Intra-uterine growth restriction (IUGR) is defined as impaired growth and development of the embryo and/or its organs during gestation( Reference Wu, Bazer and Wallace 1 ). Approximately 5–10 % of human neonates and 15–20 % of piglets suffer from IUGR, which has been a common problem in human health and animal production( Reference Wu, Bazer and Wallace 1 , Reference McMillen and Robinson 2 ). Neonates with IUGR have been found to have increased morbidity and mortality during the early life period, and have been associated with long-term effects on the risk of adult-onset diseases( Reference Aucott, Donohue and Northington 3 , Reference Garite, Clark and Thorp 4 ). Because of IUGR, the development and function of immune system is impaired in neonates( Reference Cromi, Ghezzi and Raffaelli 5 ). Also, IUGR has been found to cause immunodeficiency and increased vulnerability to infectious diseases in later life( Reference Raqib, Alam and Sarker 6 – Reference Ferguson 8 ). In animal models, poor proliferation of lymphocytes in thymus and decreasing cytokine levels in peripheral blood had been observed in rats and sheep with IUGR( Reference Contreras, Yu and Hale 9 , Reference Gao, Liu and Zhang 10 ).
Neonates with IUGR are generally fed high nutritional diet to achieve rapid growth, which predisposes to postnatal metabolic syndrome such as obesity, diabetes and CVD( Reference Claris, Beltrand and Levy-Marchal 11 – Reference Berends, Fernandez-Twinn and Martin-Gronert 13 ). Furthermore, the recent study has demonstrated that high nutrient intake could impair systematic and intestinal innate immunity of piglets with IUGR( Reference Han, Hu and Xuan 14 ). In contrast, postnatal milk restriction by cross-fostering feeding was able to protect rats with IUGR from the metabolic syndrome( Reference Dai, Thamotharan and Garg 15 , Reference Desai, Gayle and Babu 16 ). In addition, the low nutrient density has been shown to be beneficial for the immune system of broilers( Reference Guo, Li and Chen 17 ) and primates( Reference Messaoudi, Warner and Fischer 18 ), probably by enhancing antioxidant capability and T-lymphocyte response( Reference Jolly 19 ).
Gastrointestinal tract is the major location for digestion and absorption of nutrients, and the gut-associated lymphoid tissue is the largest immune organ in the body( Reference Zhong, Li and Huang 20 ). In the present study, therefore, we investigated the effects of postnatal nutritional restriction on systematic and intestinal immune function of piglets with IUGR. Because of the structural and physiological similarities of gastrointestinal tract between pigs and human beings, pigs have been recognised as an ideal model for the study of clinical nutrition( Reference Sangild 21 , Reference Ferenc, Pietrzak and Godlewski 22 ). As a multi-fetal domestic animal, pigs have exhibited severe naturally occurring IUGR due to utero-placental insufficiency( Reference Sangild 21 ).
Materials and methods
Animal care and formula milk
The experiment followed the actual law of animal protection and was approved by the Animal Care and Use Committee of the Sichuan Agricultural University, and was performed in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals. The basic formula milk powder (Table 1) was formulated according to our previous studies( Reference Han, Hu and Xuan 14 ). The basic nutrient-level formula milk was prepared by mixing 1 kg of formula milk powder (DM 87·5 %) with 4 litres of water to a milk solution, which was prepared to supply the same amount of nutrient as sow milk.
Table 1 Composition and nutrient level of the basal formula milk powder (87·5 % DM basis, %)
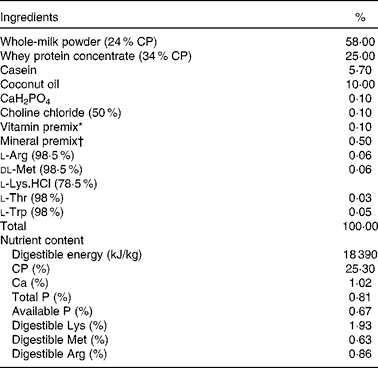
CP, crude protein.
* Vitamin premix provided per kg powder diet: vitamin A, 0·94 mg; vitamin D3, 0·01 mg; vitamin E, 20 mg; vitamin K3, 1 mg; vitamin B12, 0·04 mg; riboflavin, 5 mg; niacin, 20 mg; pantothenic acid, 15 mg; folic acid, 1·5 mg; thiamin, 1·5 mg; pyridoxine, 2 mg; biotin, 0·1 mg.
† Mineral premix provided per kg powder diet: Zn, 90 mg; Mn, 4·0 mg; Fe, 90 mg; Cu, 6·0 mg; I, 0·2 mg; Se, 0·3 mg.
Postnatal nutritional restriction model
A total of twelve pairs of newborn boars (Pig Improvement Company 327 × 1050) of normal-birth weight (NBW) with a body weight (BW) of 1·56 (sd 0·05) kg and IUGR with a BW of 0·91 (sd 0·03) kg from twelve healthy sows with the same litter size (ten piglets/litter) were chosen according to the previous studies( Reference Che, Thymann and Bering 23 ). All piglets were weaned at 7 d of age, and were moved to be individually fed with formula milk per meal every 3 h by bottle feeding between 06.00 and 24.00 hours in nursing cages (0·8 m × 0·7 m × 0·4 m). For nutritional treatments, six pairs of NBW and IUGR piglets were randomly assigned to have adequate nutrient intake (ANI) and the other six pairs were allocated to have restricted nutrient intake (RNI) in pairs. Thus, in total, four groups (birth weight-nutrient intake) of male piglets were created and studied: NBW-ANI; IUGR-ANI; NBW-RNI; IUGR-RNI (n 6). Among them, NBW-ANI and IUGR-ANI piglets had formula milk ad libitum, NBW-RNI piglets were provided the same amount of formula milk as IUGR-ANI piglets, while IUGR-RNI piglets were provided approximately 70 % of formula milk intake by IUGR-ANI pigs (Table 3). All piglets had free access to drinking-water. Room temperature was maintained at approximately 30°C, and humidity was controlled between 50 and 60 %. BW and formula milk intake of piglets were recorded daily. The average daily DM intake was calculated via multiplying the average daily intake of formula milk by its DM content (%), while formula milk intake was calculated as the difference between the offered amounts and the refusals.
Blood sampling and analyses
Blood samples were collected by venepuncture on the morning (08.00 hours) of day 21 after an overnight fast, and were injected into two vacuum tubes containing sodium heparin. The vacuum tubes were immediately placed on ice until they arrived at the veterinary hospital for the examination of leucocytes and flow cytometry analysis, respectively (within 2 h). Leucocyte examinations (neutrophil, lymphocyte and monocyte counts) were done through an automatic blood analyser. Total peripheral blood lymphocytes were separated from heparinised peripheral blood by separation medium, and were then stained with mouse anti-porcine CD3e-SPRD (PE-Cy5; catalogue no. 4510-13), CD4a-FITC (catalogue no. 4515-02) and CD8a-PE (catalogue no. 4520-09), which were purchased from Southern Biotechnology Associates. PBS (1 × ; Gibco) and 1·0 % bovine serum albumin (ICN Biomedicals) were used as diluent and washing buffer. Flow cytometry analysis was performed on a FACSCalibur flow cytometer (Becton Dickinson) and was repeated for the same sample and compared for repeatability.
Tissue sample collection
Piglets were weighed, and crown-rump length (CRL) was taken (the supine length of the piglet from the crown of its head to the base of its tail) at days 7 and 28. BMI (BW/CRL2) was calculated for each piglet. At day 28, all piglets were anaesthetised with an intravenous injection of sodium pentobarbital (15 mg/kg BW) and killed. The liver, spleen, kidney, heart and pancreas of each piglet were weighed immediately. The length and weight of the small intestine were measured after the removal of luminal contents. Duodenal, jejunal and ileal samples of approximately 2 cm in length were stored in 4 % paraformaldehyde solution for histological analyses. The rest of the ileum was snap-frozen and then stored at − 80°C until further analysis.
Small intestinal morphology and goblet cell counting
The duodenal, jejunal and ileal samples were preserved in 4 % paraformaldehyde solution and then embedded in paraffin. Each sample (duodenum, jejunum and ileum) was used to prepare five slides, and each slide had three sections (5 μm thickness), which were stained with eosin and haematoxylin for intestinal morphology measurement by twenty well-oriented villi and crypts each section (Optimus software version 6.5; Media Cybergenetics), and villi:crypt ratio (VCR) was calculated. The goblet cell number per villus was measured (NIS-Elements BR 2.3; Nikon France SAS). The values obtained from ten villi from each small intestinal segment per piglet were averaged.
Enzyme analyses
After thawing, the frozen jejunal sample was weighed and homogenised (5 min) in the nine times volume of 50 mm-Tris–HCl buffer, pH 7·0, centrifuged (3000 g , 10 min), and the supernatant was collected and stored at − 20°C for the enzyme assay. Total proteins were extracted, and their concentration was determined according to the procedure of bicinchoninic acid (Solarbio, Inc.), with bovine serum albumin as the standard. Disaccharidase (including maltase, sucrase and lactase) and alkaline phosphatase (AP) were measured using commercial kits (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer's instructions. The absorbance was determined with spectrophotometer (Beckman Coulter DU-800; Beckman Coulter, Inc.). The activities of disaccharidases were presented as U/mg protein, while the AP activity was presented as U/g protein. One unit (U) was defined as 1 nmol maltose, sucrose, lactose or AP as substrate for the enzymatic reaction.
Total RNA extraction and real-time RT-PCR
Total RNA was extracted from frozen placenta using TRIzol Reagent (catalogue no. 15 596-026; Invitrogen) according to the manufacturer's instructions. The quality and purity of RNA samples were assessed by electrophoresis on 1·0 % agarose gel and nucleic acid analyser (A260/A280, Beckman DU-800; Beckman Coulter, Inc.), respectively. Subsequently, the RNA was performed at 37°C for 15 min, followed by RT inactivation at 85°C for 5 s using PrimeScript™ RT reagent Kit (catalogue no. RR047A; Takara). A 1 μl portion of the RT products was used directly for real-time PCR. Real-time PCR was performed on ABI-7900HT instrument (Applied Biosystems). Oligonucleotide primers were used to detect the gene expressions of the target gene and the reference gene (β-actin) using the SYBR green system (catalogue no. RR820A; Takara). The sequences of primers and length of the products were presented in Table 2. The reaction mixture (10 μl) contained 5 μl of fresh SYBR®
Premix Ex TaqII (Tli RNaseH Plus) and 0·2 μl ROX Reference Dye II (50 × ), 0·8 μl of the primers, 1 μl of RT products and 3 μl of diethylpyrocarbonate-treated water. The following PCR protocol was used: one cycle (95°C 30 s); forty cycles (95°C 5 s, 60°C 31 s); one cycle (95°C 15 s, 60°C 1 min and 95°C 15 s). The standard curve of each gene was run in duplicate and three times for obtaining reliable amplification efficiency values as described previously(
Reference Chen, Chen and Tian
24
). The correlation coefficients (r) of all the standard curves were >0·99, and the amplification efficiency values were between 90 and 110 %. At the end of amplification, melting curve analysis was performed to identify amplification specificity. β-Actin transcript was used to standardise the results by eliminating variations in mRNA and complementary DNA quantity and quality, and each mRNA level was expressed as its ratio to β-actin mRNA. The relative quantification of gene expression among the treatment groups was analysed by the
![]() $$2^{ - \Delta \Delta C _{t}} $$
method(
Reference Livak and Schmittgen
25
).
$$2^{ - \Delta \Delta C _{t}} $$
method(
Reference Livak and Schmittgen
25
).
Table 2 Primer sequences of the target and reference genes

TLR, Toll-like receptor; MyD88, myeloid differentiation factor 88; TRAF-6, TNF receptor-associated factor 6; SIGIRR, single Ig IL-1-related receptor; TOLLIP, Toll-interacting protein; NOD2, nucleotide-binding oligomerisation domain 2; DNMT, DNA methyltransferase.
Statistical analysis
Results are presented as means with their standard errors. Data of intestinal morphology were analysed as repeated measures using the MIXED procedure of Statistical Product and Service Solutions 20.0 (SPSS, Inc.) according to the following model:
where Y ijkl represents the dependent variable, μ is the mean, α i is the effect of BW (i =IUGR, NBW), β j is the effect of NI (j =ANI, RNI), (αβ) ij is the interaction between BW and NI, U k ~N (0, σ2) is the litter (k= 1, 2,…, 12), ω l is the segment (duodenum, jejunum and ileum), (αω) il refers to the interaction between BW and segment, (βω) jl refers to the interaction between NI and segment, (αβω) ijl refers to the interaction between BW, NI and segment, and ε ijkl represents the error term. Data on growth performance, organ indices, blood leucocytes, lymphocyte percentages, enzyme activities and gene expressions were analysed according to the model, but omitting the effect of segment and the interaction between segment, BW and NI. Differences between groups were also analysed using general linear model procedure followed by Duncan's test. P< 0·05 was considered as statistically significant.
Results
Growth performance
In the present study, IUGR piglets had lower BW ( − 33–38 %, P< 0·001) and shorter CRL ( − 10–17 %, P< 0·001) than NBW piglets, and these differences persisted until 4 weeks of age (Table 3). Regardless of BW, RNI markedly decreased the net weight gain ( − 25 %, P= 0·002), average daily gain ( − 26 %, P= 0·002) and average daily DM intake ( − 29 %, P< 0·001) of piglets; however, no difference in feed conversion ratio was observed. Throughout the experimental period, moreover, there was similar average daily gain and net weight gain between the IUGR-ANI and the NBW-RNI piglets due to their similar average daily DM intake and feed conversion ratio.
Table 3 Effects of the level of nutrient intake (NI) on the growth performance of intra-uterine growth restricted (IUGR) and normal-birth weight (NBW) neonates (Mean values with their standard errors)

ANI, adequate nutrient intake; RNI, restricted nutrient intake; BW, body weight; CRL, crown-rump length; ADG, average daily gain; ADMI, average daily DM intake; FCR, feed conversion ratio.
a,b,c,dMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* FCR was calculated by dividing the ADMI by its corresponding ADG.
Organ indices
Regardless of NI, weights of internal organs such as intestine, heart, liver, spleen, kidney, brain and pancreas were markedly decreased ( − 7–33 %, P< 0·010) in IUGR relative to NBW piglets (Table 4). However, the relative intestinal length, heart and brain weights to BW were significantly higher (+21–41 %, P< 0·050) in IUGR relative to NBW piglets. Meanwhile, BMI values at both days 7 and 28 were significantly decreased by IUGR ( − 11–19 %, P< 0·001) relative to NBW piglets. Regardless of BW, moreover, the weights of heart (P= 0·019), liver (P= 0·010) and kidney (P= 0·014) were markedly reduced by RNI, while NI and BW had significant interaction on the weight of heart (P= 0·003).
Table 4 Effects of the level of nutrient intake (NI) on the organ indices of intra-uterine growth restricted (IUGR) and normal-birth weight (NBW) neonates (Mean values with their standard errors)

ANI, adequate nutrient intake; RNI, restricted nutrient intake; BW, body weight; L, length; wt, weight.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Composition of peripheral leucocytes and lymphocyte percentages
In Fig. 1, regardless of BW, the counts of leucocytes, lymphocytes and monocytes were significantly decreased ( − 20–45 %, P< 0·010) by RNI. Moreover, the percentage of lymphocytes decreased (P= 0·028) while the percentage of neutrophils increased (P= 0·036) by RNI. In addition, IUGR-RNI piglets had lower counts of leucocytes and lymphocytes (P< 0·050) than IUGR-ANI piglets, respectively. The percentage of CD8+ T cells had the tendency to decrease (P= 0·083) by RNI, resulting in the increased (P= 0·034) ratio of CD4+ to CD8+ (Fig. 2(D)). Furthermore, the percentage of CD8+ of IUGR-RNI piglets was lower (P< 0·050) than that in IUGR-ANI piglets (Fig. 2(C)).

Fig. 1 Effects of the level of nutrient intake on the count and percentage of blood leucocytes (A), neutrophils (B and E), lymphocytes (C and F) and monocytes (D and G) in intra-uterine growth restricted (■) and normal-birth weight (□) neonates. Values are means, with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05). Mean values were significantly different from those of the adequate nutrient intake (ANI) group: * P< 0·05, ** P< 0·01 (significant effect of level of nutrient intake). There was no significant interaction between body weight and nutrient intake. RNI, restricted nutrient intake.

Fig. 2 Effects of the level of nutrient intake on the percentage of CD3+ (A), CD4+ (B), CD8+ (C) T-lymphocytes and the ratio of CD4+ to CD8+ (D) in intra-uterine growth restricted (■) and normal-birth weight (□) neonates. Values are means, with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05). * Mean values were significantly different from those of the adequate nutrient intake (ANI) group (P< 0·05; significant effect of level of nutrient intake). There was no significant interaction between body weight and nutrient intake. RNI, restricted nutrient intake.
Intestinal morphology and goblet cell density
BW and NI had a significant interaction effect on villous height (P= 0·001) and the VCR (P< 0·001). The villous height (P< 0·001), the crypt depth (P< 0·001) and the VCR (P< 0·035) were significantly affected by the segment in the small intestine, with the duodenum having the highest villous height and the deepest crypt depth and the jejunum having the highest VCR (Table 5). Moreover, the density of the total goblet cells per intestinal villus of the IUGR-RNI piglets was higher (P< 0·050) than that of the IUGR-ANI piglets in the small intestine (Table 6).
Table 5 Effects of the level of nutrient intake (NI) on the intestinal morphology of intra-uterine growth restricted (IUGR) and normal-birth weight (NBW) neonates (Mean values with their standard errors)
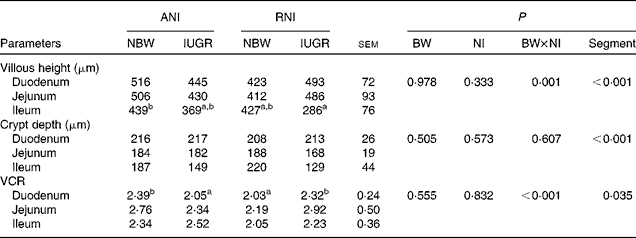
ANI, adequate nutrient intake; RNI, restricted nutrient intake; BW, body weight; VCR, villi:crypt ratio.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05). There was no significant interaction between NI and Segment.
Table 6 Effects of the level of nutrient intake (NI) on the density of goblet cells in the small intestine of intra-uterine growth restricted (IUGR) and normal-birth weight (NBW) neonates (Mean values with their standard errors)

ANI, adequate nutrient intake; RNI, restricted nutrient intake; BW, body weight.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05). There was no significant interaction between NI and Segment.
Jejunal enzyme activities
Irrespective of NI, the activity of jejunal AP was markedly lower (P= 0·028), while the activity of lactase was markedly higher (P= 0·046) in IUGR piglets relative to NBW piglets (Table 7). Furthermore, IUGR-RNI piglets had similar AP activity as NBW-RNI piglets, while it was much lower in IUGR-ANI piglets relative to NBW-ANI piglets (P< 0·050).
Table 7 Effects of the level of nutrient intake (NI) on enzyme activities in the jejunum of intra-uterine growth restricted (IUGR) and normal-birth weight (NBW) neonates (Mean values with their standard errors)
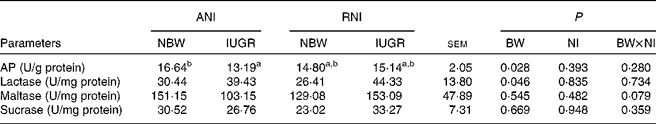
ANI, adequate nutrient intake; RNI, restricted nutrient intake; BW, body weight; AP, alkaline phosphatase.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Gene expression in the ileum
Regardless of NI, the mRNA abundance of Toll-like receptor 9 (TLR-9) was markedly increased (P= 0·030), and DNA methyltransferase 1 (DNMT1) tended to increase (P= 0·070) in the ileum of IUGR piglets relative to NBW piglets. Regardless of BW, moreover, the mRNA abundance of TLR-9 and DNMT1 was significantly higher (P< 0·010), while the mRNA abundance of TNF receptor-associated factor 6 (TRAF-6) and nucleotide-binding oligomerisation domain 2 (NOD2) was significantly lower (P< 0·010) in the ileum tissues of piglets with RNI than that of piglets with ANI. In addition, the significant interaction between BW and NI was observed for the mRNA abundance of DNMT1 (P= 0·007) in the ileum (Fig. 3).

Fig. 3 Effects of the level of nutrient intake on the mRNA abundance of Toll-like receptor 9 (TLR-9) (A), TNF receptor-associated factor 6 (TRAF-6) (B), nucleotide-binding oligomerisation domain 2 (NOD2) (C) and DNA methyltransferase 1 (DNMT1) (D) in the ileum of intra-uterine growth restricted (■) and normal-birth weight (□) neonates. Values are means, with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P< 0·05). ** Mean values were significantly different from those of the adequate nutrient intake (ANI) group (P< 0·01; significant effect of level of nutrient intake). For TLR-9 (A), there was a significant effect of body weight (P< 0·05). There was a significant interaction between body weight and nutrient intake on the mRNA abundance of DNMT1 (P< 0·05). RNI, restricted nutrient intake.
Discussion
Neonates with IUGR have been shown to present immature immune system compared with their normal counterparts( Reference D'Inca, Gras-Le Guen and Che 26 , Reference Wang, Chen and Li 27 ). In the present study, we sought to elucidate the role of postnatal nutritional environment on growth performance and immune function of neonates with IUGR using piglets as model. Interestingly, the results of the present study demonstrated that postnatal nutritional restriction may delay the growth and development of the small intestine, as well as immune response of IUGR piglets through altering intestinal morphology, enzyme activities, composition of peripheral leucocytes and innate immunity-related gene expressions. However, previous studies have reported the improved immune response in low nutrient intake of broilers and primates( Reference Guo, Li and Chen 17 , Reference Messaoudi, Warner and Fischer 18 ); this differential response may be related to the degrees of nutritional restriction, restricted nutrients and species.
In agreement with previous reports( Reference Han, Hu and Xuan 14 ), piglets with IUGR had lighter BW than NBW piglets at 28 d of birth, which could be resulting from the lower intake of nutrients on day 21 of the suckling period, as indicated by the decreased intake of average daily DM intake in piglets with IUGR. However, the growth rate was similar between IUGR and NBW piglets once they received the same amount of nutrients; however, feed conversion ratio was not markedly different between IUGR and NBW piglets. This finding is consistent with the previous study, which demonstrated that piglets with IUGR had comparable growth response to a high protein diet as NBW piglets( Reference Morise, Seve and Mace 28 ). In fact, piglets with IUGR have physiological basis for catch-up growth when they are followed by ad libitum milk intake( Reference Dai, Thamotharan and Garg 15 ).
Along with the smaller body size and weight, piglets with IUGR had lighter relative organ weights than NBW piglets. Consistent with previous results( Reference Alvarenga, Chiarini-Garcia and Cardeal 29 ), the relative weight of the intestine, heart and brain to BW was markedly increased in piglets with IUGR compared with NBW piglets. These phenotypic changes could be further explained by the programming of ‘trade-offs’( Reference Monaghan 30 ), which selectively allocated maternal nutrition to optimise the growth of internal organs to adapt the chronic placental restriction( Reference Maruyama and Koizumi 31 ).
The small intestine histological analysis could reflect the renewal rate of intestinal epithelial cells. Piglets with IUGR have been shown to have longer and thinner small intestine with reduced villous height relative to NBW piglets( Reference Xu, Mellor and Birtles 32 , Reference Wang, Huo and Shi 33 ). In the present study, consistently, BW affected intestinal morphology, as indicated by the lower ileum villous height of piglets with IUGR relative to NBW piglets was found. Moreover, there was an interactive effect on intestinal morphology between BW and NI; nutritional restriction to piglets with IUGR would increase jejunum villous height and VCR. When it comes to distal intestine (ileum), however, RNI tends to decrease villous height of piglets with IUGR. This segmental difference in intestinal morphology might be related to the amount of NI. The proximal intestine had priority to be nursed by nutrition, whereas the distal intestine might be starved with relatively less enteral nutrition in piglets with IUGR-RNI. It has been reported that inadequate enteral nutrition is detrimental for intestinal development( Reference Burrin, Stoll and Chang 34 ).
Regardless of NI, there were markedly lower villous height and crypt depth in the ileum of piglets with IUGR; accordingly, intestinal activities of AP, which is expressed exclusively in villus-associated enterocytes( Reference Goldberg, Austen and Zhang 35 ), had been compromised in the jejunum of piglets with IUGR. Because AP has been shown to detoxify lipopolysaccharide and to prevent bacterial invasion across the gut mucosal barrier( Reference Goldberg, Austen and Zhang 35 ), lower AP activity suggested that intestinal barrier functions may be impaired in the intestine of piglets with IUGR. In the present study, however, the relatively higher lactase activity in piglets with IUGR may be a compensatory response to lack of nutrition supply. The previous study has also shown that survived piglets with IUGR had an enhanced intestinal tropic response to feeding relative to NBW piglets( Reference Che, Thymann and Bering 23 ). Since lactose is the main component of carbohydrate in milk, higher lactase activity in piglets with IUGR indicated their priority to utilise lactose as energy under the shortage of nutrient( Reference Qiu, Huang and Shen 36 ).
Intestinal epithelial cells could provide an immunological barrier to microbial invasion through both innate and adaptive immune response( Reference Suarez-Souto, Lara-Padilla and Reyna-Garfias 37 ). TLR and Nod-like receptors are two major forms of innate immune sensors( Reference Fukata, Vamadevan and Abreu 38 ). TLR are a family of membrane-bound receptors in the activation of innate immunity, whereas Nod-like receptors reside within the cytoplasm to detect microbial motifs that enter into the host cell( Reference Athman and Philpott 39 ). In the present study, the nutritional restriction to piglets with IUGR markedly up-regulated the intestinal gene expression of TLR-9, but inhibited the gene expression of NOD2 and its downstream molecule (TRAF-6) relative to normal piglets. These findings suggest that nutritional restriction to piglets with IUGR may impair intestinal innate immune response. As a matched nutritional strategy, nutritional restriction has been widely proved to improve the metabolic syndrome of IUGR offspring( Reference Garg, Thamotharan and Dai 40 ); however, it may not be true when it comes to the evaluation of immunological traits of piglets with IUGR receiving RNI. Moreover, the ileal mRNA level of DNA methyltransferase 1 in piglets with IUGR-RNI was markedly higher than piglets in other groups, which indicates that there may be abnormal DNA methylation, the genomic methylation status has been shown to influence gene expression and recognised as an epigenetic mechanism to link the intra-uterine environment to adult diseases( Reference Pham, MacLennan and Chiu 41 ).
The immunotype of blood is an important tool in the diagnosis of immunological disorders( Reference Comans-Bitter, de Groot and van den Beemd 42 ). The cellular immune response of piglets with IUGR to postnatal nutritional restriction is determined by immune cells and lymphocyte subpopulations. In the present study, RNI markedly decreased the number and/or percentage of lymphocytes, leucocytes and monocytes; particularly, RNI disturbed the balance of T-lymphocyte subsets of piglets with IUGR. Similarly, it has been demonstrated that IUGR leads to the lower lymphocyte counts and alterations in CD4+ and CD8+ populations of thymus and spleen in rats( Reference Contreras, Yu and Hale 9 ). The ratio of CD4+ to CD8+ has been widely used to determine cellular immune status during disease, nutritional stress and autoimmune problem( Reference Deng, Cui and Peng 43 ). Neonates with IUGR have been shown to compromise immune system, which may be caused by inadequacy of cell-mediated immune response( Reference Cromi, Ghezzi and Raffaelli 5 ).
In conclusion, postnatal nutritional restriction delayed growth and intestinal development in piglets with IUGR. Most importantly, the present study demonstrated that the immunological traits were abnormal in piglets with IUGR receiving postnatal nutritional restriction. Further investigation is required to determine whether this impact by an early nutrition intervention would persist in adult life.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (31101727); the International Cooperation in Science and Technology Project of Sichuan Province (2014HH0034); the Program for Changjiang Scholars and Innovative Research Team in University (IRT13083); and the Natural Science Foundation of Sichuan Province (12ZA110).
The authors' contributions are as follows: L. C. designed the study; L. H., Y. L., C. Y., X. P., Q. X., Y. X., F. H. and G. T. carried out the study; L. H., Z. F., Y. L., S. X., K. Z., D. C. and D. W. performed the analysis and analysed the data; L. H. wrote the paper; L. C. made some modifications in the manuscript.
The authors declare that there are no conflicts of interest.













