined transportation time as the difference in time between the time of injury and the time of arrival at a neurosurgical trauma centre. We evaluated discharge disposition location (either expired or palliative; transfer to nursing care facility, rehabilitation facility or other acute care facility; or home including with or without supports and leaving against medical advice), as a secondary outcome.
We collected additional information including age, sex, home postal code, injury time and location, injury type and mechanism, arrival time of EMS to the scene, EMS transportation mode, first and second healthcare facility transportation time and location (if applicable), injury severity score (ISS), AIS scores, vital signs at multiple time points, revised trauma score (RTS) at multiple time points, neurosurgical trauma centre arrival time and location, use of mechanical ventilation, and length of stay in hospital and ICU. Procedure codes in the BCTR were linked with the Canadian Classification of Health Interventions (CCI) codes from the Canadian Institute for Health Information. 17 We collected information on neurosurgical procedures including the performance of a burr hole, craniotomy, craniectomy and insertion of an intracranial pressure (ICP) monitor.
The BCTR was linked to the Vancouver Area Neighborhood Deprivation Index (VANDIX), using home postal code, to determine socio-economic status (SES) for each patient. The VANDIX has been demonstrated to be a reliable predictor in the assessment of SES and health status in paediatric TBI. Reference Bell and Hayes18,Reference Amram, Schuurman and Pike19 The VANDIX classifies SES on an ordinal scale of 1–5, with 5 representing the most deprived census dissemination area. We linked the postal code of where the injury occurred to classify the location of injury as either urban or rural. We defined urban based on Statistics Canada definitions. 20 Geospatial data were generated with ArcGIS (Esri, Redlands, California, USA), linking postal code of the injury and the VANDIX scores.
Statistical Analyses
All analyses were performed using Stata/MP version 15.1 (StataCorp, College Station, Texas, USA). Data were reported as numbers (with proportions) or medians (with interquartile range [IQR]) when appropriate. Differences between groups were assessed using Student’s t-test or Wilcoxon rank sum test for continuous variables, and χ2 or Fisher’s exact test for categorical variables.
Univariable logistic regression analysis was performed, examining transportation time as a continuous variable and as a binary variable (<1 hour versus ≥1 hour, or <2 hours versus ≥2 hours). We chose these specific binary time cut-offs a priori as they represented the “golden hour” time cut-offs described in prior studies. Reference Dinh, Bein, Roncal, Byrne, Petchell and Brennan21–Reference Lerner and Moscati24 Multivariable logistic regression was used to calculate the adjusted odds ratio (ORs) for in-hospital mortality from increased transportation time, adjusting for variables determined a priori including age, sex, year of injury, VANDIX score, RTS at the scene, ISS score, direct (versus indirect) transportation to a level I or II neurosurgical centre and location of injury (urban versus rural). We also analysed the data, using a second regression model, with additional adjustment for the presence of severe chest or abdominal trauma (as defined by an AIS score ≥4) and the type of injury (blunt versus non-blunt). Multinomial logistic regression was used to evaluate discharge disposition. We evaluated for potential effect modification on the primary outcome by sex, age (<65 versus ≥65 years), head AIS score, injury location (urban versus rural), transportation by air, performance of a neurosurgical procedure (i.e. ICP monitor insertion, burr hole, craniotomy or craniectomy) and direct transportation to a neurosurgical centre. Effect modification had previously been described in prior trauma studies with these variables in relation to mortality. Reference Linn, Levi, Grunau, Zaidise and Zarka25–Reference Shackelford, del Junco and Reade27 Effect modification was also evaluated for the years after 2006 compared to before 2006 to further evaluate the effect of the new autolaunch programme.
Missing data were handled using indicator variables. There was no missing data for the primary outcome of in-hospital mortality. All results were reported with ORs or relative risk ratios when appropriate, with associated 95% confidence intervals (CIs). A two-sided p-value <0.05 was considered statistically significant.
Sensitivity Analyses
First, we performed multiple imputation for missing data using a multivariate normal distribution and a Markov Chain Monte Carlo method, with 10 imputations. Second, we re-performed the primary analyses on the BCTR using a population of critically ill adults with severe TBI with an initial scene Glasgow Coma Scale (GCS) ≤8. Third, we did an analysis of our primary outcome, limiting the analysis to patients with a transportation time <6 hours, to ensure we selected patients in whom transport was time critical. Fourth, we performed a post hoc analysis of our primary outcome, using our original multivariable model, but also with additional adjustment for hypotension at the scene and intubation at the scene. These additional variables were selected as they could be potential factors influencing patient outcome. Reference Brorsson, Rodling-Wahlström, Olivecrona, Koskinen and Naredi28–Reference Gravesteijn, Sewalt and Nieboer31 Fifth, we performed an additional post hoc analysis of our primary outcome, using our original multivariable model, but with additional adjustment for modality of transport (either private vehicle, land ambulance or air transport). Next, we also performed a post hoc analysis of our primary outcome, limiting the population to those who had received neurosurgical interventions for TBI. Seventh, we also re-performed analysis of our primary outcome, also focusing on the “Platinum Ten” minutes. Reference Daban, Falzone, Boutonnet, Peigne and Lenoir32 The “Platinum Ten” minutes refer to the amount of time (i.e., 10 minutes or less) that EMS providers should remain on scene prior to definitive transport. We classified patients in those that were transported within the “Platinum Ten” and those that were not. Finally, we performed a competing-risks survival regression analysis, using Fine and Gray’s proportional hazards model, evaluating transportation time as binary variable (<1 hour versus ≥1 hour) and its effect on mortality (either in-hospital death or transfer to palliative care). Reference Fine and Gray33 Discharges to home (home, home with supports or against medical advice) or to a care facility (rehabilitation facility or nursing care facility) were considered as competing events.
Results
Demographic and Clinical Characteristics of Critically Ill Patients with Severe TBI
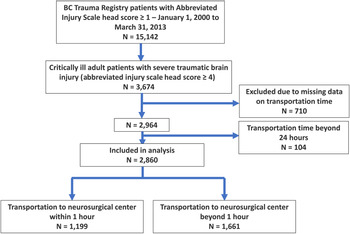
We identified 15,142 patients within the BCTR who had a head AIS score ≥ 1, of which 2,860 patients (18.9%) met the inclusion criteria of our study (Figure 1). A summary of the baseline characteristics of the population, including the number of individuals with missing data, is described in Table 1. The median age of all patients was 43 years (IQR 26–59). Six hundred and seventy-six (23.6%) patients were female. The median GCS score at the scene was 8 (IQR 4–13). Seven hundred and forty-six (26.1%) patients had a head AIS score of 4, 2,106 (73.6%) patients had a head AIS score of 5 and 8 (0.3%) patients had a head AIS score of 6. Two thousand seven hundred and sixty-eight (96.8%) critically ill patients with severe TBI had blunt injuries, of which vehicular crashes (58.0%) were the most common mechanism of injury, followed by falls (31.6%). In this study, 2,439 (86.7%) were transported by land ambulance, 65 (2.3%) by private vehicle, 300 (10.7%) by helicopter ambulance and 8 (0.3%) by fixed-wing ambulance. The median transportation time from the time of injury to time of arrival at a neurosurgical trauma centre was 80 minutes (IQR 40–315). Injuries predominantly occurred in the lower mainland area of Vancouver (Supplementary Appendix eFigure 1).

Figure 1: Flow diagram of the study.
Table 1: Demographic, injury, transportation and clinical characteristics of the population

AIS = abbreviated injury scale; EMS = Emergency Medical Services; GCS = Glasgow Coma Scale; ICU = intensive care unit; IQR = interquartile range; ISS = injury severity scale; N = number; RTS = revised trauma score; VANDIX = Vancouver Area Neighborhood Deprivation Index.
* Variables with missing data.
† Neurosurgical procedures include craniotomy, craniectomy, burr hole insertion and insertion of intracranial pressure monitor.
‡ Comparing <1 hour versus ≥1 hour.
Six hundred and ninety-six (24.3%) patients had died in-hospital. They had a median length of stay in ICU of 6 days (IQR 2–12) and in hospital of 20 days (IQR 7–42). Patients with head AIS scores of 5 and 6 had the highest in-hospital mortality (30.6% [644/2,106] and 75.0% [6/8], respectively). Among the patients that survived to hospital discharge, 699 (33.0%) were discharged home, home with supports or against medical advice. Five hundred and thirty-four (66.9%) were discharged to a nursing care facility, rehabilitation facility or another acute care facility. Two (0.1%) were discharged to palliative care.
Univariable and Multivariable Analysis of Primary and Secondary Outcomes
A summary of the univariable and multivariable analyses can be found in Tables 2 and 3. When performing a univariable analysis, we found a reduction in the odds of in-hospital mortality for each 1-hour increase in transportation time to a neurosurgical trauma centre (OR 0.96, 95% CI 0.94–0.98). After multivariable analysis, we did not find any statistically significant difference (adjusted OR [aOR] 0.98, 95% CI 0.95–1.01 for Model #1 and aOR 0.98, 95% CI 0.95–1.01 for Model #2). We similarly did not find any difference from the effect of transportation time on discharge disposition after adjustment.
Table 2: Effect of transportation time on in-hospital mortality in 2,860 patients with severe traumatic brain injury

* For each 1-hour increase in transportation time to a neurosurgical centre.
Model #1: Adjusting for age, sex, direct transportation to neurosurgical centre, revised trauma score at the scene, injury severity scale score, year of injury, VANDIX and location of injury (urban versus rural).
Model #2: Additional adjustment for the type of injury (blunt versus penetrating), and concomitant severe chest/abdominal trauma with abbreviated injury score ≥4.
Table 3: Effect of transportation time on discharge disposition in 2,860 patients with severe traumatic brain injury

* For each 1-hour increase in transportation time; adjusting for age, sex, direct transportation to neurosurgical centre, revised trauma score at the scene, injury severity scale score, year of injury, VANDIX and location of injury (urban versus rural).
Next, we analysed the effect of transportation time to a neurosurgical centre in <1 hour (versus ≥1 hour) and <2 hours (versus ≥2 hours), and we did not find any statistically significant association with in-hospital mortality (aOR 0.92, 95% CI 0.72–1.17 and aOR 0.96, 95% CI 0.67–1.37, respectively) (Supplementary Appendix eTable 1 and eTable 2). There were also no statistically significant differences in discharge disposition.
When analysing for effect modification on transportation time on the primary outcome, we found effect modification by injury location (P interaction = 0.003). Among individuals transported from an urban injury location, each 1-hour delay in transportation time was associated with increased odds of in-hospital mortality (aOR 1.07, 95% CI 1.01–1.14). Among individuals transported from a rural injury location, there was no association between transportation time and in-hospital mortality (aOR 0.95, 95% CI 0.89–1.00). We did not find any other evidence of effect modification by sex, age, head AIS score, transportation by air, year (before 2006 versus 2006 onwards), direct transportation to a neurosurgical centre or performance of a neurosurgical procedure (Supplementary Appendix eFigure 2).
Sensitivity Analysis
We performed a few sensitivity analyses. First, we used multiple imputation to account for missing data, and we found no difference from the effect of transportation time to a neurosurgical centre on in-hospital mortality (aOR 0.98; 95% CI 0.95–1.01). We additionally found no difference when comparing transportation time in <1 hour versus ≥1 hour (aOR 0.96, 95% CI 0.76–1.22), or when comparing transportation time in <2 hours versus ≥2 hours (aOR 0.98, 95% CI 0.69–1.39). Second, we re-analysed our data by selecting 1,139 critically ill patients with severe TBI using the GCS score. After adjustment, we found no significant difference from the effect of transportation time on in-hospital mortality, when analyzing transportation time either as a continuous variable or as a binary variable. When we further limited our population to those who were transported to a neurosurgical centre in <6 hours, we still did not observe any significant difference.
Next, we re-analysed our data for the primary outcome, additionally including adjustment for intubation at the scene and hypotension at the scene to our original model. After adjustment, we still found no significant difference for the effect of transportation time on in-hospital mortality (p = 0.24). Next, we also re-analysed for the primary outcome, including additional adjustment for the mode of transport (land ambulance, private vehicle or air transport) to our original model. After adjustment, transport time was still not associated with in-hospital mortality (p = 0.25). Next, we also re-analysed our data, limiting the population to those who had performance of a neurosurgical procedure, and did not find any association between transportation time and mortality (p = 0.13). Next, we re-performed our analysis of our primary outcome, classifying as patients that were transported within the “Platinum Ten” minutes and those that were not. There was no significant association between transportation within the “Platinum Ten” and in-hospital mortality (aOR 1.06; 95% CI 0.80–1.41, p = 0.68).
Finally, we performed a competing risks regression, and we modelled the cumulative incidence function of different disposition outcomes (Supplementary Appendix eFigure 3). We found that the estimated subdistribution hazard ratio for in-hospital mortality or transfer to palliative care was 0.90 (95% CI 0.74–1.09) for patients transported in <1 hour compared to ≥1 hour.
Discussion
Prior research in Canada has demonstrated that delays in transportation may result in worse outcomes in trauma patients. Reference Tansley, Schuurman and Bowes34–Reference Fleet, Lauzier and Tounkara36 The incidence of patients with TBI in Canada has been rising over the years, with an increasing number of patients presenting to emergency departments for assessment and treatment. Reference Rao, McFaull, Thompson and Jayaraman37,Reference Fu, Jing, Fu and Cusimano38 We report one of the first Canadian studies to examine the effect of transportation time to a neurosurgical level I or II trauma centre on hospital outcomes in critically ill adult patients with severe TBI. We were able to analyse a substantial number of critically ill patients using the BCTR over several years. Overall, we found that in-hospital mortality of these patients was approximately 24%. We did not find any effect of transportation time on in-hospital mortality or hospital discharge disposition. Nevertheless, we did observe higher odds of in-hospital mortality with longer transportation times in a subgroup of patients that were injured in urban areas.
Prior studies in trauma care have suggested to prioritise early transportation, ideally within the “golden hour.” Reference Cowley39 We did not find any differences in mortality for patients with TBI transported to hospital in <1 hour or <2 hours. While older studies found that shorter transportation times have been associated with improved outcomes, contemporaneous studies of trauma patients have not demonstrated benefit with faster transportation time. Reference Dinh, Bein, Roncal, Byrne, Petchell and Brennan21–Reference Newgard, Schmicker and Hedges23,Reference Cowley39–Reference Sampalis, Denis, Frechette, Brown, Fleiszer and Mulder42 There may be several explanations for the lack of finding an association between transportation time and outcome, in our analysis and other more recent studies. First, the stabilisation of these patients (i.e., endotracheal intubation and support of haemodynamics) may be more important rather than the actual time to transport. Reference Manley, Knudson, Morabito, Damron, Erickson and Pitts29,Reference Gravesteijn, Sewalt and Nieboer31 Earlier studies demonstrating benefit to faster transportation time occurred prior to systematic and improved organisation of trauma care and EMS delivery, particularly within the Canadian context. Reference Moore, Stelfox and Evans43 Since the reorganisation of trauma services and establishment of Canadian accreditation guidelines, there have been improving trends in trauma outcomes. Reference Moore, Stelfox and Evans43,Reference Simons and Kirkpatrick44 Similarly, there have been substantial improvements in the critical care and TBI, with reducing trends in mortality observed through the years. Reference Gerber, Chiu and Carney5,Reference Zimmerman, Kramer and Knaus45
Second, while timely care may be important in certain TBI patients requiring neurosurgical interventions, the identification of these patients may be challenging pre-hospital. Reference Shackelford, del Junco and Reade27,Reference Barthélemy, Melis, Gordon, Ullman and Germano46,Reference Bullock, Chesnut and Ghajar47 In this study, many (52%) were managed medically and hence may not require time-dependent intervention. Of those who required neurosurgical intervention, the majority (70%) arrived at a neurosurgical centre within 4 hours. Additionally, the majority (62%) of our patients were directly transported to a neurosurgical centre, which we had accounted for in our analysis. Prior studies had found that direct transportation to a neurosurgical centre resulted in improved outcomes, rather than the time to transport. Reference Hartl, Gerber, Iacono, Ni, Lyons and Ghajar10,Reference Sampalis, Denis, Frechette, Brown, Fleiszer and Mulder42,Reference Sugerman, Xu, Pearson and Faul48 Third, the mortality of critically ill severe TBI patients in Canada has commonly been associated with the withdrawal of life-sustaining therapy after the determination of poor neurological prognosis. Reference Turgeon, Lauzier and Simard49 Previously identified factors associated with the decision to withdraw life-sustaining therapies have included the evidence of brain herniation on computed tomography (CT) scan, while the performance of surgical interventions or the presence of epidural haematoma on CT scan were associated with reduced odds of death. Reference Turgeon, Lauzier and Simard49,Reference Côte, Turgeon and Lauzier50 Neurological prognostication may occur within a few days after admission and may not be wholly dependent on pre-hospital transportation time. It is therefore possible that transportation within the “golden hour” may have minimal impact on the mortality outcomes of critically ill patients with severe TBI.
Overall, delayed admission in TBI is still common, and in our study, we found that the median time to transport to hospital for these patients was 80 minutes. Previous studies have identified risk factors associated with delayed admission including male sex, injury occurring at a public place (versus private residence), low-energy trauma, the absence of pre-hospital physician involvement, the presence of stable vital signs, the absence of major extracranial injuries and the presence of concurrent alcohol intoxication. Reference Raj, Siironen and Kivisaari51 Other studies have also noted similar delays in transport. Reference Fuller, Woodford, Lawrence, Coats and Lecky11,Reference Gravesteijn, Sewalt and Nieboer31,Reference Raj, Siironen and Kivisaari51 In a large multicentre prospective observation trial in Europe of patients with TBI, the median time to arrival of EMS services was 20 minutes (IQR 11–30), with an on-scene time of 35 minutes (IQR 25–51) and travel time from the scene to hospital of 20 minutes (IQR 12–35). Reference Gravesteijn, Sewalt and Nieboer31 An analysis of a national UK trauma registry database found that the median transportation time was 60 minutes (IQR 45–80). However, analyses of North American trauma registries found shorter transportation times to hospital. Reference Newgard, Meier and Bulger22,Reference Sugerman, Xu, Pearson and Faul48 In the Resuscitation Outcomes Consortium Epistry-Trauma registry, which captures 51 trauma centres across North America, the median total EMS time was 36.3 minutes (IQR 28.4–47.0). Reference Newgard, Meier and Bulger22 Similarly, an analysis of the American College of Surgeons National Trauma Databank from 2007 to 2009 found that the median transport time of severe TBI patients was 36.5 minutes in those directly transported to a level I or II trauma centre and 122.3 minutes in those indirectly transported to a level I or II trauma centre. Reference Sugerman, Xu, Pearson and Faul48 The delays in transportation in BC may be in part due to the large mountainous geography of the province, remoteness of some communities and the potential for severe weather in northern areas. Reference Hameed, Schuurman and Razek16,Reference Amram, Schuurman and Pike19,Reference Schuurman, Hameed, Fiedler, Bell and Simons52
Interestingly, we observed that patients transported from urban areas had worse outcomes when delays in transport were present. On the other hand, we did not observe any effect of transportation from rural areas on in-hospital mortality. There may be some explanations for this phenomenon, which may not have been captured by our study. It is possible that rural patients may have died pre-transport or pre-arrival to a neurosurgical trauma facility. A prior study in BC found that a large number of pre-hospital deaths occurred in rural areas. Reference Simons, Brasher and Taulu35 Additionally, many trauma patients injured in rural areas may be admitted to hospitals outside of major trauma centres. Reference Newgard, Fu and Bulger53 An additional consideration is the effect of SES on transportation time. Prior studies have found that lower SES was associated with a higher prevalence of injuries. Reference Amram, Schuurman and Pike19,Reference Schuurman, Hameed, Fiedler, Bell and Simons52 Additionally, patient ethnicity has been associated with differences in patient outcomes and transportation time in other previous studies. Reference Sugerman, Xu, Pearson and Faul48,Reference Cuthbert, Corrigan and Harrison-Felix54 In our study, we did not observe any substantial effect of SES on transportation time or on TBI outcomes; however, we did not capture information on patient ethnicity.
Finally, we evaluated the effect of transportation time on discharge disposition. Following their injury, many critically ill patients with severe TBI require supportive services in the form of rehabilitation, subacute care or long-term care. In a large database study of patients with moderate-to-severe TBI, a substantial proportion (>30%) of those patients required rehabilitation or subacute care following hospital discharge. Reference Cuthbert, Corrigan and Harrison-Felix54 Among the survivors of severe TBI in our study, approximately 67% of patients did not return to home upon hospital discharge. While we did not find any impact of transportation time on discharge disposition, we were unable to measure any functional outcomes of the discharged TBI patients in our study. A prior study has suggested that functional outcomes may not be impacted by pre-hospital time in rural TBI patients. Reference Jordan, Lewis and Frank55 Further research will be needed in this area with the conduct of large observational studies.
Limitations
There are several limitations to our study. First, the secondary use of databases or registries to perform research on patients with TBI is limited by the ability to correctly identify patients with TBI. Reference Carroll, Cochran, Price, Guse and Wang56 The AIS score is commonly used as a surrogate marker for TBI severity in databases as the GCS score is frequently undercoded. Reference Rogers and Trickey57 Also, the GCS score may misclassify patients with severe TBI, in the context of sedation, concomitant alcohol or drug use, intubation, or distracting injuries. On the other hand, the AIS score may incorrectly identify patients with severe TBI, especially at lower scores. Reference Rogers and Trickey57 We specified an AIS score ≥4 to try to correctly classify most patients. In addition, we selected critical care patients to further minimise misclassification. To address some of these concerns, we performed a sensitivity analysis by classifying patients with severe TBI using the GCS, and we still did not find substantial differences in our results. We also were not able to determine the urgency of the neurosurgical procedures performed as this information was not captured in the database, and the time to performance of these procedures may be impacted by pre-hospital transportation time. Nevertheless, we did not find any effect modification on transportation time by the performance of any neurosurgical procedures. We also did not find any substantial differences in our results when limiting the population to those who had received neurosurgical procedures.
Second, our study may be prone to residual confounding and confounding by indication. Faster transportation to a neurosurgical centre may be reflective of severity of illness, as the most severely injured are prioritised over stable patients. These patients may have worse outcomes in hospital. We attempted to control for severity of illness using the RTS, but this was limited by the lack of additional physiologic or clinical information in the registry to control for ICU severity of illness such as the sequential organ failure assessment score. Additionally, we were not able to control for some factors that may impact transportation time such as seasonality. Seasonal differences may account for differences in trauma volume and time to presentation to hospital. Reference Stonko, Dennis and Callcut58
Third, there were variables with substantial missing data in the registry. Some variables such as the length of mechanical ventilation and performance of neurosurgical procedures had up to 70% of data missing. We did not have information on level of training of the paramedics involved in transport; however, critical care paramedics would be responsible for transports by air (either fixed-wing or helicopter) in BC. 59 As a result, these variables were not included in the analysis. For the remainder of variables, we did account for missing data in this study, using indicator variables and multiple imputation methods. With these methods, there were no appreciable changes in the results for our primary outcome. On the other hand, we were not able to account for missing data for transportation time, or patients who may have died prior to arriving at a neurosurgical trauma centre. This may have introduced some bias, particularly if these groups of patients had worse outcomes with faster or shorter transportation times. However, of the patients who had missing data for transportation time, in-hospital mortality was similar (approximately 25%) compared to patients without missing data. Additionally, a prior study from the Resuscitation Outcomes Consortium would suggest that death in the field in TBI patients will be low (<1%).
Fourth, our study was not powered to detect small differences in subgroups (i.e., helicopter transportation) or evaluate the risk factors associated with worse outcome. While we were not able to detect any statistical interaction between helicopter transportation and transportation time, it is difficult to discern the effect of the autolaunch programme. Less than 11% of all patients were transported by air and most (>90%) took over an hour to reach hospital. It is likely that ground transportation from these locations would have been slower than air transportation. Finally, our study is limited by the relative age of our data. It is difficult to know whether these findings still hold true to this day. Despite this, we were able to analyse many sequential years to inform our conclusions.
Conclusions
In conclusion, there it does not appear to be an associated benefit with shorter transportation times on in-hospital mortality or discharge disposition in critically ill patients with severe TBI. However, this may be limited by residual confounding. Further study will need to be done to determine whether transportation time influences other important clinical outcomes, such as time to functional recovery and disability status following injury.
Funding
DEGG is supported through a Heath-Professional Investigator Award through the Michael Smith Foundation for health research.
The funders had no influence on the conception, design, statistical analysis or reporting of this study.
Otherwise, there were no grants/funding from any other public, private or commercial interests.
Disclosures
No conflicts of interests to declare.
Statement of Authorship
Study design: ES, DEGG and HJB. Acquisition of the data: SMH and DEGG. Geospatial data: OA. Statistical analysis: ES and HJB. Drafting and review of the manuscript: ES, OA, SMH, DEGG and HJB.
Ethics
Ethics approval obtained through UBC Research Ethics board (H13-01198).
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2021.5.