Worldwide, as the population ages, the probability of people developing neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease (PD) is increasing. PD is the second most common neurological disorder, affecting 2 % of the population over the age of 60 years; it is characterised by the selective loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc)(Reference Lo Bianco, Schneider and Bauer1). Although the cause of PD remains unclear, evidence from many studies suggests the involvement of oxidative stress(Reference Mandel, Grunblatt and Riederer2, Reference De Rijk, Tzourio and Breteler3). Oxidative stress is thought to be one of the factors that reduce cognitive and motor performance in neurodegenerative disease; oxidative defence mechanisms such as catalase and GSH decline, while oxidative damage molecules, such as hydroxyl radicals and peroxynitrite, increase. Thus, a high level of antioxidant activity has been linked with protection against neurodegenerative diseases(Reference Mandel, Grunblatt and Riederer2).
Various berries have long been reported to have preventative or positive effects in many disease states including cancer(Reference Li, Zhang and Stoner4), CVD(Reference Ghosh and Scheepens5) and age-related brain diseases(Reference Balk, Chung and Raman6). Berries contain numerous phytochemicals such as flavonoids, tannins and phenolic acids, and recently, the antioxidant effects of berry components have been studied(Reference Khknen, Hopia and Heinonen7). Fruit-derived polyphenolic compounds are known to have antioxidant properties and to potentially be neuroprotectants(Reference Joseph, Shukitt-Hale and Casadesus8–Reference Youdim, Spencer and Schroeter10). Moreover, research has revealed that some berries have neuroprotective effects; strawberries and blueberries were shown to reverse declines in cognition, motor behaviour, neuronal signal transduction and memory; furthermore, blueberries showed neuroprotective effects in an Alzheimer's disease model(Reference Joseph, Shukitt-Hale and Denisova11–Reference Andres-Lacueva, Shukitt-Hale and Galli16). Shukitt-Hale et al. (Reference Shukitt-Hale, Lau and Joseph17) reported that berry fruits may lower the risk of developing age-related neurodegenerative diseases, suggesting that polyphenolic compounds in berry fruits may reduce oxidative stress and inflammation.
Mulberry (Morus alba L.), a member of the Moraceae family, has been naturalised and widely cultivated; mulberry fruit is commonly eaten, often dried, or made into wine. In traditional oriental medicine, it has been used to treat premature grey hair, to nourish the blood, to treat constipation and diabetes and to generate body fluids, which generally means enhancing health and promoting longevity(Reference Chen, Chen and Crampton18). In addition, it has been reported to ameliorate inflammation-related haematological parameters in carrageenan-induced arthritic rats(Reference Kim and Park19), to promote recovery from physical stress(Reference Hwang20), to have neuroprotective effects against cerebral ischaemia(Reference Kang, Hur and Kim21) and to have radical-scavenging properties(Reference Isabelle, Lee and Ong22).
Like other berry fruits, mulberry fruit contains not only high amounts of anthocyanins(Reference Liu, Lee and Shin23, Reference Shih, Chan and Liao24), a subset of the flavonoids that are important natural antioxidants(Reference Dugo, Mondello and Errante25, Reference Moyer, Hummer and Finn26), but also non-anthocyanin phenolics including rutin and quercetin known to have multi-bioactive functions including neuroprotective effects(Reference Isabelle, Lee and Ong22–Reference Shih, Chan and Liao24, Reference Zhang, Han and He27). It may be assumed that these compounds have neuroprotective, antioxidant and anti-inflammatory effects in PD models. However, few studies have actually examined the possible neuroprotective outcomes in experimental settings. Thus, we examined the effects of the ethanol extract of mulberry fruit (ME) on dopaminergic neuron protection in in vitro PD models using the SH-SY5Y neuroblastoma stressed with 6-hydroxydopamine (6-OHDA) and mesencephalic dopamine neurons stressed with 6-OHDA and 1-methyl-4-phenylpyridinium (MPP+). We also investigated the effects of ME in an in vivo PD model induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).
Experimental methods
Materials
Dulbecco's modified Eagle's medium, minimal essential medium, fetal bovine serum and penicillin–streptomycin were purchased from Gibco Industries, Inc. (Auckland, New Zealand). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 6-OHDA, MPTP, MPP+, glucose, l-glutamine, dimethyl sulphoxide, paraformaldehyde, poly-l-lysine, 3,3-diaminobenzidine, Griess reagent, NaCl, sucrose, ethanol, trifluoroacetic acid, acetonitrile, PBS, glycine, JC-1 and 2,7-dichlorodihydrofluorescein (DCF) diacetate were purchased from Sigma-Aldrich (St Louis, MO, USA). Tetramethylethylenediamine, protein assay reagent, Tween 20, ammonium persulphate, acrylamide, enzyme-linked chemiluminescence reagent and skimmed milk were purchased from Bio-Rad Laboratories (Hercules, CA, USA). Cyanidin-3-O-β-glucopyranoside was purchased from Polyphenols Laboratories AS (Sandnes, Norway). A caspase-3 assay kit and a mitochondria/cytosol fractionation kit were purchased from BioVision, Inc. (Mountain View, CA, USA). Rabbit anti-tyrosine hydroxylase (TH) and rabbit anti-cleaved caspase-3 antibodies were obtained from Chemicon International, Inc. (Temecula, CA, USA). Rabbit anti-Bax, rabbit anti-Bcl-2 and mouse anti-β-actin antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-rabbit and mouse-horseradish peroxidase secondary antibodies were purchased from Assay Designs, Inc. (Ann Arbor, MI, USA). Biotinylated anti-rabbit antibody, normal goat serum and an avidin–biotin–peroxidase complex standard kit were purchased from Vector Laboratories (Burlingame, CA, USA).
Preparation of extract
Dried mulberry fruit was purchased from Jung Do Herbal Drug Company (Seoul, South Korea), and was extracted with 70 % ethanol for 24 h at room temperature. Then, the extract was filtered, evaporated on a rotary vacuum evaporator and finally lyophilised. The powder (yield, 20·53 %) was kept at 4°C. Before each experiment, the extract was dissolved in an appropriate vehicle and was vortex-mixed for 2 min at room temperature. ME was standardised based on the contents of C3g, one of the active components of mulberry(Reference Kang, Hur and Kim21, Reference Bae and Suh28, Reference Chen, Bower and Xu29), using reverse-phase HPLC (Agilent 1100 HPLC system; Agilent technologies, Inc., Santa Clara, CA, USA) equipped with a photodiode array detector. Separation was carried out using a J'sphere ODS-H80 column (250 × 4·6 mm, 4 μm; YMC Company Limited, Shin Kyoto, Japan) at 25°C. The mobile phases (A: 0·1 % trifluoroacetic acid in acetonitrile and B: 0·1 % trifluoroacetic acid in water) were 10–10 % A for 0–10 min; 10–30 % A for 10–20 min; and 30–100 % A for 20–30 min at a flow rate of 1·0 ml/min. The detector wavelength was set at 520 nm. Four concentrations of C3g were prepared at 1, 2·5, 5 and 10 μg/ml, where 10 μl were injected as an external standard. ME and reference to the calibration curve obtained with C3g were analysed in triplicates. C3g was found in ME at a mean level of 0·43 (sem 0·02) mg/g.
Cell culture and treatment
The SH-SY5Y cell line, a human neuroblastoma, was obtained from the Korea Cell Line Bank (Seoul, South Korea). Cells were maintained in Dulbecco's modified Eagle's medium, supplemented with 10 % heat-inactivated fetal bovine serum, 100 units/ml penicillin and 100 μg/ml streptomycin in a water-saturated atmosphere of 5 % CO2 at 37°C. The culture medium was changed every 3 d, and the cells were sub-cultured about twice a week. All experiments were carried out 48 h after the cells had been seeded in ninety-six-well plates and 60-mm dishes, at densities of 2 × 105 cells/ml and 2 × 106 cells/dish, respectively. After cells were about 80 % confluent, various concentrations (0·1–100 μg/ml) of ME were added to the cells for 24 h at 37°C with or without 150 μm-6-OHDA for the last 6 h of ME treatment.
Measurement of cell viability
Treated cells were incubated with 1 mg/ml of MTT at 37°C in a CO2 incubator for 3 h. MTT medium was carefully aspirated from the wells, and the formazan dye was eluted using dimethyl sulphoxide. The plate was shaken on a shaker to dissolve the blue MTT-formazan. Absorbance was measured using a spectrophotometer (Versamax microplate reader, Molecular Device; Sunnyvale, CA, USA) at a wavelength of 570 nm, and then expressed as a percentage of the value in the vehicle-treated control culture.
Determination of intracellular reactive oxygen species
Intracellular ROS generation was measured using a fluorometer. DCFH diacetate passively enters the cells, and it is converted to non-fluorescent DCFH. ROS react with DCFH to form the fluorescent product, DCF. The cells were seeded in ninety-six-well plates and were treated with ME and/or 6-OHDA. Then, the cells were incubated with 20 μm-DCFH diacetate for 30 min. The fluorescence intensity was measured at 480 nm excitation and 530 nm emission using a fluorescence microplate reader (SpectraMax Gemini EM; Molecular Device).
Determination of extracellular nitric oxide
The accumulated level of nitrite (an indicator of NO) in culture supernatants was measured using the colorimetric reaction with the Griess reagent. The supernatants (100 μl) were transferred to a separate plate, and were reacted with 100 μl of Griess reagent in the dark for 10 min at room temperature. Absorbance at 550 nm was measured. For each experiment, freshly prepared NaNO2 that had been serially diluted was used as a standard, in parallel with culture supernatants.
Assessment of mitochondrial membrane potential (ΔψM)
The ΔψM was measured using a fluorescent dye, JC-1 reagent. The treated cells were incubated with 1X JC-1 reagent solution at 37°C for 15 min. The red and green fluorescence were measured using a fluorescence microplate reader, with excitation at 585 and 510 nm and emission at 590 and 527 nm, respectively. The ratio of red to green fluorescence was quantified from the cells of interest.
Measurement of caspase-3 activation
The caspase-3 assay was performed according to the manufacturer's protocol. Briefly, treated cell lysates were incubated with chilled lysis buffer on ice for 15 min. Then, they were centrifuged (14 000 g, 4 min, 4°C), and the supernatants were transferred to ninety-six-well plates. Reaction buffer containing 10 mm dithiothreitol was added to each well, and the 1 mm-Asp-Glu-Val-Asp (DEVD) 7-amino-4-trifluoromethyl coumarin substrate was added and mixed. The mixture was incubated for 2 h at 37°C before protease activity was detected using a fluorescence microplate reader, with 360 nm excitation and 450 nm emission filters.
Western blot analysis
The treated cells were lysed with a triple-detergent lysis buffer to detect cleaved caspase-3. For the detection of Bax and Bcl-2 proteins, the cells were fractionated into mitochondria and cytosol using a mitochondria/cytosol fractionation kit according to the manufacturer's instructions. Cell lysates were separated by 15 % SDS-PAGE, and were then transferred to a membrane. Membranes were incubated with 5 % skimmed milk in Tris-buffered saline with Tween for 1 h and then with the primary antibodies overnight at 4°C, followed by incubation with horseradish peroxidase-conjugated secondary antibodies for 1 h. Immunoreactive bands were detected using an ECL detection kit, and a LAS-4000 mini system (Fujifilm Corporation, Tokyo, Japan) was used for visualisation. The intensities of the bands were normalised to the β-actin band intensity using Multi Gauge software (Fujifilm Corporation).
Cultures of mouse mesencephalic dopaminergic cells
Cell cultures were prepared from the mesencephalons of 14 d embryos of timed pregnant Sprague–Dawley rats (Orient Bio, Osan, South Korea). Mesencephalons were dissected, collected, dissociated and plated in twenty-four-well plates with cover slips pre-coated with poly-l-lysine at a density of 1·5 × 105 cells per well. Cultures were maintained in a humidified incubator of 5 % CO2 at 37°C in a minimal essential medium with 6·0 g/l glucose, 2 mm-glutamine and 10 % fetal bovine serum. On day in vitro 6, the cells were treated with ME and were stressed with 10 μm-6-OHDA or 10 μm-MPP+ for a further 18 and 23 h, respectively. Then, the cells were fixed with 4 % paraformaldehyde at room temperature for 30 min. The cells were stored in PBS at 4°C for immunocytochemistry.
Animals
Animal maintenance and treatment were carried out in accordance with the Principles of Laboratory Animal Care (NIH publication no. 85-23, revised 1985) and the Animal Care and Use guidelines of Kyung Hee University, Seoul, South Korea. Male C57bl/6 mice (7 weeks) were purchased from Samtako, Inc. (Osan, South Korea). Animals were housed at an ambient temperature of 23 ± 1°C and at a relative humidity of 60 ± 10 % under a 12 h light–dark cycle, and they were allowed free access to water and food.
Drug administration
Animals were assigned to three groups: (1) control group (n 8, intraperitoneally vehicle injected plus intraorally vehicle-treated group); (2) MPTP group (n 8, intraperitoneally MPTP injected plus intraorally vehicle-treated group); (3) MPTP+ME group (n 8, intraperitoneally MPTP plus intraorally ME-treated group). ME that was dissolved in 10 % dimethyl sulphoxide was administered at 500 mg/kg per d for 15 d. MPTP (MPTP base form) in normal saline was injected at 30 mg/kg per d for the last 5 d of ME treatment.
Behavioural test and brain tissue preparation
We performed the pole test, which measures motor coordination, on the seventh day after the last MPTP injection. The mice were placed head upward near the top of a vertical rough-surfaced pole (diameter 8 mm, height 55 cm). The time it took for the mice to turn completely downward (time to turn) and the time it took to reach the floor (locomotion activity time) were recorded, with a cut-off limit of 30 s. After the pole test, the mice were anaesthetised with 50 mg/kg Zoletil (intramuscularly) and were rapidly perfused transcardially with PBS, followed by 4 % paraformaldehyde in 0·1 m-phosphate buffer. Then, the brains were rapidly taken out, post-fixed in 4 % paraformaldehyde solution and processed for cryoprotection in 30 % sucrose at 4°C. Frozen brains were cut into 30-μm coronal sections using a cryostat microtome (CM3000; Leica, Wetzlar, Germany). Then, the tissues were stored in storing solution containing glycerine, ethylene glycol and phosphate buffer at 4°C for immunocytochemistry.
Immunocytochemistry
Mesencephalic cells on the cover slips and free-floating sections were rinsed in PBS at room temperature before immunostaining. They were pre-treated with 1 % H2O2 in PBS for 15 min to remove endogenous peroxidase activity. Then, they were incubated overnight at room temperature with a rabbit anti-TH antibody (1:2000 dilution) for dopaminergic neuron detection and a rabbit anti-Bax antibody (1:100 dilution) for apoptotic protein detection. They were then incubated with a biotinylated anti-rabbit IgG for 90 min, followed by incubation in avidin–biotin–peroxidase complex solution for 1 h at room temperature. The peroxidase activity was visualised with 3,3-diaminobenzidine for 3 min. After every incubation step, the cells and tissues were washed three times with PBS. Finally, the mesencephalic cells on the cover slips were mounted on gelatin-coated glass slides, air dried and photographed with a research microscope. The free-floating brain tissues were mounted on gelatin-coated slides, dehydrated, cleared with xylene and cover slipped using histomount medium. For quantification of the effect of ME in the mesencephalic dopaminergic cells, TH immunopositive cells were counted on at least four cover slips from independent experiments for each condition. Quantification of the effect of ME in brain tissues was performed by counting the TH-immunopositive cell number in SNpc at × 100 magnification under a microscope (AxioSkop 2; Carl Zeiss, Inc., Göttingen, Germany). The TH-immunopositivity in the striatum was measured at × 40 magnification using a StereoInvestigator (MBF Bioscience, Inc., Williston, ND, USA). Bax-immunopositive cells in SNpc were captured at × 200 magnification under a microscope. Data are presented as percentages of the control group values.
Statistical analyses
All quantitative data were analysed. The results are expressed as mean values with their standard errors. Statistical significance was determined by one-way ANOVA followed by the least significant difference test using SPSS (12.0K for Windows; Chicago, IL, USA). P values < 0·05 were deemed to be statistically significant.
Results
Ethanol extract of mulberry fruit protects SH-SY5Y cells against 6-hydroxydopamine-induced neurotoxicity
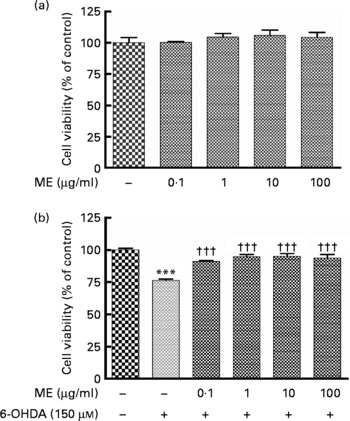
To evaluate the protective activity of ME in SH-SY5Y cells, we used the MTT assay. Treatment with ME for 24 h at various concentrations had no influence on cell proliferation and caused no cell toxicity (Fig. 1(a)). Decreased cell viability induced by 150 μm-6-OHDA was reduced or prevented by ME pre-treatment at 0·1–100 μg/ml (Fig. 1(b)).

Fig. 1 Neuroprotective effects of ethanol extract of mulberry fruit (ME) in SH-SY5Y cells. After cells were confluent, they were treated with ME for 18 h and incubated without (a) or with 150 μm-6-hydroxydopamine (6-OHDA) (b) for a further 6 h. Cell viabilities are expressed as a percentage of the controls (cells treated with vehicle for 24 h). Values are indicated as the mean values with their standard errors. *** Mean values were significantly different from the control group (P < 0·001). ††† Mean values were significantly different from the 6-OHDA-only treated group (P < 0·001).
Ethanol extract of mulberry fruit inhibits 6-hydroxydopamine-induced stress in SH-SY5Y cells
To investigate whether the protective effect of ME on 6-OHDA-induced apoptosis involved ROS and NO generation, we used DCFH diacetate and the Griess reagent, respectively. Exposure to 150 μm-6-OHDA led to significant (P < 0·001) ROS and NO elevation in SH-SY5Y cells, 151·86 (sem 1·67) % and 468·76 (sem 3·37) %, respectively, relative to the control. ME pre-treatment inhibited ROS (Fig. 2(a)) and NO (Fig. 2(b)) generation at 10 and 100 μg/ml and at 1, 10 and 100 μg/ml, respectively.

Fig. 2 Antioxidant effects of ethanol extract of mulberry fruit (ME) on 6-hydroxydopamine (6-OHDA)-induced accumulation of reactive oxygen species and nitric oxide in SH-SY5Y cells. The fluorescence intensity of 2,7-dichlorodihydrofluorescein (DCF) was measured after SH-SY5Y cells were exposed to 150 μm-6-OHDA for 1 h, followed by 20 μm-DCFH diacetate for 30 min (a). Nitric oxide production in SH-SY5Y cells with ME pre-treatment for 18 h before 150 μm-6-OHDA treatment was assayed by measuring the levels of nitrite in the supernatant fluid using the Griess reagent (b). Representative results from experiments. Values are indicated as the mean values with their standard errors. *** Mean values were significantly different from the control group (P < 0·001). ††† Mean values were significantly different from the 6-OHDA-only treated group (P < 0·001).
Ethanol extract of mulberry fruit inhibits 6-hydroxydopamine-induced apoptosis in SH-SY5Y cells
To determine the protective effects of ME on 6-OHDA-induced apoptosis, we measured Bax and Bcl-2 expression levels, ΔψM and caspase-3 cleavage activity in SH-SY5Y cells. 6-OHDA-induced toxicity increased the Bax protein level in the mitochondria and decreased the Bcl-2 protein level, while ME protected SH-SY5Y cells from it (Fig. 3(a)). The ratio between green fluorescence (monomeric form, low ΔψM) and red fluorescence (aggregate form, high ΔψM) indicates the depolarisation of ΔψM. 6-OHDA-induced toxicity significantly decreased ΔψM (P < 0·001), whereas ME pre-treatment at 10 and 100 μg/ml prevented depolarisation of the mitochondrial membrane (Fig. 3(b)). Caspase-3 activation was increased in the 6-OHDA group compared with the control group (P < 0·001), whereas ME pre-treatment inhibited caspase-3 activity dose dependently (Fig. 3(c)); a maximal effect was obtained at 100 μg/ml ME. The expression level of cleaved caspase-3 in Western blot assay was consistent with its activity (Fig. 3(c)).

Fig. 3 Inhibitory effect of ethanol extract of mulberry fruit (ME) on 6-hydroxydopamine (6-OHDA)-induced apoptosis. Cells were pre-treated with ME for 18 h before exposure to 6-OHDA for a further 6 h. Bax and Bcl-2 proteins were expressed using Western blot assay (a). The degree of ΔψM was measured as the ratio of red:green fluorescence of the JC-1 reagent (b). Caspase-3-like activity was determined by fluorescence using cleavage of a substrate, and cleaved caspase-3 level was expressed using Western blot assay (c). Values are indicated as the mean values with their standard errors. *** Mean values were significantly different from the control group (P < 0·001). Mean values were significantly different from the 6-OHDA-only-treated group: † P < 0·05, †† P < 0·01, ††† P < 0·001.
Ethanol extract of mulberry fruit has protective effects against 6-hydroxydopamine and 1-methyl-4-phenylpyridinium toxicity in mesencephalic dopaminergic neurons
To examine the protective effects of ME against 6-OHDA and MPP+ toxicity in primary dopaminergic neurons, we counted cell bodies with immunoreactivity to the anti-TH antibody. TH-positive cells were 500–700 cells per cover slip in control cultures. 6-OHDA neurotoxicity was defined as a 49·40 (sem 1·15) % reduction in the survival rate, whereas pre-treatment with ME at 10 μg/ml protected dopaminergic cells, showing 65·04 (sem 1·18) % (P < 0·01) of TH-positive cells compared with the control group (Fig. 4(a)). MPP+ neurotoxicity was defined as a 48·04 (sem 1·56) % reduction in the survival rate, whereas pre-treatment with ME also protected them, showing 69·55 (sem 0·97) % (P < 0·01) and 64·25 (sem 4·50) % (P < 0·05) of survival rates compared with the control group at 10 and 100 μg/ml, respectively (Fig. 4(b)).

Fig. 4 Protective effect of ethanol extract of mulberry fruit (ME) against 6-hydroxydopamine (6-OHDA) and 1-methyl-4-phenylpyridinium (MPP+) toxicity in primary dopaminergic culture. Cells were treated with ME (1, 10 and 100 μg/ml, 6 h) or vehicle before exposure to 6-OHDA (10 μm, 18 h; (a), (c)–(g)) or treated with ME (1, 10, and 100 μg/ml, 6 h) or vehicle before exposure to MPP+ (10 μm, 23 h; (b), (h)–(l)). After fixation with 4 % paraformaldehyde, the cells were stained with an anti-tyrosine hydroxylase (TH) antibody. The numbers of TH-positive neurons were counted ((a) and (b)), and representative images of experiments are shown ((c)–(l)): ((c), (h)) control groups; (d) 6-OHDA-only-treated group; (e–g) 6-OHDA+ME-treated groups (1, 10 and 100 μg/ml, respectively); (i) MPP+-only-treated group; (j–l) MPP+ME-treated groups (1, 10, and 100 μg/ml, respectively). * Mean value was significantly different from the control group (P < 0·05). †† Mean value was significantly different from the 6-OHDA-only-treated group (P < 0·01). Mean values were significantly different from the MPP+-only-treated group: ‡ P < 0·05, ‡‡ P < 0·01.
Ethanol extract of mulberry fruit has neuroprotective effects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity in a mouse Parkinson's disease model
We observed previously that ME protected mesencephalic dopaminergic neurons from 6-OHDA and MPP+ toxicity. In this experiment, we investigated whether ME could relieve motor symptoms and protect dopaminergic cells in the MPTP-induced mouse PD model. The pole test showed that the time to turn and locomotion activity time in the MPTP group were significantly prolonged compared with the control group, whereas they were significantly shortened in the MPTP+ME group (Fig. 5). Thus, ME prevented MPTP-induced bradykinesia at 500 mg/kg per d. Fig. 6(a) shows dopaminergic neurons in the SNpc. In the control group, many TH-positive cells were observed with normal shapes and neurite lengths, whereas in the MPTP group, few TH-positive cells were observed, and they had shrunken cell bodies and neurites. In contrast, the MPTP+ME group showed less dopaminergic neuronal cell loss than did the MPTP group. The immunoreactivity of TH-positive optical density in the ST showed a similar tendency (Fig. 6(b)). In the Bax immunostaining in the SNpc, Bax-immunopositive cells were increased in the MPTP group, whereas they were decreased in the MPTP+ME group (Fig. 6(i)–(k)).
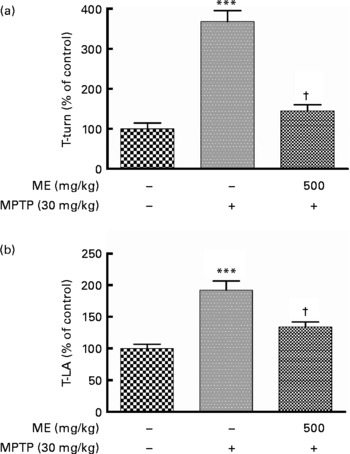
Fig. 5 Protective effect of ethanol extract of mulberry fruit (ME) against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced behavioural dysfunction in a mouse Parkinson's disease model. Saline or 500 mg/kg ME was administered orally once per day for 15 d, and MPTP (30 mg/kg, intraperitoneally) was injected for the last 5 d. Seven days after the last MPTP injection,the time to turn completely downward (a, T-turn) and the time to arrive at the floor (b, T-LA) were recorded with the cut-off limit of 30 s. Values are indicated as the mean values with their standard errors. *** Mean values were significantly different from the control group (P < 0·001). † Mean values were significantly different from the MPTP-only-treated group (P < 0·05).

Fig. 6 Protective effect of ethanol extract of mulberry fruit (ME) on dopaminergic neurons in a mouse Parkinson's disease model. Saline or 500 mg/kg ME was administered orally once per day for 15 d, and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; 30 mg/kg, intraperitoneally) was injected for the last 5 d. Dopaminergic neurons were visualised with tyrosine hydroxylase (TH) immunostaining. The numbers of TH-positive neurons in the substantia nigra pars compacta (SNpc) (a) were counted, and the optical density in the striatum (ST) (b) was measured. Representative photomicrographs of SNpc ((c)–(e)) and ST ((f)–(h)) were taken. Bax, an apoptotic protein, was visualised with Bax immunostaining in the SNpc ((i)–(k)). ((c), (f) and (i)) Control group; ((d), (g) and (j)) MPTP group and ((e), (h) and (k)) MPTP+ME group. Scale bar = 250 μm. Values are indicated as the mean values with their standard errors. *** Mean values were significantly different from the control group (P < 0·001). ††† Mean values were significantly different from the 6-MPTP-only-treated group (P < 0·001).
Discussion
The present study showed that the ME protected dopaminergic cells from neurotoxicity in SH-SY5Y and primary mesencephalic cultures. Moreover, the present study confirmed the effect of ME in an MPTP-induced animal PD model, which included bradykinesia, nigral dopaminergic neuronal loss and striatal dopamine depletion. First, we used the MTT assay to investigate the protective effects of ME against 6-OHDA-induced neurotoxicity in SH-SY5Y cells. 6-OHDA, a neurotoxin which plays dominant neurotoxic roles in selectively damaging catecholaminergic neurons including dopaminergic neurons, has widely been used in experimental models of PD, and it can operate in extracellular or intracellular oxidation, yielding ROS that lead to toxic downstream molecules and resulting in neuronal damage(Reference Choi, Yoon and Oh30). It has been demonstrated that 6-OHDA is involved with disturbing mitochondrial outer membrane permeability, leading to increased cytosolic cytochrome C and apoptotic proteins, including caspase-3(Reference Mandel, Grunblatt and Riederer2). In the present study, ME showed a significant protective effect against 6-OHDA-induced toxicity and showed no toxicity to SH-SY5Y cells. In the measurement of ROS, ME significantly reduced intracellular ROS generation induced by 6-OHDA in SH-SY5Y cells in a dose-dependent manner. Because interaction between ROS and NO can stimulate the oxidative stress cascade and initiate a neurotoxic cascade(Reference Bonfoco, Krainc and Ankarcrona31, Reference Lander32), the inhibitory effect of ME on 6-OHDA-induced NO accumulation, which was only about 10 % but statistically significant, seems to contribute to the protective effect of ME.
Next, because ROS generated by 6-OHDA contribute to mitochondrial dysfunction, we measured Bax and Bcl-2 expression levels, ΔψM and caspase-3 activation in SH-SY5Y cells to investigate the effect of ME on mitochondria-mediated apoptosis induced by 6-OHDA. Bax and Bcl-2 proteins have a role in apoptotic signal transduction by regulating the permeability of the mitochondrial membrane(Reference Offen, Beart and Cheung33). ΔψM, an important factor in apoptosis, is disrupted by ROS, and it interacts with Bcl-2 family proteins(Reference Ly, Grubb and Lawen34). Caspase-3, which is produced by pro-apoptotic agent such as ROS and cascaded by 6-OHDA toxicity, appears to be essential in neuronal apoptosis through the apoptotic pathway common in many cell types(Reference Salvesen and Dixit35). Present treatments for PD are focused on relieving symptoms; anti-apoptotic activity in a natural compound may improve the therapeutic options for treating PD and may be able to reduce the neurodegenerative progression(Reference Mandel, Grunblatt and Riederer2). The present study showed that pre-treatment with ME regulated 6-OHDA-induced apoptotic pathway. We also examined the effect of ME on GSH, an antioxidant protecting cells from toxins such as free radicals. 6-OHDA caused significant GSH depletion, whereas ME had no effect on GSH (data not shown). Thus, the neuroprotective effects of ME against 6-OHDA in SH-SY5Y cells may be mediated by ROS and NO inhibition, Bcl-2 and Bax regulation, ΔψM stabilisation and inhibition of caspase-3 activation.
We also investigated the effects of ME on cultured primary mesencephalic dopaminergic neurons, which have been used to develop target agents for PD. MPP+, the active metabolite of MPTP, induces selective dopaminergic neuronal loss; it inhibits the respiratory complex I chain, leading to cell death(Reference Przedborski and Vila36, Reference Schmidt and Ferger37). ME significantly protected dopaminergic neurons from 6-OHDA at a dose of 10 μg/ml. Against the other toxicity, MPP+, ME showed the effect at a wide range of concentrations of 10 and 100 μg/ml. From these results, we assumed that ME would be more effective in MPTP-induced mouse PD model than in other toxin-induced models.
Then, to evaluate the effect of ME in an in vivo PD model, we performed behavioural test and brain tissue stereology after MPTP and/or ME treatment. The MPTP mouse model is widely used for studying neuroprotective effects of candidate drugs because the characteristics of PD, such as oxidative stress, mitochondrial dysfunction and apoptosis, are reproduced pathologically and biochemically by MPTP(Reference Przedborski and Vila36, Reference Schmidt and Ferger37). In the pole test, a commonly used behavioural test in the mouse model of PD(Reference Matsuura, Kabuto and Makino38), ME treatment, 500 mg/kg per d for 15 d, could maintain movement ability in MPTP-induced bradykinesia. In the histological analysis, whereas TH immunoreactivity was reduced in the SNpc and ST by MPTP, ME significantly reduced MPTP-induced dopaminergic neuronal damage in the SNpc and ST by inhibition of apoptotic protein, Bax. From these results, we confirmed that ME had neuroprotective effects in both in vitro and in vivo PD models.
In our previous studies, mulberry fruit showed a total phenolic content (TPC) of 1·78 %, which is similar to that of the black bean, 1·82 %, and more than that of red or white beans, 1·10 % or 0·46 %, respectively(Reference Bressani, Hernandez and Braham39). Phenolic compounds are well known to have antioxidant effects, and they have been reported to inhibit 6-OHDA- and MPTP-induced apoptosis via the attenuation of oxidative stress(Reference Nie, Cao and Zhao40). It has been reported that the value of oxygen radical absorbance capacity is correlated with phenolic contents in mulberry fruit, and that the total antioxidant capacity and TPC are comparable to those in other berries(Reference Isabelle, Lee and Ong22, Reference Wu, Beecher and Holden41). Also, C3g, an aglycon of anthocyanin, has a neuroprotective effect against cerebral ischaemia via reduction of ROS generation(Reference Kang, Hur and Kim21). Polyphenols and other antioxidant compounds such as δ- and γ-tocopherols in ME(Reference Isabelle, Lee and Ong22–Reference Shih, Chan and Liao24, Reference Zhang, Han and He27, Reference Bae and Suh28) may have important roles in protecting against 6-OHDA- and MPTP-induced neurotoxicity via their antioxidant and anti-apoptotic effects, blocking of ROS and NO generation, regulating Bcl-2 family protein, stabilising mitochondrial membrane and inhibiting caspase-3 activity.
In summary, the mulberry fruit extract, by its antioxidant and anti-apoptotic effects, significantly protected neurons against neurotoxins in in vitro and in vivo PD models. These results suggest that mulberry fruit or compounds in it may provide neuroprotective candidates for use in treating or preventing PD.
Acknowledgements
This research was supported by the Programme of Kyung Hee University Research Fund in 2007 (KHU-20070713). M. S. O. and H. G. K. were involved in the designing, writing and editing the paper. M. S. J. and J. S. S. performed the sample extraction and total polyphenol contents measurement. S. Y. K. and M. C. K. performed HPLC analysis for the sample standardisation. H. G. K. and S.-H. L. contributed to the in vitro study. H. G. K. and Y. H. participated in the in vivo study. All authors reviewed the final manuscript. There are no conflicts of interest to declare.