Introduction
The importance of dietary amino acids (AAs) in protein synthesis and breakdown in health and disease has been widely discussed. AAs are ingested daily via the diet, followed by digestion, absorption and ultimately distribution to many important sites in the body. The net balance between protein synthesis and breakdown in the body during a day is the result of metabolic and physiological homeostasis. When this homeostasis is disturbed by disease, between meals a shift towards a more negative protein balance often occurs, ultimately resulting in loss of (skeletal muscle) protein. In these circumstances, an overall increase in protein turnover, a stimulation of protein breakdown in muscle, and an enhanced uptake of AAs by the splanchnic bed are key factors.
Alterations in the metabolic function and regulation of the gut and liver are often present in acute and chronic disease states, associated with an increased stress-response (i.e. inflammation). As a consequence, the extraction of AAs between meals is often increased to support a higher energy expenditure, hepatic gluconeogenesis, the production of acute phase proteins and other substrates related to the present condition. Importantly, the increased need for AAs by the splanchnic tissues potentially stimulates the breakdown of protein in muscle, draining muscle protein stores.
We and others have shown that high-grade inflammatory states such as sepsis and burn injury as well as low-grade systemic inflammatory conditions like cancer, chronic obstructive pulmonary disease (COPD), and cystic fibrosis (CF) are all characterized by changes in the interorgan exchange of several AAs.
When during a day, net protein breakdown exceeds net protein synthesis, catabolism will occur. To optimize the anabolic response to a meal, more specifically of muscle, detailed insight in the underlying mechanisms is needed.
Stimulation and inhibition of muscle protein synthesis are two metabolic pathways potentially regulated by different mechanisms(Reference Wolfe1). However, both depend on the availability of AAs in the extracellular pool (i.e., blood stream). As dietary protein is digested and absorbed by the gut, dietary AAs are extracted by the splanchnic area (i.e. portal drained viscera and liver) during their first pass or alternatively released into the systemic circulation (Fig. 1). The extent of their extraction reflects on their availability in the systemic circulation. Likely, it is the increase in extracellular AA concentrations after a meal that gives signal for protein synthesis(Reference Wolfe and Miller2) and consequently AAs are transported into the muscle. To the contrary, a fall in extracellular AA concentrations below basal levels inhibits the stimulation of muscle protein synthesis(Reference Wolfe1).

Fig. 1 Concept of splanchnic extraction of amino acids during feeding and availability to muscle for protein synthesis (PS).
Animal and human clinical trials in health and disease have investigated whether dietary protein and amino acid diets can positively modify the accretion of protein during a meal, thereby improving the overall protein balance throughout the day. However, there is concern for an anabolic resistance of skeletal muscle related to aging, disuse and the presence of inflammation that in various disease states could ultimately lead to more protein wasting. In the diets that have been introduced it appears that the quantity, as well as the dietary protein or amino acid composition of a meal is of crucial importance. Most recent studies have focused on the effects of adding the AA leucine (LEU) in combination with other ingredients to the diet. It appears that muscle protein synthesis can be stimulated with LEU diets under certain conditions. However, we still lack insight into the role of specific dietary amino acids like LEU in attenuating or preventing the loss of skeletal muscle in chronic or critically ill patients, which is of crucial importance in relation to their recovery, functional status, and overall outcome. Our main goal is to develop dietary modifications that lead to improved outcome and function of chronic and acute disease states based on translation of available knowledge from animal and human clinical research.
Important modifying factors for protein anabolism
There are two factors that play a key role in modifying the capacity for protein anabolism during feeding in situations where a chronic or acute disease is present: first-pass splanchnic extraction (SPE) of dietary AAs and anabolic resistance to dietary AAs.
First-pass splanchnic extraction of dietary amino acids
The splanchnic tissues play a key role in the regulation of protein turnover, because these tissues are responsible for the first-pass extraction of the dietary AAs and their release to the peripheral tissues (Fig. 1)(Reference Stoll and Burrin3). Dietary AAs absorbed by the splanchnic bed are used either for incorporation into protein of the splanchnic area (constitutive or labile proteins (e.g. pancreatic enzymes, mucus, mucosal villi)) or are converted into other products such as other AAs that subsequently can be used for protein synthesis or are to be oxidized(Reference Stoll and Burrin3). Splanchnic tissues are known for their high protein turnover rates. In situations of an altered metabolic function of these tissues (i.e. acute or chronic disease) this could potentially limit the flow and availability of dietary AAs to the peripheral tissues, thereby negatively influencing the metabolic response to a meal.
In previous studies, we and others have shown that the first-pass SPE of the AAs LEU and phenylalanine increases with age(Reference Tessari, Inchiostro and Barazzoni4–Reference Luiking, Deutz and Jakel8) (Fig. 2). Although the exact reason for this elevated SPE in the elderly is still unknown, it is suggested that it may contribute to the development of sarcopenia as hypothetically less AAs could be available to the peripheral tissues. Interestingly, Boirie and colleagues(Reference Boirie, Gachon and Beaufrere5) found a positive correlation between the SPE of dietary LEU and BMI in a small study group and this relationship appeared to be more pronounced in the elderly. The relatively large contribution of adipose tissue to an increased BMI in the elderly as well as the fat distribution could possibly enhance levels of inflammatory markers and therefore lead to a higher hepatic extraction of LEU. Limited data are available as to whether acute or chronic diseases further aggravate the age-related disturbances found in splanchnic amino acid extraction, Interestingly, a lower SPE was found in patients with moderate COPD associated with an enhanced anabolic response to a protein meal (Fig. 3), suggesting that the metabolic efficiency of feeding was increased in this COPD group(Reference Engelen, Rutten and De Castro9, Reference Engelen, Rutten and De Castro10). The reduced SPE in COPD is remarkable as COPD is a low-grade systemic inflammatory condition(Reference Gan, Man and Senthilselvan11, Reference Pinto-Plata, Mullerova and Toso12). Besides an adaptation to increased needs elsewhere in the body, the reduced SPE might reflect a reduced splanchnic protein turnover rate rather than a reduced splanchnic amino acid net utilization. Factors known to influence splanchnic protein turnover are nicotine use and the intake of certain drugs (e.g. non-steroidal anti-inflammatory drugs known to reduce blood flow in the splanchnic region)(Reference Hashimoto, Yoneda and Shimada13). We hypothesize that a reduced first-pass SPE is a compensatory mechanism of the body to keep an overall protein balance (Fig. 4). When this adaptive mechanism starts to fail, patients will lose their ability to counteract the protein loss during catabolic conditions.
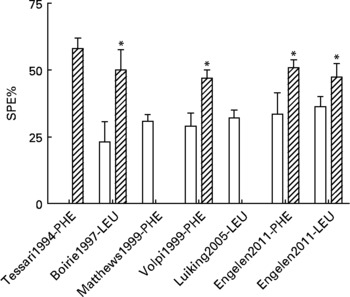
Fig. 2 Values are means ± SE. Splanchnic extraction (SPE) of phenylalanine (PHE) or leucine (LEU) stable isotopes in young (open bar) and elderly (striped bar) individuals(Reference Tessari, Inchiostro and Barazzoni4–Reference Luiking, Deutz and Jakel8) and unpublished results from Engelen et al., 2011. Significance of difference between young and elderly individuals *P < 0·05.

Fig. 3 Values are means ± SE. Net anabolism in COPD patients (striped bar) as reflected by the net increase in whole-body protein synthesis (WbPS) is associated with a reduced splanchnic extraction (SPE) of meal-derived amino acids as compared to healthy controls (open bar)(Reference Engelen, Rutten and De Castro9, Reference Engelen, Rutten and De Castro10) and unpublished results from Engelen et al., 2011. Significance of difference between COPD patients and healthy controls *P < 0·05, **P < 0·01.
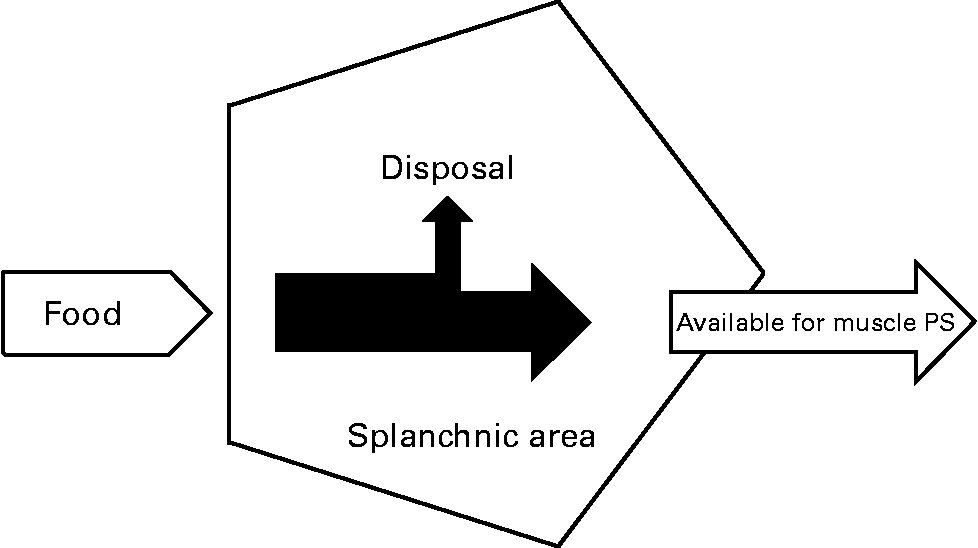
Fig. 4 Reduced splanchnic extraction during disease as an adaptive mechanism (refer Fig. 1) to make more amino acids available for muscle protein synthesis (PS) to compensate for the stimulated protein breakdown between meals.
Anabolic resistance to dietary protein
Aging
A relatively large portion of human research on protein metabolism has been performed in healthy young and elderly individuals. It has been shown that elderly who are active and have no evidence of an inflammatory condition, have comparable values for basal muscle protein turnover (synthesis and breakdown rates) as young adults(Reference Volpi, Sheffield-Moore and Rasmussen14–Reference Symons, Schutzler and Cocke17). Recent research does indicate the possibility of a gender related difference in muscle protein synthesis(Reference Smith, Atherton and Villareal18), explaining the slower progression of muscle loss in elderly women compared to men.
Although there are strong indications that the first-pass SPE of dietary AAs is higher in the elderly(Reference Tessari, Inchiostro and Barazzoni4–Reference Luiking, Deutz and Jakel8), Volpi and others showed that the amino acid availability to the muscle, subsequent amino acid uptake and muscle protein synthesis were not affected(Reference Volpi, Mittendorfer and Wolf7, Reference Koopman, Walrand and Beelen19). In line with this, but on a whole-body level, we found that although the SPE of several dietary AAs was higher in response to a whey protein meal in the elderly, protein synthesis was not affected (Engelen et al., unpublished results) (Fig. 5). Alternatively, Cuthbertson and colleagues did observe a blunted anabolic response on a muscle level to a mixture of free dietary essential amino acids (EAAs), ranging from 2·5 to 40 g, independent of insulin availability or dose of AAs(Reference Cuthbertson, Smith and Babraj16). A possible explanation for this could be a decreased activation of amino acid signaling proteins, such as mammalian target of rapamycin (mTOR), p70 S6 kinase and eukaryotic initiation factors, in the elderly.
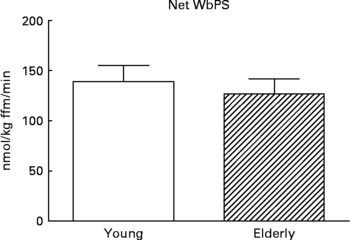
Fig. 5 Values are means ± SE. Net whole-body protein synthesis (Net WbPS) 2 h after continuous intake of a whey protein+carbohydrate meal in young and elderly individuals (unpublished results from Engelen et al., 2011). The complete drink supplied 8·1 g protein and 21 g maltodextrin (based on a 75 kg subject) over 2 h. No statistically significant interaction was observed between group and protein.
Disuse
Exercise, besides dietary protein, is an important stimulus of muscle protein synthesis. However in chronic as well as in acute disease states, patients are often subject to prolonged inactivity, caused by hospitalization, bed rest or a more gradual decline in daily activities. The disuse of skeletal muscle triggers certain metabolic alterations in protein turnover, resulting in disuse atrophy. As described in a recent review by Rennie et al. (Reference Rennie, Selby and Atherton20), disuse primarily affects muscle protein synthesis, with no concomitant increase but rather a reduction in muscle protein breakdown. Different models of disuse (i.e. knee immobilization or bed rest for 10 to 28 d) have shown this down regulation of muscle protein synthesis(Reference Paddon-Jones, Sheffield-Moore and Cree21–Reference Ferrando, Paddon-Jones and Hays23). In addition, it could be that longer periods of disuse are associated with a more progressive reduction in protein synthesis, as a study in healthy elderly showed that the total mean nitrogen balance was significantly lower in the second half of a 10 d period of bed rest(Reference Kortebein, Ferrando and Lombeida24).
More importantly, attempts to increase protein synthesis after inactivity, by amino acid supplementation has revealed an anabolic resistance of skeletal muscle to feeding. After 14 d of unilateral knee immobilization in healthy young adults, 4 h of infusion with a low as well as a high dose of mixed AAs was not able to acutely stimulate muscle protein synthesis to the same extent as in the mobilized leg(Reference Glover, Phillips and Oates22). The low and high dose resulted in an increase (55 % vs. 70 %, respectively) in anabolic response in the mobilized leg(Reference Glover, Phillips and Oates22). Interestingly, a recent study by Ferrando and colleagues(Reference Ferrando, Paddon-Jones and Hays23) showed that supplementation with dietary EAAs (high in LEU) was capable of maintaining muscle protein synthesis at baseline level during 10 d of bed rest, which otherwise decreased by 30 %, indicating the anabolic potential of dietary EAAs.
Systemic inflammation
It is suggested that a systemic inflammatory response contributes to the pathogenesis of muscle wasting. Systemic inflammation is a condition characterized by increased plasma concentrations of several inflammatory markers, such as C-reactive protein, fibrinogen, leukocytes, IL-6 and 8 and tumor necrosis factor-α. In healthy elderly, increased concentrations of plasma C-reactive protein, IL-6 and tumor necrosis factor receptor II were all negatively related to muscle protein synthesis(Reference Toth, Matthews and Tracy25). The liver, as the main production site of acute phase reactants, extracts an increased proportion of alimentary AAs to support the accelerated acute phase response. Thus, if and when the availability of AAs to muscle is decreased, the response of muscle protein synthesis could be blunted. In addition, the delivery of dietary AAs to the liver appears to result in an enhanced splanchnic consumption of certain dietary EAAs(Reference Reeds, Fjeld and Jahoor26) and therefore the amino acid profile may be unbalanced for the skeletal muscle, thereby negatively influencing skeletal muscle anabolism.
In conclusion, several factors such as aging, disuse and systemic inflammation play a key role in modifying the anabolic capacity of the body. It is expected that these effects might be even more pronounced when a chronic or acute disease state is present.
Protein and amino acid supplementation
Can we overcome the discussed factors by modifying the diet? Dietary protein and amino acid supplements are the most likely candidates to do this and both appear to be primarily capable of stimulating muscle protein synthesis, whereas muscle protein breakdown rates often remain unchanged(Reference Biolo, Tipton and Klein27, Reference Volpi, Mittendorfer and Wolf7, Reference Katsanos, Kobayashi and Sheffield-Moore28, Reference Dillon, Volpi and Wolfe29).
Protein quantity and quality
Supplementation of dietary protein in healthy individuals stimulates muscle protein synthesis and is responsible for a net anabolic response to feeding. However, the capacity for muscle protein synthesis is determined to a certain degree by the amount of protein intake. A recent study in healthy young and elderly showed that a threefold higher dietary protein intake was not favourable above a single dose of 30 g dietary protein ( = 0·4 g /kg body weight for a person of 75 kg, ~1/3th of average daily protein intake)(Reference Symons, Sheffield-Moore and Wolfe30) and muscle protein synthesis was equally stimulated by 50 %. On the other end, a study by Katsanos and others showed that a small protein meal (7 g) did not result in comparable stimulation of muscle protein synthesis in young and elderly, indicating a higher threshold for protein synthesis in the elderly(Reference Katsanos, Kobayashi and Sheffield-Moore28). This higher threshold for protein synthesis could be another important factor in the development of sarcopenia and therefore a recommended dietary allowance for protein intake of 1·5 g protein/day should be aimed for to maintain muscle mass and function in the elderly(Reference Wolfe, Miller and Miller31).
There is still some controversy as to whether the quality of dietary protein is an important factor influencing muscle protein synthesis. We recently performed a study in healthy young individuals and showed that the quality of the protein provided did not differentially influence muscle protein kinetics(Reference Luiking, Engelen and Soeters32). The mixed muscle fractional synthetic response and net muscle protein breakdown were comparable after intake of soya protein versus an isonitrogenous amount of casein protein (Fig. 6). Adversely, in a recent study by Tang(Reference Tang, Moore and Kujbida33), the proteins whey and soya, were favourable in comparison to the casein protein in stimulating skeletal muscle protein synthesis in healthy young men. The differences found between casein, whey and soya protein may largely be related to how quickly the proteins are digested (fast vs. slow) instead of the quality of the amino acid composition of the proteins. A possible explanation for the greater muscle synthetic response after whey and soya protein ingestion could be the more rapid and high plasma appearance of AAs compared to the slower and moderate hyperaminoacidaemia resulting from casein intake(Reference Boirie, Dangin and Gachon34). However, it may well be the case that the observed differences in the capacity of different proteins to stimulate muscle protein synthesis are related to the duration of the observation period after intake of the protein meals. A recent study from Denmark(Reference Reitelseder, Agergaard and Doessing35) clearly showed that when muscle protein synthesis was measured in the first 3 h after the meal, whey protein was more anabolic, whereas casein protein was more anabolic when measuring between 3 and 6 h after the meal. Interestingly, no differences were found between casein and whey proteins in their muscle anabolic effect when measuring over the whole 0–6 h period after meal intake. This might also explain our own observation when comparing soy and casein proteins(Reference Luiking, Engelen and Soeters32). It could well be that the essential amino acid content and composition of the different dietary proteins, and especially the LEU content are more decisive factors for a positive effect on muscle protein synthesis in situations where a chronic or acute disease is present. The differences between the types of proteins that we eat are probably not large enough to make a difference in healthy, young individuals in an unstressed condition. However, it might well be possible that the prandial observation periods when comparing different proteins are often chosen to be too short and that therefore certain (wrong) conclusions have been made.

Fig. 6 Values are means ± SE. Mixed muscle fractional synthesis rate (FSR) 4 h after continues intake of dietary casein protein (CAPM) or soya protein (SOPM) in healthy young subjects(Reference Luiking, Engelen and Soeters32). The complete drink supplied 0·2 g protein/kg BW/4 h (about 14 g/subject/4 h) and 42 g carbohydrates. No significant interaction was observed between group and protein.
Free total and dietary essential amino acids
Muscle protein synthesis in the elderly can be stimulated by continuous infusion of mixed AAs in the post-absorptive state(Reference Volpi, Ferrando and Yeckel36) and also by a bolus ingestion of dietary EAAs(Reference Paddon-Jones, Sheffield-Moore and Zhang15). However, increasing evidence indicates that only dietary EAAs appear to be required for the stimulation of muscle protein synthesis in healthy elderly(Reference Volpi, Kobayashi and Sheffield-Moore37, Reference Paddon-Jones, Sheffield-Moore and Katsanos38). A research study performed in healthy young men showed a similar effect, as only the dietary EAAs were able to increase the incorporation of L-[1-13C]LEU into muscle protein.
Specific role of leucine
The dietary EAA leucine (LEU), isoleucine and valine (identified as the branched-chain amino acids (BCAA)) form a group of AAs primarily prevalent in skeletal muscle. LEU is of particular interest due to the increasing evidence indicating the diverse roles of LEU and the specific regulatory role that LEU plays in stimulation of muscle protein synthesis. LEU alters muscle protein synthesis by regulating the translation initiation of several eukaryotic initiation factors (eIF4E, eIF4G) and ribosomal protein kinase S6 (p70S6)(Reference Kimball and Jefferson39). LEU furthermore functions as a nitrogen donor for the production of alanine and glutamine (GLN) in muscle(Reference Rennie and Tipton40) and LEU is one of the relatively small number of AAs which are known to enhance insulin secretion from the primary islet cells and β-cell lines(Reference Newsholme, Brennan and Rubi41). Previous studies have shown that infusion of the amino acid LEU resulted in the strongest insulin increase compared to other AAs(Reference Floyd, Fajans and Conn42).
In a healthy population, the anabolic response to LEU appears to be distinct between the young and elderly(Reference Cuthbertson, Smith and Babraj16), which could be related to the enhanced extraction by the gut, liver or both described in the elderly by Boirie et al. (Reference Boirie, Gachon and Beaufrere5). Consistent with the notion that LEU plays a regulatory role in the stimulation of muscle protein synthesis, it has recently been shown that dietary supplementation with extra LEU improves the response of muscle protein synthesis in elderly subjects(Reference Katsanos, Kobayashi and Sheffield-Moore43–Reference Rieu, Balage and Sornet45), whereas in young adults the addition of extra LEU did not facilitate an increased stimulatory effect(Reference Katsanos, Kobayashi and Sheffield-Moore43). Therefore, raising the plasma concentration of LEU by the provision of a LEU-enriched medical food potentially can overcome impaired responsiveness of muscle protein synthesis in the elderly and creates a rationale for using LEU as an important anabolic substrate for patients with increased muscle loss(Reference Cuthbertson, Smith and Babraj16). It can be argued that supplementation of LEU without co-ingestion of the other BCAAs valine and isoleucine could result in a lower muscle protein synthesis, the so-called BCAA-antagonism, as all three precursors are required for skeletal muscle protein synthesis(Reference Tipton, Elliott and Ferrando46).
Citrulline as an alternative stimulator
Citrulline (CIT), produced by the gut from GLN and ARG (approximately 80–90 % of CIT is produced via this conversion) is metabolized to ARG in the kidneys. After the release by the kidneys, ARG becomes available in the circulation for uptake by peripheral (muscle) tissue. ARG availability is dependent on dietary intake and the de novo synthesis of ARG from CIT. A possible role of CIT in modifying protein kinetics is through the availability of CIT in the kidney and the conversion of CIT to ARG, regulated by plasma CIT availability(Reference van de Poll, Siroen and van Leeuwen47).
It has been observed that dietary intake of both ARG(Reference Bruins48–Reference Zhang, Chinkes and Wolfe53) and (more recently) CIT(Reference Cynober, Moinard and De Bandt54) stimulate muscle protein synthesis, and that CIT per gram is more effective than ARG. Probably this is related to the fact that CIT escapes hepatic uptake and because of a more efficient dietary absorption of CIT in the gut. Through the GLN-CIT-ARG pathway, CIT as a precursor for ARG might well be a more potent stimulator of protein synthesis than ARG itself.
Protein metabolism and the response to feeding in acute and chronic wasting diseases
It is well known that in acute and chronic diseases alterations in protein synthesis and breakdown have a detrimental effect on muscle protein stores. As muscle protein stores become depleted, patients experience muscle wasting, negatively influencing their prognosis and functional capabilities. As the available research in chronic disease states (such as cancer, COPD, CF) and acute diseases has so far mainly been on the potential anabolic properties of certain proteins (i.e. whey and casein) and AAs (i.e. EAAs, LEU, ARG and CIT), this review is specifically focused on these specific proteins and AAs.
Cancer
Cancer is often associated with a constellation of responses which together lead to cachexia, the syndrome of anorexia, muscle wasting and progressive depletion of adipose tissue. In addition to a loss of appetite, metabolic changes occur in cancer patients that may amplify the loss of muscle. These metabolic changes may result from inflammation, insulin resistance, hypogonadism, or other causes(Reference Tisdale55). To continue, the rate of muscle protein synthesis has been reported to be reduced in patients with established cancer cachexia(Reference Dillon, Volpi and Wolfe29, Reference Durham, Dillon and Sheffield-Moore56) and whole-body protein turnover was found to be elevated in cancer patients unrelated to the presence of weight loss(Reference Melville, McNurlan and Calder57). Previously, we and others showed that metabolic changes are also apparent in the plasma amino acid profile of patients with breast, colonic, pancreatic and lung cancer, even without weight loss being present, with strong indications for a disturbed ARG metabolism(Reference Melville, McNurlan and Calder57, Reference Vissers, Dejong and Luiking58). Besides, in skeletal muscle the catabolic machinery is already activated (enhanced ubiquitin mRNA levels and proteasome activity) prior to significant weight loss(Reference Bossola, Muscaritoli and Costelli59, Reference Bossola, Muscaritoli and Costelli60).
It has been suggested that the metabolic response to cancer (i.e. hepatic acute phase response) prevents the efficient use of food and in combination with a reduced intake, gains in lean mass are difficult to achieve. A recent study in cachectic patients with advanced ovarian cancer showed that patients responded less anabolically following receiving a balanced mixture of 40 g of dietary EAAs and non-EAAs compared to healthy controls. The data indicated that while these cancer patients were able to mount an acute anabolic response to a high amount of AAs during the absorptive period, anabolism was attenuated(Reference Dillon, Volpi and Wolfe29). We recently compared two different nutritional supplements and observed that muscle protein synthesis was stimulated by a high leucine/high protein supplement, but not by a conventional supplement(Reference Deutz, Safar and Schutzler61). Further human research in cancer is needed to confirm these findings and to examine whether only high dosages of dietary AAs are capable of stimulating anabolism. Although human studies examining the specific role of LEU in stimulating protein anabolism in cancer have, to our knowledge, not been described yet, animal studies show promising results. A study performed in mice bearing a cachexia-inducing tumour showed that muscle protein synthesis was increased and protein breakdown attenuated in response to a high amount of LEU (1 g kg− 1 d− 1)(Reference Eley, Russell and Tisdale62). In another study, using tumour-bearing cachectic mice(Reference Peters, van Helvoort and Kegler63), muscle-mass loss was counteracted dose-dependently by leucine supplementation (0 vs 1 vs 8 g of supplemented leucine per kg feed, for 21 d), however a change in markers of protein breakdown was not seen. Very limited research has been performed in cancer patients in relation to muscle protein stimulation by specific AAs and therefore, in our opinion, amelioration of muscle loss in these patients is still very difficult to target.
Chronic Obstructive Pulmonary Disease
In moderate to severe COPD basal protein turnover is enhanced, reflected by an increase in whole-body protein synthesis and whole-body protein breakdown (WbPB)(Reference Engelen, Deutz and Wouters64). Therefore, even though the net catabolic response resembles the response that is seen in healthy individuals, turnover rates are increased, indicating a higher metabolic demand in COPD, creating a drain on the body protein stores. Other important observations relating to interorgan metabolism in COPD are reduced levels of muscle glutamate (GLU) and plasma BCAAs(Reference Engelen and Schols65), mostly due to a fall in LEU(Reference Hofford, Milakofsky and Vogel66, Reference Engelen, Wouters and Deutz67).
Besides changes in basal protein turnover and amino acid levels, an altered inter-organ response to feeding is also seen in COPD. We observed that patients with moderate COPD have a higher anabolic response to a casein-based protein meal than healthy controls, caused by both a stimulation of whole-body protein synthesis and attenuation of WbPB(Reference Engelen, Rutten and De Castro9). The larger reduction in WbPB in COPD patients compared to healthy controls was explained by a lower SPE of phenylalanine by the gut, liver, or both during feeding, which could lead to a higher peripheral availability of dietary phenylalanine(Reference Engelen, Rutten and De Castro9). Furthermore, a recent study investigating the effects of soya feeding with or without added BCAAs in COPD and healthy controls(Reference Engelen, Rutten and De Castro10) showed that soya feeding with added BCAAs only improved whole-body protein synthesis in COPD (Fig. 7). In both groups, BCAA supplementation reduced splanchnic protein synthesis, suggesting that BCAA supplementation alters inter-organ metabolism in favour of the peripheral (i.e. muscle) compartment. When comparing whey, casein and soya protein with and without BCAAs, it is even more clear that net anabolism in COPD patients is likely associated with a reduced SPE of meal-derived AAs (Fig. 3).

Fig. 7 Values are means ± SE. Net increase in whole-body protein synthesis (WbPS) after intake of soya protein (open bar) or co-ingestion of branched-chain amino acids (BCAAs) and soya protein (striped bar) in COPD and healthy controls(Reference Engelen, Rutten and De Castro10). Significance of difference between ingestion of soya protein and soya protein with co-ingestion of BCAAs in COPD group *P < 0·05.
In summary, it can be suggested that feeding of a protein supplement high in BCAAs and the addition of LEU might counterbalance the low plasma concentration of these AAs, optimally stimulate muscle protein synthesis and reduce SPE in COPD.
Cystic Fibrosis
In CF, disease-related factors including anorexia, inflammation, hypoxia, inactivity, and impaired glucose tolerance all contribute to the loss of skeletal muscle by altering the balance between protein synthesis and breakdown. Children with CF with acute exacerbations of their lung disease appear to have a strongly attenuated protein synthesis rate as compared to healthy children (about 50 % lower) and children with chronic but stable pulmonary disease (about 43 % lower)(Reference Holt, Ward and Francis68). In the latter group, a greater WbPB was found as compared to healthy children and CF patients suffering from an acute exacerbation.
Information about the anabolic effects of specific nutritional modulation on protein metabolism in diseased children is limited and therefore strategies to improve muscle gain via food intake are difficult to achieve. However, children with stable CF prove to be capable of improving protein metabolism in response to a high dose of protein (4 g kg− 1 d− 1), since whole-body protein synthesis was increased by 23 % with no concomitant effect on WbPB, resulting in a net anabolic response(Reference Geukers, Oudshoorn and Taminiau69). In a similar group of children without CF this high dose of protein significantly increased WbPB, indicating that feeding affects protein turnover differently in children with stable CF(Reference Kien, Zipf and Horswill70). Another study in stunted children with stable CF showed similar results and a dose-response effect on protein synthesis was observed when protein was provided at 1·5, 3 and 5 g protein kg− 1 d− 1, respectively, for 4 d. The protein synthesis rate was 30 % higher in the high-protein group compared to the low-protein group and resulted in a much greater net anabolic response(Reference Geukers, Oudshoorn and Taminiau69). The specific role of insulin in inhibiting protein loss in CF should also be a focus of attention. Due to a reduced production of insulin (as a result of pancreatic insufficiency)(Reference Kien, Horswill and Zipf71) and the diminished ability of insulin to suppress endogenous protein breakdown(Reference Hardin, LeBlanc and Lukenbaugh72, Reference Moran, Milla and Ducret73) new protein accretion might be hampered.
More extensive research in CF and also in other disease states of children is required to determine the specific nutritional requirements of these children and whether they respond more anabolically to specific amino acid mixtures, with a special role for the amino acid LEU as an anabolic stimulus and for its insulinotropic properties.
Acute disease
On the Intensive Care Unit, the net catabolic response of skeletal muscle is a key observation in patients with sepsis and burn injury, and is strongly associated with their severe inflammatory condition. Mostly by reference to animal studies, it has been shown that during high levels of inflammation, muscle protein synthesis is decreased(Reference Lang, Frost and Vary74, Reference Lang, Frost and Bronson75). We previously showed that reduced plasma levels of ARG and CIT are present in the acute phase of critical illness in children, related to an increased level of C-reactive protein(Reference van Waardenburg, de Betue and Luiking76). The reduced level of plasma ARG can be explained by an increased usage of ARG for nitric oxide production, increased arginase activity(Reference Argaman, Young and Noviski77), decreased renal de novo ARG synthesis from conversion of its precursor CIT(Reference Luiking, Engelen and Deutz78), or can be the result of an elevated protein synthesis in the liver (formation of acute phase reactants) or decreased dietary intake. We hypothesize that a decreased plasma concentration of CIT could be caused by a decreased GLN and GLU availability in the gut or a decreased gut function associated with the severe inflammatory milieu. A depletion of plasma ARG and CIT in critical illness may inhibit peripheral skeletal muscle protein synthesis because ARG is needed as a precursor for the formation of a complete protein.
Goals of nutritional support in acute disease are clear in terms of stimulating synthesis of proteins in the muscle, gut and liver. At the same time, it is essential that nutritional support has no adverse effects (e.g. further blood acidification and increase in plasma urea concentration). Endogenous non-EAAs are generally available in excess during sepsis because of accelerated release from muscle protein catabolism(Reference Bruins, Deutz and Soeters79) and increased de novo synthesis resulting from oxidation of dietary EAAs(Reference Bruins, Deutz and Soeters79). Therefore, we anticipate that, as shown in elderly populations, only dietary EAAs are necessary in acute critical illness to improve net protein synthesis and would be beneficial above complete amino acid mixtures by reducing the chance of adverse effects. The initiation of protein synthesis involves a series of steps involving eukaryotic initiation factors(Reference Rhoads, Niu and Odle80) and it has been shown that LEU, and more recently ARG can activate these factors(Reference Rhoads, Niu and Odle80, Reference Corl, Odle and Niu81). And although in sepsis a “leucine resistance” is described caused by an attenuated phosphorylation of initiation factors(Reference Lang, Frost and Vary74), both LEU and ARG could play a specific role in muscle protein synthesis beyond their role as a precursor for protein synthesis. In addition, CIT instead of ARG could be preferred as a dietary stimulus, since a greater stimulation of muscle protein synthesis was found with CIT(Reference Cynober, Moinard and De Bandt54).
In conclusion, more clinical research is needed examining the specific protein metabolic effects of dietary EAAs, CIT, ARG and LEU supplementation in acute critical illness.
Conclusions
A higher SPE of dietary AAs, caused by aging and inflammation, alters the amino acid availability for the skeletal muscle and along with the anabolic resistance to dietary protein, as observed in the elderly and in increased states of inflammation, negatively affecting protein anabolism. Muscle disuse, often a characteristic of acute and chronic disease appears to amplify the anabolic resistance to dietary protein, therefore further aggravating muscle loss. Dietary interventions with specific proteins, amino acid mixtures and EAAs appear to be mostly capable of stimulating (muscle) protein synthesis. The majority of research so far has been performed in healthy subjects (young and old). The available research in disease states shows a most promising role for the dietary EAAs LEU and ARG, with new perspectives on the role of CIT that need to be further explored. Moreover, it would be of scientific and clinical interest to investigate in more detail whether in certain disease states combinations of proteins and/or amino acids have the potential to induce even higher levels of anabolism. More research in disease states is crucial to establish specific dietary recommendations for various chronic and acute diseases.
Acknowledgements
The authors declare no conflict of interest.
The work described was supported in part by The National Heart, Lung and Blood Institute (award number R-01HL095903). The content is solely the responsibility of the authors and does not necessarily represent official views of the National Heart, Lung and Blood Institute or the National Institutes of Health.
All authors have made substantial contributions and have given approval to the conception, drafting, and final version of the manuscript.









