Introduction
The marijuana plant, Cannabis sativa, Cannabis indica, and Cannabis ruderalis (for some considered a subspecies of C. sativa), contains more than 500 chemical species with more than 100 different phytocannabinoid (i.e. derived from the plant) compounds.Reference Husni, McCurdy and Radwan1 Two of these compounds – Δ-9-tetrahydrocannabiniol (THC) and cannabidiol (CBD) – have generated the most interest in terms of their putative effectiveness as an anti-seizure agent. Most of the psychoactive properties of marijuana are mediated by THC. CBD is the most abundant nonpsychoactive cannabinoid in the marijuana plant. As a plant-derived oral solution, both CBD and THC have shown some anti-seizure effects in a number of laboratory models of seizures and epilepsyReference Friedman and Devinsky2,Reference O‘Connell, Gloss and Devinsky3 and in both open-label and randomized control trials (RCTs) in Dravet syndrome and Lennox-Gastaut syndrome.Reference Szaflarski, Bebin and Comi4–Reference Thiele, Marsh and French10 However, other studies have failed to replicate THC anti-seizure effect and a few showed pro-convulsant effects.Reference O‘Connell, Gloss and Devinsky3 There is some evidence that THC (recreational use) causes cognitive impairment and chronic psychiatric disturbances in adolescents and adults. For these reasons, the focus of most research and clinical use has been on another cannabinoid, namely, CBD.Reference O‘Connell, Gloss and Devinsky3
The potential beneficial effects of nonpurified medical marijuana with a high CBD-THC ratio has motivated discussions in recent years, leading to an increased demand for the high CBD and low THC cannabis herbal extract oil (CBD-THC oil) for both adults and children with drug-resistant epilepsy. This increased demand and specific issues about the use of cannabinoids in CanadaReference McLachlan11 has prompted the Canadian League Against Epilepsy (CLAE) to develop a formal statement about its current position regarding the use of CBD-THC, particularly the oil formulation, for epilepsy. As the Canadian government uses the term cannabis to refer to regulated marijuana (for recreational or medical use), for the purpose of this statement, the term cannabis will refer to both regulated marijuana and its derivatives within the framework of federal, provincial, and territorial regulations for recreational or medical purpose. We have purposely avoided the term “hemp” as it is loosely used by the media, creating some confusion. According to the Industrial Hemp Regulations (SOR/2018-145, https://laws-lois.justice.gc.ca/PDF/SOR-2018-145.pdf), “industrial hemp” means a cannabis plant or any part of that plant in which the concentration of THC is 0.3% or less in the flowering heads and leaves. However, some commercially available high CBD-THC ratio hemp oils may not meet this specific legal criterion.
This position statement is based on a literature search, expert opinion, and consumer (epilepsy) representatives’ input with final approval by the CLAE Board of Directors and Executive Committee; however, it may not necessarily represent the opinion of all CLAE members. It addresses three key elements: (1) the current legal framework for cannabis medical use, (2) the published evidence for the anti-seizure efficacy of CBD-derived products, and (3) our current understanding of its utilization, doses, and safety with regard to both the short- and the long-term effects of cannabis use for medical purposes.
Legal Considerations of Cannabis for Medical Use
The use of recreational cannabis in adults (defined as 18-year-old or older) was legalized in Canada on October 17, 2018. As per the Department of Justice website (https://www.justice.gc.ca/eng/cj-jp/cannabis/) and subject to provincial or territorial restrictions, the current legal framework (legalization and regulation) for cannabis use in adults is addressed in the Cannabis Act (S.C. 2018, c.16) and summarized in Table 1.
Table 1: Summary of regulations instituted in the Cannabis Act (S.C. 2018, c.16)
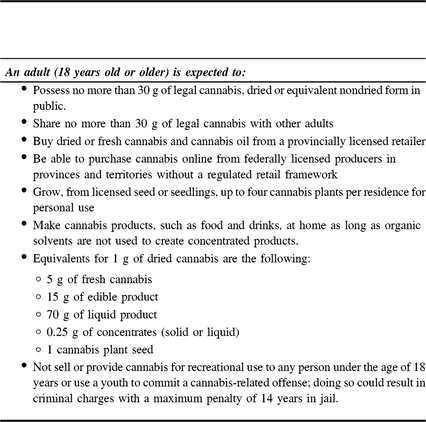
The Government of Canada has several online resources for the general public. A detailed explanation on how to access cannabis for medical purposes from a licensed producer can be found at https://www.canada.ca/en/health-canada/services/getting-cannabis-from-licensed-producer/accessing-from-licensed-producer.html. General information about the consumption of cannabis can be accessed at https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/licensed-producers/consumer-information-cannabis.html.
The process to access cannabis and its derivatives for medical purposes for Canadian patients was recently summarized by the Canadian Pediatric Society (CPS)Reference Rieder12 and by the Government of Canada (https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/understanding-new-access-to-cannabis-for-medical-purposes-regulations.html). Cannabis for medical use was sanctioned by Health Canada in 2001. Since then multiple revisions occurred. The most up-to-date version of the Access to Cannabis for Medical Purposes Regulations (ACMPR, last amended on January 15, 2019) is available at https://laws-lois.justice.gc.ca/PDF/SOR-2018-144.pdf under Part 14.13
The process to obtain cannabis for medical purposes is summarized in Table 2.

Figure 1: Example of a medical document obtained from Health Canada (http://www.hc-sc.gc.ca/dhp-mps/alt_formats/pdf/marihuana/info/med-eng.pdf). The medical document could also be obtained from the licensed producer websites or the respective colleges of each province or territory.
Table 2: Summary of the required steps to obtain cannabis for medical purposes

Despite the legalization of the consumption of medical cannabis in Canada, there are no regulations regarding when it is appropriate to use medical cannabis. This leaves the final decision to the discretion of physicians in consultation with the person living with epilepsy and/or their care partners/parents. Table 3 includes a list of resources for physicians practicing in different provinces or territories in Canada with links to access provincial regulatory policies. These regulations do not apply to out-of-country utilization where the use of cannabis could be considered a criminal offense.
Table 3: List of provincial policies and regulations for the use of medical cannabis
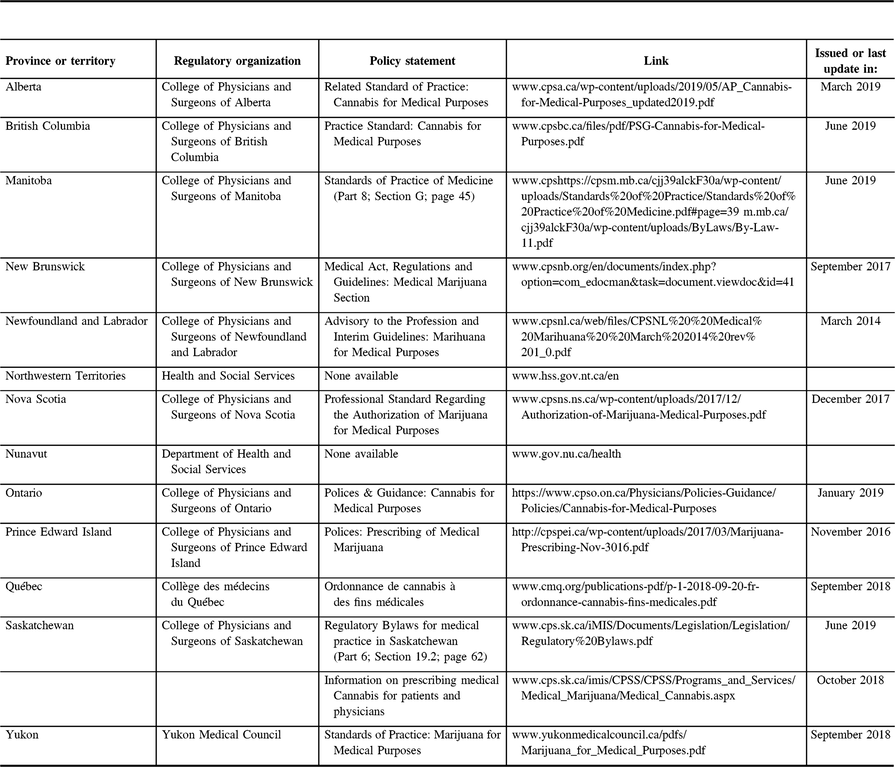
Summary of provincial prescribing regulations for medical cannabis. Notice that these regulations may include the use of medical cannabis for other purposes and not only for epilepsy. Each province has slightly different requirements and proceedings. Physicians working in different provinces should be familiar with local regulations. At the current time, Northwest Territories and Nunavut do not have prescribing regulations for medical cannabis, thus, physicians practicing in these areas should watch for new information or policies presented by regulatory entities or contact them directly for specific enquiries.
Of note, the Canadian Medical Association submitted a proposal to Health Canada to unify the cannabis regulations system for medical and recreational cannabis. If this were to happen, the maximum monthly cannabis supply allowed may need to be modified as some medical doses may require larger amounts of cannabis (https://www.cma.ca/sites/default/files/pdf/News/proposed-approach-regulation-cannabis-e.pdf).
Therapeutic Considerations
A pharmacological review of the anticonvulsant activity of the cannabinoids is beyond the scope of this document. For a detailed review we recommend the following references: Gaston and FriedmanReference Gaston and Friedman14 and Reddy and Golub.Reference Reddy and Golub15 In addition, we refer the reader to Dow-Edwards and SilvaReference Dow-Edwards and Silva16 for a review of the endocannabinoid system and its effects on brain plasticity during development.
A Cochrane reviewReference Gloss and Vickrey17 and a systematic review by the American Academy of NeurologyReference Koppel, Brust and Fife18 (both published in 2014) concluded that there was a lack of adequate data from randomized, controlled trials of THC, CBD, or any other cannabinoid to support the use of these compounds in the treatment of epilepsy. Since these publications however, there have been four published randomized placebo-controlled clinical trials using a purified CBD oil (Epidiolex®) to treat two well-recognized and very specific drug-resistant pediatric epilepsy syndromes, namely, Dravet syndromeReference Devinsky, Patel and Thiele7,Reference Devinsky, Cross and Laux8 and Lennox-Gastaut syndrome.Reference Devinsky, Patel and Cross9,Reference Thiele, Marsh and French10 Epidiolex® is a CBD solution with a concentration of 100 mg/ml. Results to date show CBD to be beneficial in treating drug-resistant epilepsy, with a reasonable safety profile for short-term use in the above syndromes. When compared to placebo, there was an overall relative improvement in seizure frequency of 20%–25% for all seizure types.Reference Devinsky, Patel and Thiele7–Reference Thiele, Marsh and French10,Reference Elliott, DeJean and Clifford19 The first study in Dravet syndrome showed a statistically significant reduction only of convulsive seizures reported by the parents of affected children but not in other seizure types.Reference Devinsky, Cross and Laux8 Furthermore, 5% of all patients were seizure free on CBD treatment and 57% of patients had a seizure reduction of less than 50%. Common side effects were noted in more than 30% of patients, in particular gastrointestinal disturbance and somnolence.Reference Devinsky, Cross and Laux8 CBD can increase the serum levels of some anti-seizure medications, particularly clobazam and its metabolite, which may have resulted in somnolence, at times severe, in some patients. Elevations in liver enzymes have also been reported.Reference Devinsky, Cross and Laux8–Reference Thiele, Marsh and French10
Clinical trials in patients with Lennox-Gastaut syndrome showed a statistically significant improvement in the frequency of parent-reported drop seizures and overall seizures.Reference Devinsky, Patel and Cross9,Reference Thiele, Marsh and French10 Despite the statistically significant improvement, clinical significance is not clear since the median number of drop seizures decreased from 85 to 50 per month (from 3 to 2 seizures a day). CBD doses trialed were 10 and 20 mg/kg/day. Study limitations included the lack of blinding due to noteworthy side effects in the Epidiolex® group. There was also a relatively high incidence of seizure worsening, including status epilepticus (up to 15%) in patients on Epidiolex® when compared to placebo.Reference Devinsky, Patel and Cross9,Reference Thiele, Marsh and French10
The results of the RCTs published to date do not readily translate to the Canadian experience as Epidiolex® (GW Pharmaceuticals, Cambridge, UK) is not available in Canada. In these studies, the maintenance dose used was between 10 and 20 mg/kg/day and only as an add-on therapy with other anti-seizure medication(s). The wide spectrum of CBD-containing products manufactured for medical purposes in Canada further precludes the ability to make any conclusions. Canadians mainly use oil formulations with a wide range of CBD-THC ratios. A recent, prospective open-label study in Dravet syndrome, using a cannabis oil product with a CBD-THC (50:1) performed in Canada,Reference McCoy, Wang and Zak6 resulted in a median motor seizure reduction of 70.6%, a statistically significant improvement in quality of life, and a reduction in electroencephalographic spike activity over a 20-week period. The small sample size of 19 patients and unblinded intervention, however, were significant study limitations.Reference McCoy, Wang and Zak6
Relatively long-term efficacy and safety was reported in an open-label ongoing expanded-access program study for the use of Epidiolex®.Reference Szaflarski, Bebin and Comi4 Of the 607 enrolled patients, 89 (15%) withdrew due to lack of efficacy and 32 (5%) withdrew due to adverse events. Only 138 (23%) patients were followed until week 96. The 50%, 75%, and 100% reduction in convulsive seizures rates by week 12 of treatment were 52%, 31%, and 11%, respectively. These differences remained similar throughout until week 96 in those patients with available data. However, 23%–29% of patients had increased convulsive seizure frequency and 24%–27% had an increase in total seizures by the last visit at week 96 of those with available data.Reference Szaflarski, Bebin and Comi4 Thus, about 50% of patients with drug-resistant epilepsy (mainly Dravet syndrome or Lennox-Gastaut syndrome) will have a 50% or greater seizure reduction, 25% will not improve or will not tolerate the intervention, and 25% will have an increase in seizure frequency.
In Canada, the current commercially available CBD-THC cannabis oil for the treatment of epilepsy ranges from 12.5:1 to 50:1, with variable concentrations of milligram per milliliter. In the authors’ experience, management decisions cannot simply be made by only considering a ratio. For instance, when comparing two different oil products with CBD-THC of 20:1 ratio, one product may contain 100:5 mg/ml of CBD-THC, while another may have 10:0.5 mg/ml. In both samples, the ratio is the same, 20:1 but the milligram amount of CBD and THC per ml is different. Due to these differences, CBD-THC cannabis oil products also have variable dried cannabis content. A recent European study comparing the reported and the actual concentration in commercially available CBD oils supports this observation. In this study, authors carried an in-depth chemical profiling of cannabinoids, terpenes, and oxidation products of 14 commercially available CBD oils. Nine (64%) samples had concentrations differing from the declared amount with only five maintaining the optimal limits.Reference Pavlovic, Nenna and Calvi20 The maintenance dose (as used in the four RCTs) was 10–20 mg/kg/day. Depending on the patient’s weight and the product used, this maintenance dose may require more than 150 g of dried cannabis per month, exceeding the legally allowed possession limit. In such cases, the practitioner may still authorize the appropriate dose, but the patient or parent/caregiver would have to order multiple batches of supply from the licensed producer, so that they do not possess more than 150 g at any one time.
The variability between cannabis oil products is also a major topic of confusion as it has a direct impact on doses, costs, and side effects, particularly when switching suppliers. Of note, according to a published paper in Canada, a combination of CBD-THC (50:1 with 50:1 mg/ml) was used, tapering to smaller doses by the end of the 20-week period, achieving a mean dose of 13.3 mg/kg/day of CBD (range 7–16 mg/kg/day) and 0.27 mg/kg/day of THC (range 0.14–0.32 mg/kg/day)Reference McCoy, Wang and Zak6 which could potentially support the use of a smaller daily dose of CBD (and THC). The cost of an appropriate daily CBD dose could range from CAD $800 to $1500 per month; regarding Epidiolex®, the cost for 100 ml is US$1297.84 (https://www.drugs.com/price-guide/epidiolex) and the yearly cost could be up to US$32,500. At present, cannabis oil is not covered by any medical insurance companies, and the cost must be borne out of pocket by the patient or family/care partners. This situation is unlikely to change, unless more evidence is obtained to support the use of medical cannabis.
Safety Considerations
Much of the available data regarding the safety and side effect profile of cannabinoids, especially with long-term use, are obtained from studies examining the effects of recreational marijuana, which contains high levels of THC. Few studies assessed the long-term effects of purified CBD. In studies of pediatric patients with severe epilepsy, the short-term side effects of cannabis included diarrhea, gastrointestinal intolerance, fatigue, and severe somnolence. In an open-label study of purified CBD, status epilepticus was observed in 6% of patients.Reference Devinsky, Marsh and Friedman21 More chronic side effects, particularly of THC include impairment of memory, judgment, cognition and motor performance, and psychosis.Reference Volkow, Swanson and Evins22 The use of CBD alone seems to have a minor to moderate impairment effect on driving. Combining CBD with alcohol, however, is a major concern.Reference Williamson and Evans23 Cannabis use during pregnancy, especially on a daily basis, has been associated with adverse neonatal outcomes leading to neurophysiological and behavioral abnormalities.Reference Calvigioni, Hurd, Harkany and Keimpema24,Reference Petrangelo, Czuzoj-Shulman, Balayla and Abenhaim25 The potential exposure to pesticides, which are used to keep the marijuana plant healthy, is also of concern during pregnancy.Reference Leung, Silva, Palumbo, Lohstroh, Koshlukova and DuTeaux26 It is unknown whether cannabis for medical use could produce similar results.
An important point to consider is the pharmacological interactions between CBD and commonly used anti-seizure medications. It is important to be aware of potentially important and concerning serum level increases in anti-seizure medications, particularly N-desmethylclobazam, eslicarbazepine, rufinamide, topiramate, and zonisamide. Altered liver enzyme levels are common when combining CBD and valproic acid; thus, close monitoring of liver function is key.Reference Gaston, Bebin and Cutter27 High levels of the biologically active clobazam metabolite (N-desmethylclobazam) were reported when given concomitantly with CBD. Patients on this combination reported an over twofold increase in somnolence as compared to patients not on clobazam.Reference Szaflarski, Bebin and Comi4 Careful monitoring of this interaction is therefore essential to avoid the complications of excessive somnolence, particularly respiratory failure and accidents. Measuring clobazam and N-desmethylclobazam levels in serum should be considered when side effects are noticed as CBD or THC levels in blood are not reliable indicators of toxicity. Currently there is no reliable serum level assay for CBD.
An additional important issue is the lack of consistency in CBD-THC concentrations from one batch of product to another as each marijuana plant possesses different concentrations of CBD and THC. The current regulations from Health Canada are attempting to address good production practices. However, there are no CBD-based products related to epilepsy treatment with a drug identification number at the present time (https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/licensed-producers/additional-information-licensed-producers-under-access-cannabis-medical-purposes-regulations.html#a2).
Information about the long-term side effects of purified CBD or combinations at different ratios of CBD-THC is lacking even when these products are appropriately dosed and medically supervised. Furthermore, the effect of CBD and/or THC on the developing brains of neonates, infants and children is not clear. Well-designed studies investigating the long-term side effects of CBD and/or THC are lacking.
Summary
Due to social media, patient and family advocacy groups, and Internet activity, there is significant public interest in cannabis as an alternative treatment of those living with epilepsy. CBD therapy offers a potentially promising alternative for patients who have failed to respond to many traditional anti-seizure medications, the ketogenic diet and/or surgical interventions. At present, the best data are available for two specific epilepsy syndromes: Dravet syndrome and Lennox-Gastaut syndrome. The extrapolation of these data to all seizure types or other epilepsy syndromes is difficult and not recommendable. The cost of treatment with reported maintenance doses is high. This therapy is not covered by medical insurance companies, provincial health programs, or the Ministry of Health and may place financial distress on already strained patients and their care partners/families. Although few RCTs are published, the reported findings are encouraging for Dravet and Lennox-Gastaut syndromes, showing significant improvements in seizure frequency in 20%–50% of patients. However, there exists a 20%–25% potential risk of significant side effects severe enough to warrant discontinuation. Furthermore, 15%–20% of patients do not show a decrease in seizure frequency. Information regarding the side effects of long-term use in humans and the risk in pregnancy is limited. Notably, the purified CBD oil (Epidiolex®) tested in the above described RCTs is not currently available in Canada. Canada remains dependent on a variety of products of differing quality and consistency.
Recommendations
We recognize that recent publications herein examined show some benefits of purified CBD oil (Epidiolex®) in patients with Dravet syndrome and Lennox-Gastaut syndrome with daily seizures. In our opinion, purified CBD oil without THC may be considered as an add-on treatment of patients with these two specific epilepsy syndromes and daily seizure frequency utilizing the reported dose (10–20 mg/kg/day) only when those patients have failed two appropriately prescribed and utilized anti-seizure medications. However, evidence is lacking for the remaining epilepsy syndromes and epilepsies not otherwise classified as well as for the other products currently available in Canada, containing a combination of CBD-THC (i.e. vaporizing, edibles, smoking, and others including CBD-THC oil).
Should a patient or a parent/guardian opt to use cannabis, we strongly recommend that this decision be made in consultation with their health-care provider to ensure their safety. We recommend that the treatment with CBD-THC cannabis oil be managed by a physician knowledgeable and experienced with epilepsy care and anti-seizure medications, preferably with experience in CBD-THC cannabis oil. The product should be procured from a Health Canada-approved licensed producer. It is important that physicians carry out an appropriate baseline assessment of the patient, ensuring no contraindications prior to starting the therapy and that potential drug interactions are monitored for, and appropriate laboratory evaluations are obtained. Physicians involved in the care of these patients should also be equipped with or have rapid access to the necessary medical infrastructure and personnel to manage potential complications should they arise.
We encourage clinicians and researchers to continue to seek further knowledge and education about this therapeutic approach. The authors also encourage government and not-for-profit entities to support and fund research in this area to reduce the potential biases associated with industry-sponsored trials.Reference Burnham, Eakin and Pakhale28 The recent US Food and Drug Administration approval of Epidiolex® in the USA for its use in certain epilepsy syndromes is noted.
The authors recognize that further clinical studies are underway to address the aforementioned limitations in Canada. This statement will be reviewed and modified in the future as more data and knowledge become available.
Acknowledgement
The authors would like to recognize and thank Suzanne Nurse, MD, who was a crucial initial advocate of this statement.
Conflict of Interest Disclosures
Dr. Appendino has nothing to disclose. Dr. Boelman has nothing to disclose. Dr. Brna has nothing to disclose. Dr. Burneo reports supports for educational and research activities from Jack Cowin Chair in Epilepsy Research, from Eisai Canada, and from Sunovion Canada, outside the submitted work. RPh. Curtis Claassen has nothing to disclose. Dr. Connolly is a co-investigator for the Cannabidiol in Children with Refractory Epileptic Encephalopathy study. For this study, it is used Cannimed’s 1:20 THC-CBD Cannabis herbal extract. Dr. Connolly receives no financial support from Cannimed and purchases the product used in the study from Cannimed at the cost of production. She has nothing to disclose. RPh. De Guzman has nothing to disclose. Dr. Federico has nothing to disclose. Mrs. Floyd has nothing to disclose. Dr. Huntsman is the lead investigator for the Cannabidiol in Children with Refractory Epileptic Encephalopathy study. For this study, it is used Cannimed’s 1:20 THC-CBD Cannabis herbal extract. Dr. Hunstman received no financial support from Cannimed and purchased the product used in the study from them at the cost of production. Dr. Javidan has nothing to disclose. Dr. Jette received grant funding paid to her institution for grants unrelated to this work from NINDS (NIH U24NS107201, NIH IU54NS100064), PCORI, and Alberta Health. She also received an honorarium for her work as an associate editor of Epilepsia. NP. Jurasek has nothing to disclose. Dr. Keezer reports personal fees from Elsevier, personal fees from Sunovion, personal fees from Novartis, personal fees from Sage Therapeutics, grants and personal fees from UCB, grants and personal fees from Eisai, outside the submitted work. Dr. Lau has nothing to disclose. Dr. McCoy is a PI in a study of cannabinoids in resistant epilepsy, which was part funded by study drug vendor. Dr. McLachlan has nothing to disclose. Dr. Ng has nothing to disclose. Dr. Nguyen reports grants and personal fees from UCB; personal fees from Sunovion and personal fees from Eisai outside the submitted work. Dr. Reid has nothing to disclose. Dr. Rho reports other from Dr. Robert Haslam Chair in Child Neurology, personal fees from UCB Pharma, personal fees from Danone Nutricia, personal fees from Accera Pharma, outside the submitted work. Dr. Snead , Dr. Tellez-Zenteno, and RPh. Wang have nothing to disclose. NP Maria Zak was a co-investigator of a CBD Study in Children with Dravet Syndrome in which the CBD was provided in kind by Tilray.
Statement of Authorship
JPA, compiled all feedbacks from co-authors, wrote the first draft of the manuscript and produced the final version of the manuscript after revisions and editing were made. CB, PMB, JGB, CSC, MBC, MDG, PF, DF, RH, MJ, NJ, LLJ, MK, JCL, BMC, RML, MCN, DN, AR, JMR, CS, JFT-Z, LW, and MZ equally discussed the topic and provided themes to be considered for the generation of the first draft, reviewed the manuscript at different stages, provided constant feedback, and collaborated with manuscript editing.






