Reactive oxygen species play a prominent role in inflammatory lung diseases, including asthma(Reference Henricks and Nijkamp1). Although the lungs are equipped with a variety of enzymatic and non-enzymatic antioxidant systems, there are indications that asthma is associated with a local oxidant/antioxidant imbalance, particularly during exacerbations of the disease(Reference Dut, Dizdar and Birben2). One of the most prominent endogenous antioxidants in the airways is glutathione (GSH)(Reference Biswas and Rahman3). It has been demonstrated in earlier studies that GSH and other low-molecular-weight thiols relax guinea-pig trachea ex vivo (Reference Kloek, van Ark and Bloksma4). In these guinea-pig studies for allergic asthma, it has been shown that the allergen-induced early asthmatic response is paralleled by markedly decreased lung GSH levels(Reference Kloek, van Ark and Bloksma4, Reference Joris Kloek, Esmaeil Mortaz and Ingrid van5). Moreover, the allergen-induced airway contractions could be prevented by a cell-permeable GSH analogue(Reference Joris Kloek, Esmaeil Mortaz and Ingrid van5).
To demonstrate the therapeutic relevance of these findings, it would be of interest to enhance GSH levels in vivo. Administration of GSH, however, is not an effective way to increase tissue GSH levels(Reference Anderson6), while the GSH prodrug cysteine is not useful because of its toxicity(Reference Karlsen, Grofova and Malthe-Sorenssen7, Reference Olney, Ho and Rhee8). The cysteine derivative N-acetylcysteine is not toxic, but its oral bioavailability is poor(Reference Cotgreave9). An alternative way to enhance GSH levels in vivo is the food supplement undenatured whey protein concentrate (UWPC). UWPC is a mixture of proteins rich in γ-glutamylglycine, a dipeptide which is known to increase intracellular GSH(Reference Anderson and Meister10, Reference Lothian, Grey and Kimoff11). Interestingly, a case report has referred to a patient with obstructive lung diseases whose improvement of pulmonary function and increase in plasma GSH levels could be correlated with UWPC intake(Reference Lothian, Grey and Kimoff11). Finally, whey protein supplementation seemed to have an effect on the cytokine response in atopic asthma as well(Reference Lothian, Grey and Lands12).
In the present study, the effect of UWPC was assessed using the above-described ex vivo guinea-pig model of allergic asthma. Guinea pigs were fed a UWPC diet from 3 d before sensitisation with the allergen onwards until killing 3 weeks later. At that time, allergen-induced contractions of isolated lungs, as well as lung perfusate levels of indicators for oxidative stress and mediator release such as prostaglandins, were measured. Furthermore, total GSH content of the liver was measured. Blood samples were taken before and at regular intervals after sensitisation to determine allergen-specific IgG levels in the serum.
Materials and methods
Animals
Male specific pathogen-free Dunkin Hartley guinea pigs (Harlan Nederland, Horst, The Netherlands) weighing 200–250 g at the start of the experiments were housed under controlled conditions and provided with food and water ad libitum. All experiments were approved by the University of Utrecht Committee on the Use and Care of Animals.
Diet regimen
UWPC (HMS90 purchased by Immunocal; Immunotec Research Limited; Vaudreuil, QC, Canada) was gently dissolved in sterile tap water at a concentration of 0·5 g/ml immediately before use. The animals (n 17) received UPWC per os using a syringe with a polyethylene tubing that was placed at the back of the mouth of the animal. Slowly emptying the syringe made the animals swallow spontaneously. Each animal received 5 ml of whey solution twice daily, with 7 h between the administrations. Controls (n 16) received sterile tap water following the same administration regimen. Whey administration started 3 d before sensitisation.
Ovalbumin sensitisation and challenge
Animals (water-fed group, n 7; UWPC-fed group, n 8) were sensitised on day 0 by injecting a mixture of 20 μg ovalbumin (grade V; Sigma, St Louis, MO, USA) and 200 mg of the adjuvant, Al(OH)3 (Merck, Darmstadt, Germany), in 1·0 ml saline. Al(OH)3 in saline was used for sham sensitisation of the control animals (water-fed group, n 9; UWPC-fed group, n 9). Each animal received six injections: 0·5 ml was injected intraperitoneally, and five injections (0·1 ml each) were given subcutaneously in the axillar and inguinal regions and in the nuchal area. On day 20, airways were isolated and challenged ex vivo with ovalbumin as described in the following section. Control animals were treated in the same way, although without ovalbumin. A scheme of the treatment protocol and the parameters that were measured is depicted in Fig. 1.
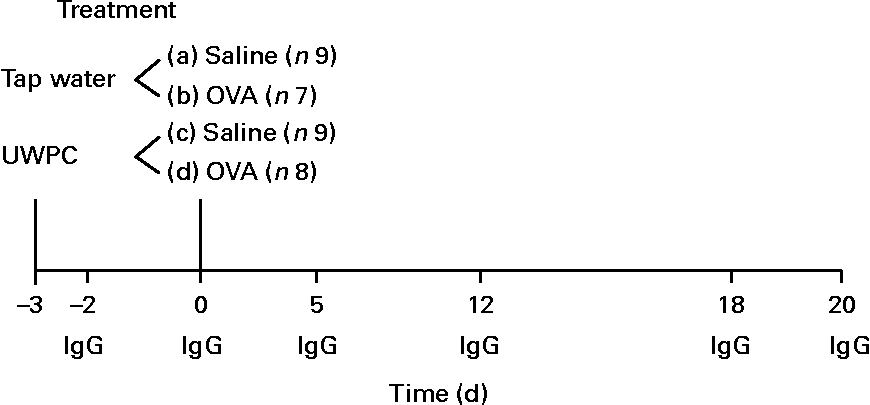
Fig. 1 Overview of the treatments and measured parameters. From day − 3, animals received tap water (n 16) or undenatured whey protein concentrate (UWPC, n 17) twice a day up to day 20. On day 0, animals were sensitised with ovalbumin (OVA, n 15) or received saline (n 18). On days − 3, 0, 5, 12, 18 and 20, blood samples were taken for IgG. At day 20, the airways were isolated and perfused with a buffer. Airway responses were measured ex vivo after the administration of OVA (challenged). In the perfusate, thiobarbituric acid-reactive substances, 8-iso-PGF2α and PGE2 were measured. Liver lobes after removal were used for the detection of total GSH as described in the Materials and methods.
Tracheally perfused lung preparation
Tracheal perfusion was performed on day 20 after sensitisation, as previously described(Reference Joris Kloek, Esmaeil Mortaz and Ingrid van5). Guinea pigs were anaesthetised by an intraperitoneal injection of 10 % (w/v) urethane (2 ml/100 g body weight). When a sufficient level of anaesthesia was achieved, a 2 cm-long polyethylene tube (inner diameter 1·67 mm, outer diameter 2·42 mm) was inserted into the trachea, and the abdominal cavity was opened to inject 500 IU/ml of heparin into the vena cava and the abdominal aorta 3 min later. Thereafter, the thoracic cavity was opened to remove heart and lungs en bloc. The lungs were dissected free and hung in a plexiglass box at 37°C and 100 % relative humidity. The lungs were perfused via the tracheal cannula with a phosphate-buffered physiological solution (pH 7·4) of the following composition: 137 mm-NaCl, 1·8 mm-CaCl2, 1·05 mm-MgCl2, 2·68 mm-KCl, 0·6 mm-NaHCO3, 0·13 mm-NaH2PO4 and 0·896 mm-Na2HPO4. The buffer was warmed to 45°C and pumped at a rate of 2 ml/min through a bubble trap before being cooled to 37°C for the actual lung perfusion. When the lungs had fully expanded, the buffer was allowed to exit the lungs through multiple small holes made in the pleura. The ‘back pressure’ resulting from the perfusion (airway opening pressure) was recorded from a side tap at the tracheal cannula with the use of a pressure transducer. We have previously shown that during continuous flow, the airway opening pressure reflects the contractile state of the lung(Reference Joris Kloek, Esmaeil Mortaz and Ingrid van5). Subsequently, lungs were perfused for 10 min to give a stable baseline pressure. For the induction of an early asthmatic response, 3·0 mg ovalbumin in 0·30 ml perfusion buffer was injected in the perfusion system as described earlier(Reference Joris Kloek, Esmaeil Mortaz and Ingrid van5).
Effluent analysis
Buffer that exited the lungs during the development of contractile responses was collected. In the case of non-sensitised animals, buffer was collected at the corresponding time. Samples were snap-frozen in liquid N2 and stored at − 80°C until analysis. Within 3 weeks after collection, the levels of PGE2, leukotrienes C4/D4/E4 and 8-iso-PGE2α were measured in the samples using commercial enzyme immunoassay kits (8-iso-PGF2α; Cayman, Ann Arbor, MI, USA; PGF2: Amersham Pharmacia Biotech, Uppsala, Sweden) according to the manufacturers' instructions. Samples (100 μl) for the measurement of thiobarbituric acid-reactive substances (TBARS) were acidified to pH 2–3 with 10 μl of 1 % (w/v) TCA. Protein was precipitated by centrifugation, and 100 μl of the supernatant was allowed to react with an equal volume of thiobarbituric acid (0·67 %, w/v) for 10 min at 100°C. After cooling to room temperature, the absorbance at 532 nm was measured photometrically(Reference Esterbauer and Cheeseman13). Perfusion buffer was treated in the same way to serve as a blank control.
Serum IgG
Blood samples ( ± 350 μl each) were collected from a femoral vein on days − 2, 5, 12, 18 and 20 (Fig. 1). Blood was allowed to clot for 30 min at room temperature before being centrifuged at 20 000 g for 10 min. Serum was collected and stored at − 20°C until analysis. IgG1 antibody responses to ovalbumin were assayed using an ELISA. Flat-bottom microplates (ninety-six-well plates; Maxisorp, Nunc A/S, Roskilde, Denmark) were coated with 100 μg ovalbumin/ml buffer (38 mm-Na2CO3 and 43 mm-KH2PO4; pH 9·5) for 60 min at 37°C. After washing with 0·05 % Tween 20 in PBS, free binding sites were blocked with 1 % bovine serum albumin (Boehringer Mannheim, Phoenix, AZ, USA) in ELISA buffer (50 mm-Tris, 2 mm-EDTA, 136·9 mm-NaCl and 0·05 % Tween 20; pH 7·2) for 60 min at 37°C. After removal of the blocking buffer and washing, serially diluted serum samples were incubated for 2 h at 37°C. After washing, the plates were incubated with horseradish peroxidase-conjugated goat anti-guinea-pig IgG1 and IgG2 (diluted 1:10 000 and 1:3000, respectively; Bethyl Laboratories, Montgomery, TX, USA) in ELISA buffer with 0·5 % bovine serum albumin. After washing, O-phenylenediamine dihydrochloride (0·4 mg/ml; Sigma) in PBS containing 0·012 % H2O2 was added. The reaction was stopped after 10 min through the addition of 4 m-H2SO4. Optical density was measured at 490 nm with a Titrek Multiscan (Flowlabs, Irvine, UK). Serum samples that had been pre-incubated (120 min at 37°C) with UWPC (25 ng/ml) underwent the same procedure to control for cross-reactivity of ovalbumin-specific IgG with UWPC, using pre-incubation with a similar amount of ovalbumin as a positive control.
Measurement of liver glutathione
After lung isolation, a liver lobe was snap-frozen in liquid N2 from each animal to be stored at − 80°C until analysis. After removal, each lobe was crushed in liquid N2 using a mortar and pestle; the resulting powder was transferred to an Eppendorf tube, and a mixture of 1 m-HClO4 with 2 mm-EDTA was added (1 ml/250 mg powder). After vigorous vortex mixing, tubes were centrifuged at 5000 g for 10 min, and total GSH (reduced and oxidised) concentrations in the supernatants were determined using a modified version(Reference Vandeputte, Guizon and Genestie-Denis14) of the GSH reductase–5,5′-dithio-bis-(2-nitrobenzoic acid) recycling assay according to Akerboom & Sies(Reference Akerboom and Sies15). Values were expressed as nmol/mg tissue (wet weight).
Data analysis and statistics
Tracings reflected the increase in airway opening pressure as a function of time. For each animal, the area under the curve was calculated from 50 to 150 s after the addition of allergen to the buffer. If data were normally distributed, they were analysed using a one-way ANOVA followed by the least significant difference post hoc test for multiple comparisons. If data were not normally distributed, they were analysed using the Kruskal–Wallis test. P values < 0·05 were considered to reflect significant differences. Student's unpaired t test was used when only two groups were compared with each other. From previous experiments, the minimum number of animals per group could be calculated to find a possible significant effect in airway responses.
Results
Liver glutathione and serum IgG levels
UWPC treatment significantly enhanced the levels of total GSH (2·75 (sd 0·7) nmol/mg, n 16, P < 0·05) in the liver as compared with water-fed guinea pigs (2·26 (sd 0·15) nmol/mg, n 17). We did not measure GSH levels in the lungs, since in an earlier study, we have described that GSH levels decrease after ovalbumin challenge in a similar set-up(Reference Joris Kloek, Esmaeil Mortaz and Ingrid van5).
Ovalbumin-specific IgG1 antibodies could not be detected in sera from sham-sensitised animals (data not shown). In the sera of ovalbumin-sensitised animals, a time-dependent increase in ovalbumin-specific IgG1 was observed (Fig. 2). UWPC feeding of sensitised animals did not alter plasma IgG1 levels as compared with the sensitised water-fed group. Furthermore, pre-incubation of serum samples with UWPC did not affect the binding of serum IgG1 to ovalbumin-coated plates, while pre-incubation with ovalbumin blocked the binding completely (data not shown).

Fig. 2 Levels of serum IgG1 from water- and undenatured whey protein concentrate (UWPC)-fed guinea pigs. IgG1 levels in the 1280-fold diluted serum of water-fed (□) and UWPC-fed (■) guinea pigs after sensitisation to ovalbumin. A time-dependent, but feeding-independent, increase in IgG1 levels was observed. Values are means, with their standard errors represented by vertical bars (water-fed group, n 7; UWPC-fed group n 8). No IgG1 was detectable in the saline-treated water-fed group (n 9) or UWPC-fed group (n 9). A490, absorbance at 490 nm.
Airway contractions
In the lungs of the control animals, ovalbumin perfusion did not change airway opening pressure from either water-fed or UWPC-fed animals. In contrast, ovalbumin provoked a clear airway contraction in the lungs from sensitised animals (Fig. 3(A)). UWPC feeding reduced the allergen-induced airway contractions in sensitised animals by 45 % as compared with sensitised animals that had received water as a control (P < 0·05). Body weights did not change between the experimental groups during the feeding period.

Fig. 3 Effects of undenatured whey protein concentrate (UWPC) on the allergen-induced early asthmatic airway contraction and PGE2 levels in perfusate samples of the lungs. Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different (P < 0·05) (A) Ovalbumin challenge induced contractions in the lungs from ovalbumin-sensitised guinea pigs (■), but not from saline-sensitised guinea pigs (□), but the ovalbumin-induced contractions were substantially reduced in the UWPC-fed group as compared with the water-fed group. Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different (P < 0·05). Group a is significantly different from b, but not from c and d. Group b is significantly different from c and d. Group c is significantly different from d. (B) Ovalbumin challenge increased PGE2 formation in the lungs from ovalbumin-sensitised animals (■) as compared with saline-treated animals (□), but less so upon UWPC feeding. Group a is significantly different from b, but not from c and d. Group b is significantly different from c but not from d. Group c did not differ from group d.
Effluent analysis
It is known that oxidative stress induces the release of several allergy-associated mediators. Indeed, the levels of PGE2 dramatically increased in the perfusates of allergen challenge of the lungs of sensitised animals compared with non-sensitised animals (P < 0·05; Fig. 3(B)). UWPC treatment decreased PGE2 levels in the perfusates of sensitised guinea-pig lungs by 55 % as compared with the corresponding water-fed control group. This effect, however, did not reach statistical significance. Leukotriene concentrations were below the detection limit of the assay.
Allergen challenge induces oxidative stress
There were no macroscopical differences of the lungs between saline- and ovalbumin-treated animals. To investigate whether allergen challenge induced oxidative stress, 8-iso-PGF2α and TBARS were measured in the effluent of ovalbumin- or sham-sensitised (control) animals challenged with ovalbumin. Allergen challenge significantly increased 8-iso-PGF2α levels (22·02 (sd 1·23) pg/ml, P < 0·01) compared with the saline group (9·6 (sd 0·6) pg/ml). Also, the levels of TBARS in the lung perfusate of sensitised animals were significantly elevated compared with the saline group (0·073 (sd 0·012) v. 0·041 (sd 0·005) optical density at A532, P < 0·05).
Discussion
We show for the first time that an anaphylactic bronchoconstriction is associated with oxidative stress, since 8-iso-PGF2α was increased in the perfusate that was obtained during the anaphylactic constrictions of the airways. Besides being an indicator of oxidative stress, 8-iso-PGF2α strongly constricts the airways(Reference Held and Uhlig16–Reference Wang, Young and Witten19). In earlier studies, we have found that GSH decreases in the lungs during the early asthmatic reaction(Reference Joris Kloek, Esmaeil Mortaz and Ingrid van5) and that GSH can decrease tracheal hyperreactivity to the contractile agent histamine(Reference Kloek, van Ark and Bloksma4). In contrast, GSH depletion renders the airways to become hyper-reactive to this mediator(Reference Phimister, Lee and Morin20). TBARS are endoperoxide breakdown products, but their formation does not require GSH(Reference Meagher and FitzGerald21). TBARS levels were increased in the perfusate after ovalbumin challenge as well, giving further support that anaphylaxis is associated with oxidative stress indeed. Therefore, oxidative stress and/or a decrease in antioxidants may lead to an enhancement in mediator release and/or the reactivity of the airways.
Furthermore, the present study demonstrates that UWPC food supplementation protects against early allergen-induced airway contractions in a guinea-pig model of allergic asthma. Since serum IgG levels were not affected by UWPC, the reduced airway contractions could not be explained by an effect of UWPC on sensitisation and, as a consequence, significant allergen-specific IgG1 levels, the antibodies that reportedly mediate immediate-type hypersensitivity in the guinea pig(Reference Fraser, Graziano and Larsen22). Liver GSH, however, was increased by 20 % by UWPC feeding, suggesting increased GSH levels in other tissues, including the lungs, as well. If so, UWPC treatment may have led to decreased allergen-induced contractions via a similar mechanism as perfusion with GSH or a GSH donor, which has been earlier shown to relax airway smooth muscle and prevent histamine- and allergen-induced airway contractions(Reference Kloek, van Ark and Bloksma4, Reference Joris Kloek, Esmaeil Mortaz and Ingrid van5). Unfortunately, lung GSH levels could not be measured reliably with the methodology used in the present study, since the contractile state of the airway influenced the amount of buffer in the lungs, making it impossible to relate GSH content to tissue weight.
The mechanism by which UWPC decreased the allergen-induced airway contractions is still unclear. The observation that an early asthmatic response is associated with decreased GSH levels and a disturbed reduced/oxidised GSH in guinea-pig lungs strongly suggested that allergen-induced airway contractions are accompanied by oxidative stress in the guinea pig(Reference Kloek, van Ark and Bloksma4, Reference Joris Kloek, Esmaeil Mortaz and Ingrid van5). This suggestion is substantiated by observations that two indicators of oxidative stress, 8-iso-PGF2α(Reference Roberts and Morrow23) and TBARS(Reference Aghdassi and Allard24), were increased in the effluent of lungs during the early asthmatic response.
It is conceivable that allergen-induced oxidative stress modulates the production of mediators. Indeed, besides 8-iso-PGF2α, another cyclo-oxygenase metabolite, namely PGE2, was profoundly increased after ovalbumin challenge. Interestingly, in the UWPC-fed group, which had 20 % higher GSH levels and a 40 % decreased airway contraction, the PGE2 levels were decreased by 55 %. PGE2 production is known to be stimulated by oxidative stress(Reference Becker, Madden and Newman25) and inhibited by a reactive oxygen species scavenger(Reference Brigham, Meyrick and Berry26). Additionally, it is known that PGE2 can dampen Th1 type of immune reactions leading to the up-regulation of the classical Th2-mediated allergy reaction. If PGE2 can be down-regulated, it might be very successful in skewing the immune response from Th2 towards Th1, which will lead to less severe allergic immune reactions with proteins.
This all suggests that oxidative stress probably contributes to the release of inflammatory mediators during anaphylaxis. Indeed, it has been shown by others that allergen-induced contractions of guinea-pig trachea were paralleled by histamine and PGE2 release(Reference Whigham, Cook and Stahl27). The contractile mediators, histamine and leukotrienes, which are known to be released upon allergen-induced mast cell activation(Reference Barnes, Chung and Page28) and whose release is potentiated by oxidative stress(Reference Masini, Palmerani and Gambassi29), appeared hardly or not at all detectable in the perfusate of ovalbumin-challenged lungs from sensitised animals, regardless of the feeding. Possibly, the flow rate (2 ml/min) of the perfusion buffer has caused too much dilution of mediators to detect them in the perfusate.
In conclusion, we demonstrated that an early asthmatic reaction is associated with oxidative stress. Increasing endogenous GSH levels by UWPC largely prevented the anaphylactic airway constriction. UWPC did not influence the production of IgG1 and thus sensitisation to ovalbumin. Therefore, besides the mediators released from mast cells by cross-linking of IgG1, it is likely that the oxidative stress induces additional mediator release which can be inhibited by UWPC. This opens a new therapeutic area for patients with allergic diseases, who may benefit from a whey-enriched diet.
Acknowledgements
The present study was funded by the Division of Pharmacology and Pathophysiology, Utrecht Institute for Pharmaceutical Sciences, Utrecht University, The Netherlands. The authors have no conflicts of interest. J. K., N. B., E. M., F. P. N. and G. F. conceived and designed the experiments. J. K., E. M. and I. V. A. performed the experiments. J. K., E. M., N. B., J. G., F. P. N. and G. F. analysed the data. J. K., E. M., I. V. A., N. B. and G. F. contributed the reagents/materials/analysis tools. J. K., E. M. and G. F. wrote the manuscript.





