Introduction
Ischemic stroke often leads to disabling sequelae or even death. Early prediction of functional outcomes after acute ischemic stroke could facilitate appropriate treatment and management. Several clinical factors have been identified as predictors of outcome, including age, sex, initial stroke severity, white matter hyperintensities (WMH), and vascular risk factors.Reference Johnston, Connors and Wagner 1 - Reference Meijer, Ihnenfeldt and de Groot 3
Cerebral microbleeds (CMBs) are small foci of chronic hemorrhages that have been increasingly detected with the widespread application of MRI techniques that are sensitive to magnetic susceptibility. CMBs are more prevalent in patients with ischemic stroke than healthy elderly subjects and are associated with increased risk of intracerebral hemorrhage, worse cognitive function, and poststroke depression.Reference Koennecke 4 - Reference Tang, Chen and Lu 7
More commonly observed in patients with small-vessel disease than subgroups with large-vessel atherosclerosis or cardioembolism,Reference Ovbiagele, Saver and Sanossian 8 CMBs are also a marker of cerebral small-vessel disease along with lacunes and WMH.Reference Poels, Ikram and van der Lugt 5 Although both lacunes and WMH are associated with poor functional outcomes and disability,Reference Kang, Stewart and Park 2 , Reference Koton, Schwammenthal and Merzeliak 9 - Reference Viswanathan, Gschwendtner and Guichard 11 whether CMBs are associated with poor functional outcomes after ischemic stroke has not been verified.
We tested the hypothesis that preexisting CMBs are associated with poor functional outcomes after ischemic stroke. We examined the associations between CMBs and functional outcomes at discharge and 6 months after acute ischemic stroke.
Methods
Patients
The ethics committee of Seoul St. Mary’s Hospital approved this study. We studied consecutive patients with acute ischemic stroke who were admitted to the Department of Neurology at Seoul St. Mary’s Hospital between May 1, 2011, and April 30, 2012. Clinical information obtained included age, sex, history of hypertension, diabetes mellitus, dyslipidemia, ischemic heart disease, previous stroke, and current cigarette smoking. All patients underwent clinical evaluation, including neurological examination, laboratory tests, chest radiography, electrocardiography, 24 h Holter monitoring, brain MRI, and contrast-enhanced magnetic resonance angiography (MRA). Stroke was defined on the basis of clinical history and the neurological examination with compatible new lesions on MRI. Patients were excluded if had any of the following conditions applied: (1) preexisting significant disability (defined as modified Rankin scale [mRS] ≥2 from any conditions); (2) history of stroke in the past 3 months; (3) hemorrhagic stroke; (4) transient neurologic symptoms without ischemic lesions on diffusion-weighted imaging; (5) received intravenous fibrinolysis or endovascular recanalization reperfusion therapy; (6) physical illness that was life-threatening or interfered with recovery from ischemic stroke; (7) subarachnoid hemorrhage, or symptoms suggestive of same even if CT scan was normal; and (8) any of the following comorbid neurological diseases: dementia, Parkinson’s disease or atypical Parkinsonism, brain tumor, intracranial vascular malformations, peripheral nerve or muscle diseases.
Assessment of Stroke Severity and Functional Outcomes
Severities and functional outcomes of stroke were assessed at admission, 24 h after admission, discharge, and 6 months after admission. Assessments used the National Institutes of Health Stroke Scale (NIHSS; scores range from 0 to 42, with higher scores indicating greater deficits)Reference Brott, Adams and Olinger 12 and the mRS (scores range from 0 [no symptoms] to 6 [death]).Reference van Swieten, Koudstaal and Visser 13 All assessments were performed by study investigators unaware of all clinical and image data.
Brain MRI
We executed multisequence MRI protocols within 24 h of admission on a 3-Tesla scanner (Verio, Siemens Medical Solutions, Erlangen, Germany). The conventional MRI protocol consisted of transverse T2/T1-weighted, fluid-attenuated inversion recovery sequences and sagittal T1 with 5-mm slices. Diffusion-weighted imaging and three-dimensional time-of-flight MRA of the intracranial arteries and contrast-enhanced MRA of the head and neck were also performed on the same system. A T2*-weighted gradient-recalled echo sequence with high spatial resolution and long echo time was used to detect microbleeds.
Analysis of CMBs
All gradient-recalled echo scans were reviewed by an experienced neuroradiologist (H.S.C.) who was unaware of all clinical data. Microbleeds were defined as focal areas of very low signal intensity. Signal voids caused by sulcal vessels, symmetric calcifications in the basal ganglia and/or choroid plexus, pineal calcifications, and signal averaging from bone were excluded.Reference van Swieten, Koudstaal and Visser 13
Size definitions of CMBs (maximum diameter) are not consistent among previous studies. Nonetheless, an upper diameter limit of 10 mm was applied in almost 80% of subjects in previous CMB studies.Reference Koennecke 4 , Reference Greenberg, Vernooij and Cordonnier 14 Therefore, we used an upper diameter limit of 10 mm and classified location of CMBs as lobar, deep, or infratentorial.Reference Vernooij, van der Lugt and Ikram 15
WMH Scoring
We rated WMH by using the semiquantitative visual rating system described by Scheltens and colleagues.Reference Scheltens, Barkhof and Leys 16 This rating scale provides four sum scores in a semiquantitative manner: periventricular hyperintensities (0-6), deep WMH (0-24), basal ganglia hyperintensities (0-30), and infratentorial hyperintensities (0-30). Lacunes (well-defined hypointense lesions >2 mm with a hyperintense rim on fluid-attenuated inversion recovery images), perivascular spaces (well-defined rounded area with the same signal characteristics as CSF), and old territorial infarcts (asymmetric ischemic lesions involving large-artery brain territory) were not counted.
Assessment of Demographic and Clinical Covariates
History of cardiovascular risk factors such as hypertension, diabetes, dyslipidemia, previous stroke, and ischemic heart disease were obtained from patients or their caregiver, or by medical records, as appropriate. Stroke location by hemisphere was divided into left, right, and bilateral by using brain diffusion-weighted MRI. Potential risk factors for stroke outcomes that we considered covariates were age, sex, initial NIHSS, stroke location by hemisphere, history of hypertension, diabetes, dyslipidemia, previous stroke, ischemic heart disease, and WMH.Reference Kang, Stewart and Park 2
Statistical Analyses
Dependent variables were functional outcomes measured by the mRS at both discharge and 6 months after ischemic stroke. Patients’ outcomes were dichotomized into good or poor functional outcomes by using a mRS score cutoff of 2 (slight disability) or 3 (able to walk without help but requiring some help in daily living activities), in accordance with previous studies.Reference Kang, Stewart and Park 2 , 17 , Reference Liou, Chen and Guo 18
Demographic and clinical characteristics and the presence of CMBs in either the whole brain area or in each categorized anatomical location were compared between the two groups by using independent-sample t tests, χ2 tests, or Fisher’s exact tests as appropriate. Associations between WMH and CMBs were determined using Spearman’s rank correlation. Associations between the number of CMBs in either the whole brain area or specific anatomical locations and poor functional outcome were also determined. Finally, logistic multivariable regression analysis of all variables with a probability value less than 0.2 in the univariate analysis was performed. Because the number of CMBs was highly skewed, consistent with previous CMBs studies,Reference Poels, Ikram and van der Lugt 5 , Reference Biffi, Halpin and Towfighi 19 , Reference Soo, Yang and Lam 20 we classified CMBs as present or absent in each anatomical region in the logistic multivariable regression analysis. For all tests, we set the significance level at p<0.05.
Results
Figure 1 shows a flow diagram of patients included in our study. Of 432 patients initially assessed, 194 were excluded on the basis of applied inclusion and exclusion criteria. Two hundred thirty-eight patients with acute ischemic stroke were evaluated and discharged from the hospital. Of these, 13 patients were lost to follow-up.

Figure 1 Flow diagram for recruitment. DWI, diffusion-weighted images.
Among the 225 ischemic stroke patients, 123 (54.7%) were male. Mean (±standard deviation) age was 67.6±13.7 years. One hundred forty-one patients (62.7%) had hypertension, 64 (28.4%) had diabetes, 87 (38.7%) had dyslipidemia, 19 (8.4%) had ischemic heart disease, and 44 (19.6%) had a history of stroke. Median time between symptom onset and examination was 17 h. Mean time from admission to hospital discharge was 7.3±8.7 days (median, 4 days). Initial NIHSS and mRS averaged 5.2±5.5 and 2.9±1.4, respectively. WHM were observed in 184 patients (81.8%) and the average WMH score was 8.6±8.6. WMH were most prevalent in the lobar (76%) area, followed by the periventricular (68%), deep (32.9%), and infratentorial (9.8%) areas (Table 1).
Table 1 Demographics and laboratory data of patients with and without CMBs

CBM; cerebral microbleed, LDL; low-density lipoprotein, WMH; white matter hyperintensity, NIHSS; National Institutes of Health Stroke Scale, mRS: modified Rankin scale.
Values represent mean with standard deviation or numbers of patients with percentage in parentheses.
Analyses were performed by independent-sample t tests and χ2 tests as appropriate.
*, p<0.05; **, p<0.01.
We found CMBs in 87 patients (38.7%), the presence of which was significantly related to older age [CMBs (–) vs. CMBs (+); 65.5±14.4 vs. 71.1±11.9 years; p=0.003], arterial hypertension (55.8% vs. 73.6%; p=0.007), and the presence of WMH (76.8% vs. 89.7%; p=0.015) (Table 1). Also, the number of CMBs was positively correlated with increasing WMH score (correlation coefficient r=0.430; p<0.001).
One hundred twenty-one (53.8%) patients were classified as having a good functional outcome (mRS≤2) at discharge, and 142 (63.1%) patients had good functional outcomes 6 months later. The group with a good functional outcome comprised younger patients with less severe stroke symptoms (lower initial NIHSS) and lower WMH scores than patients in the poor functional outcome group at both periods. Poor functional outcomes at 6 months were related to presence of previous stroke history than observed in those patients with good functional outcomes at 6 months (Table 2).
Table 2 Baseline characteristics by stroke outcome status at discharge and at 6 months

WMH; white matter hyperintensity, NIHSS; National Institutes of Health Stroke Scale, mRS: modified Rankin scale, CMB; cerebral microbleed.
Values represent mean with standard deviation or numbers of patients with percentage in parentheses.
a Good functional outcome, mRS 0–2; poor functional outcome, mRS ≥3.
b p value using independent-sample t tests, χ2 tests, or Fisher’s exact tests as appropriate.
*, p<0.05; **, p<0.01.
The presence of CMBs except was associated with a poor functional outcome at discharge [CMBs (+) patients in poor vs. good functional group; 48.1% vs. 30.6%; p=0.007 by χ2 test] and 6 months [53.0% vs. 30.3%; p=0.001 by χ2 test] (Table 2). After adjustment for confounding factors, only the presence of infratentorial CMBs was associated with a poor functional outcome at discharge (odds ratio, 2.6; 95% confidence interval, 1.2-6.0; p=0.021) and 6 months (odds ratio, 4.8; 95% confidence interval, 2.0-12.0; p=0.001) (Table 3). The poor functional outcome group more CMBs than did the good outcome group at 6 months (3.0±5.5 vs. 1.4±4.0; p=0.025 by t test).
Table 3 Associations of CMBs with poor functional outcomes (mRS score ≥3)
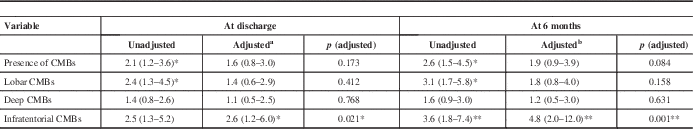
CMBs; cerebral microbleeds.
Data are odds ratio with 95% confidence interval in parentheses.
Analyses were performed with multiple logistic regression tests.
a Adjusted for age, history of previous stroke, WMH, initial scores on NIHSS.
b Adjusted for age, sex, history of hypertension and previous stroke, WMH, initial scores on NIHSS.
Discussion
In this study of patients with acute ischemic stroke, CMBs tended to be associated with poor functional outcomes. In particular, the presence of infratentorial CMBs was clearly associated with a worse outcome not only in the early phase of ischemic stroke but also in the chronic phase.
To the best of our knowledge, we are the first to report an association between CMBs and functional recovery after stroke. Our findings are consistent with the proposition that microbleeds could be a general marker for underlying vascular disease, in particular cerebral amyloid angiopathy or hypertensive arteriolosclerosis, and as such may influence functional outcomes after stroke.Reference Poels, Ikram and van der Lugt 5 In our study, the burden of CMBs was correlated with a higher WMH score. WMH, as a marker of cerebral small-vessel disease, can predict poorer functional outcomes after stroke.Reference Kang, Stewart and Park 2 , Reference Miyao, Takano and Teramoto 10 , Reference Viswanathan, Gschwendtner and Guichard 11 Severe white matter changes probably reflect chronically reduced tissue perfusion and cerebrovascular reactivity, which may lead to reduced penumbra survival after acute ischemic stroke.Reference Kang, Stewart and Park 2 , Reference Ay, Arsava and Rosand 21 , Reference Brown and Thore 22
Another possible explanation is that CMBs may reflect focal damage to brain tissue. Therefore, if they are located in areas important for motor regulatory functions and functional recovery after stroke such as the corticospinal tract, CMBs could interfere with recovery from functional deficits after stroke. We also found that especially infratentorial CMBs were associated with poor functional outcomes. Previous studies indicated that lobar microbleeds were associated with worse cognitive function.Reference Poels, Ikram and van der Lugt 5 , Reference Werring, Gregoire and Cipolotti 6 Different microbleed locations in the brain probably reflect differences in underlying etiologiesReference Poels, Vernooij and Ikram 23 ; deep or infratentorial microbleeds are related to hypertensive vasculopathy, whereas lobar microbleeds are thought to be a marker of pathologies associated with cerebral amyloid angiopathy, such as vascular deposition of amyloid or neuritic plaques.Reference Gilbert and Vinters 24 , Reference Greenberg, Eng and Ning 25 CMBs in different locations may therefore reflect differences in cognition or functional recovery after stroke due to different underlying pathologies. Infratentorial CMBs can affect many densely packed fiber pathways, including all the ascending and descending pathways linking the brain with functional peripheral nerves and muscles. Therefore, in contrast to lobar or deep microbleeds, infratentorial CMBs may lead to disconnection of functionally important structures for recovery after stroke.
In our study, CMBs affect long-term functional outcomes much more than short-term outcomes after ischemic stroke. Functional outcomes during the acute period may be associated with other clinical factors such as age, subtype of ischemic stroke, location of the involved vessels, infarct volume, and other comorbid medical conditions such as infection or hyperglycemia, whereas functional recovery during the chronic period may be more affected by preexisting structural lesions such as previous stroke lesions, WMH, or even CMBs. This assertion implies that patients with preexisting brain damage have less ability to compensate functionally than those without preexisting damage. These findings are supported by a previous study showing that WMH better predicted poorer functional outcomes in the chronic phase of ischemic stroke than in the acute phase.Reference Kang, Stewart and Park 2
This study had several strengths but also some limitations. A major strength was that this study was a prospective cohort study, and therefore was not likely to have been affected by informational or survival rate bias. Second, the cohort at follow-up had a low censoring rate (13/238, or 5.4%). However, several limitations should also be considered. First, because the number of microbleeds was highly skewed to both extremes, appropriate analysis of the burden of CMBs was difficult. Therefore, evaluation of the correlation between CMBs burden and functional outcome was challenging. We thus dichotomized microbleeds as present or absent in the logistic multivariable regression analysis. Second, excluding premorbid disability, dementia, and hemorrhagic stroke may have biased the results because these groups are most likely to have CMBs. Although we excluded patients with comorbid neurological diseases such as dementia or Parkinson’s disease, patients with mild cognitive impairment may have been included. Mild cognitive impairment is known as a prodromal stage of dementia and CMBs are associated with Alzheimer disease pathology burden. Therefore, care should be taken when interpreting the results because premorbid mild cognitive decline may have confounded findings. Finally, because NIHSS tends to overestimate dominant hemisphere and anterior circulation ischemic strokes, it would also introduce a confounding factor when used as a surrogate marker of stroke severity.
In conclusion, infratentorial CMBs were significantly associated with worse functional outcomes not only in the early phase of ischemic stroke but also in the chronic phase. These findings suggest that the presence of CMBs can predict long-term poor functional outcome after acute ischemic stroke.
Disclosures
The authors report no disclosures.






