More than twenty million low birth weight (LBW) infants (15 % of all births) are born worldwide annually( 1 ). In China, the prevalence of LBW fell from 6 % in 2000 to 2·7 % in 2008; however, LBW still accounts for about one million newborn babies every year because of the large population( 2 ). LBW is associated with mortality and morbidity in infancy and childhood, and also has long-term effects on health in adult life.
It is currently recommended by the World Health Organization( 3 ) that all pregnant women, regardless of baseline Hb status, take prenatal supplementation with Fe-containing supplements such as Fe–folic acid (IFA) to ameliorate maternal anaemia, Fe deficiency and LBW. In addition to Fe and folic acid (FA), supplements can be formulated to include other vitamin and minerals according to the United Nations International Multiple Micronutrient Preparation (UNIMMAP) to ameliorate maternal anaemia and overcome other possible maternal micronutrient deficiencies( 4 ). This guidance has mainly been based on studies in patients with low Hb levels at baseline. However, most clinical trials performed in women with mild/no anaemia in the USA( Reference Meier, Nickerson and Olson 5 ), UK( Reference Harvey, Dainty and Hollands 6 ), Australia( Reference Makrides, Crowther and Gibson 7 ), Norway( Reference Eskeland, Malterud and Ulvik 8 ), Finland( Reference Hemminki and Rimpela 9 , Reference Puolakka, Jäne and Pakarinen 10 ) and Iran( Reference Falahi, Akbari and Ebrahimzade 11 – Reference Ouladsahebmadarek, Sayyah-Melli and Taghavi 13 ) have shown no benefit of Fe supplementation on birth weight( Reference Meier, Nickerson and Olson 5 – Reference Ouladsahebmadarek, Sayyah-Melli and Taghavi 13 ), although two studies found that birth weight was increased with Fe-containing supplements( Reference Cogswell, Parvanta and Ickes 14 , Reference Siega-Riz, Hartzema and Turnbull 15 ). Thus, there are inconsistencies in the literature regarding the benefit of Fe-containing supplements in a well-nourished population with mild/no anaemia.
High maternal Hb concentrations may increase the risk of adverse pregnancy outcomes including preterm birth( Reference Murphy, O’Riordan and Newcombe 16 ), stillbirth( Reference Stephansson, Dickman and Johansson 17 ), perinatal death( Reference Murphy, O’Riordan and Newcombe 16 ), LBW( Reference Steer, Alam and Wadsworth 18 ) and gestational hypertension( Reference Gaillard, Eilers and Yassine 19 ). Steer et al. ( Reference Steer, Alam and Wadsworth 18 ) found that a U-shaped distribution of LBW incidence and preterm delivery by maternal Hb group with the highest incidence of LBW and preterm delivery occurred among white women with very low (<85 g/l) and very high (>145 g/l) Hb levels. Peña-Rosas et al. ( Reference Peña-Rosas, De-Regil and Dowswell 20 ) reported that women who received higher amounts of Fe (≥60 mg/d) were more likely than controls to have high Hb levels (>130 g/l) at term. One small trial found that daily Fe supplementation (50 mg) in women with high Hb levels (>132 g/l) significantly increased the incidence of adverse pregnancy outcomes, including small-for-gestational-age babies and hypertension( Reference Ziaei, Norrozi and Faghihzadeh 12 ). Therefore, concerns have been raised about the risks of routine Fe supplementation among women without anaemia, especially among those with high Hb levels. To the best of our knowledge, no large clinical trial has thus far determined whether the association of Fe-containing supplements with birth weight varies by baseline Hb levels, and specifically whether the effect of supplementation differs between women with normal and high Hb levels.
Recently, we conducted a large randomised controlled trial (RCT) of FA, IFA and multiple micronutrient (MMN) supplementation among pregnant women with low/no anaemia in north China. We reported that, compared with FA alone, prenatal IFA or MMN supplementation prevented anaemia in late pregnancy, but did not affect infant birth weight or gestational duration( Reference Liu, Mei and Ye 21 ). However, this finding did not eliminate the increasing concerns about potential adverse pregnancy outcomes due to routine Fe supplementation among women with high maternal Hb levels. In addition, data from the clinical studies did not address whether baseline Hb concentrations modify the association of Fe supplementation during pregnancy and birth weight.
Thus, the objectives of the present study were to determine whether the association between Fe supplementation and birth weight differs among women with normal/low Hb concentrations and those with high (>132 g/l) and very high (>145 g/l) baseline Hb concentrations.
Methods
Study setting and design
The present study was a double-blind RCT (Clinicaltrials.gov ID: NCT00133744) conducted according to the guidelines laid down in the Declaration of Helsinki. Detailed information on this trial has been published elsewhere( Reference Liu, Mei and Ye 21 ). In brief, the RCT was conducted in five rural counties in Hebei Province, China, from May 2006 to April 2009. Doctors from village clinics and township hospitals provided prenatal care services, and doctors from county hospitals provided delivery services. During the period from early pregnancy (<20 weeks) to delivery, eligible pregnant women were enrolled and individually randomised in a 1:1:1 ratio to receive a daily supplement containing the following: (1) FA (400 μg) (FA group); (2) FA (400 μg)+Fe (ferrous fumarate, 30 mg) (IFA group); or (3) FA, Fe and thirteen additional vitamins and minerals (MMN group, the UNICEF/WHO/UNU UNIMMAP supplement included FA (400 μg), Fe (ferrous fumarate, 30 mg), vitamin A (800 μg), vitamin E (10 mg), vitamin D (5 μg), vitamin C (70 mg), thiamine (1·4 mg), riboflavin (1·4 mg), vitamin B6 (1·9 mg), vitamin B12 (2·6 μg), niacin (18 mg), Zn (15 mg), Cu (2 mg), I (150 μg) and Se (65 μg)).
The inclusion criteria were as follows: (1) had a Hb concentration ≥100 g/l; (2) had recorded dates of menstruation for 2 or more months before becoming pregnant; (3) were nulliparous; (4) were at least 20 years old; (5) were ≤20 weeks of gestation; (6) were legally competent; (7) had not consumed micronutrient supplements other than FA in the previous 6 months; (8) resided in and received prenatal care in one of five counties; and (9) consented to participate. Peri-conceptional FA is taken as a single nutrient supplement in China. The study protocol was approved by the Institutional Review Boards of the Centers for Disease Control and Prevention (Atlanta, Georgia, USA) and Peking University (Beijing, China), and was renewed annually. All the women enrolled in this trial provided informed verbal consent.
Sample size
The estimated mean of birth weight was 3290·6 (sd 391·5) g in the FA group based on our previous report. Compared with the FA group, a 2 % increase in the IFA group and a 4 % increase in the MMN group were hypothesised. With a power of 80 % and α 0·05, a sample size of 172/group would be needed to detect a 2 % increase in the IFA group and a 4 % increase in the MMN group. As this study was a post hoc analysis, no dropouts were considered.
Data collection and outcome measures
The primary outcome of our analysis was infant birth weight. All the women delivered in a health facility, and infants were measured within the 1st hour of birth using an electronic scale (BD 585; Tanita) with precision to the nearest 10 g. The scales were routinely checked and calibrated. Maternal Hb concentration was measured with finger-punctured blood using the HemoCue Hb 201system (HemoCue AB) at enrolment. The average of two measurements was used. Maternal weight and height were measured at enrolment using an electronic scale (BW 150; UWE) with precision to the nearest 50 g and a collapsible height board to the nearest 0·1 cm, respectively. Gestational age was calculated based on the last menstrual period. Menstrual cycle was closely monitored by trained county or township physicians for 2 or more months before enrolment among volunteers who planned to get pregnant.
Statistical analysis
We conducted stratified analyses by maternal Hb levels according to the cut-off points for high (132 g/l) and very high maternal Hb levels (145 g/l). High (133–145 g/l) and very high (>145 g/l) Hb concentrations were defined according to the studies of Murphy et al.( Reference Murphy, O’Riordan and Newcombe 16 ), Ziaei et al.( Reference Ziaei, Norrozi and Faghihzadeh 12 ) and Steer et al.( Reference Steer, Alam and Wadsworth 18 ).
We used general linear models to assess the association between birth weight and Fe supplements after adjusting for week of enrolment and gestational age at delivery, as those two variables could have been confounding factors. Preterm infants were not excluded because preterm birth is one of the major reasons for LBW, and the study focused on all LBW infants. We examined the interaction between Fe supplements and maternal Hb concentration (≤132, 133–145 and >145 g/l) by adding a product term to the regression model. A figure was drawn to show birth weight means with 95 % CI by maternal baseline Hb concentrations (2 g/l interval) in each study group (data points were not shown after 160 g/l because of small sample size). Pearson’s correlation analysis between birth weight and Hb concentration was carried out among women with high and very high baseline Hb concentrations in each study group. P values<0·05 were considered to be statistically significant. All statistical analyses were performed using Empower (R) (www.empowerstats.com, X&Y Solutions Inc.) and R (http://www.R-project.org).
Results
A total of 18 775 women underwent randomisation. After exclusion of women who intentionally terminated their pregnancy or who died, moved or dropped out of the study (n 878), 17 897 (95·3 %) women remained with known pregnancy outcomes( Reference Liu, Mei and Ye 21 ). Of these, 67 (0·4 %) women had multiple births, 82 (0·5 %) had stillbirths, 37 (0·2 %) had offspring with malformations and 157 (0·9 %) had no information on birth weight (there were some overlaps among these exclusions), leaving a total of 17 705 (98·9 %) women in the study (online Supplementary Table S1). No differences were found between participants and those who dropped out of the study (online Supplementary Table S1).
The baseline characteristics among the Fe supplement groups are shown in online Supplementary Table S2. Mean maternal age, BMI, gestational week at enrolment, gestational week at delivery and Hb concentration were 23·6 (sd 2·8) years, 22·3 (sd 2·8) kg/m2, 12·1 (sd 4·6) weeks, 39·6 (sd 1·7) weeks and 124·4 (sd 10·1) g/l, respectively. Almost all the participants were of Han ethnicity, 1·6 % had primary or less education and 90·9 % were farmers. About 81·7, 15·3 and 3·0 % of women had Hb concentrations of <132, 133–145 and >145 g/l, respectively. The baseline characteristics among the three groups were presented by different Hb concentrations (Table 1), and were balanced among the Fe supplement groups across each Hb stratum.
Table 1 Baseline maternal characteristics by study intervention and Hb concentrationsFootnote * (Number of participants and percentages)
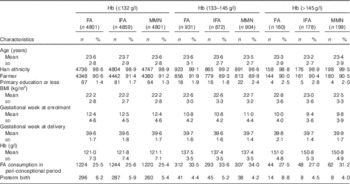
FA, folic acid; IFA, Fe–FA; MMN, multiple micronutrients.
* χ 2 tests were used to examine statistical differences in categorical variables and ANOVA to examine differences in means. P>0·05 for all comparisons of characteristics.
Maternal baseline Hb concentration and birth weight by each study group are presented in Fig. 1. We did not observe any difference in birth weight by study group until the Hb level reached approximately 144 g/l; however, the CI overlapped.

Fig. 1 Birth weight by maternal baseline Hb concentration in the study groups. Data are not shown for women with Hb concentrations >160 g/l (n 6, 10, and 10 in folic acid (FA, ![]() ) iron-FA (IFA,
) iron-FA (IFA, ![]() ) and multiple micronutrients (MMN,
) and multiple micronutrients (MMN, ![]() ) groups, respectively).
) groups, respectively).
The effects of IFA and MMN supplements on birth weight were modified by baseline Hb level (Table 2). When the baseline Hb concentration was ≤132 or 133–145 g/l, the mean birth weight did not differ with Fe supplementation. However, when the baseline Hb level was >145 g/l, the mean birth weight was 3195 (sd 468), 3280 (sd 382) and 3311 (sd 415) g in the FA, IFA and MMN groups, respectively (Table 2). After controlling for week of enrolment and gestational age at delivery, the mean birth weight was significantly higher in the IFA group (91·4; 95 % CI 3·4, 179·5 g) and the MMN group (107·6; 95 % CI 22·0, 193·3 g) compared with the FA group. This modifying effect of maternal Hb concentration on infant birth weight in women receiving Fe supplements was statistically significant (P<0·05).
Table 2 Stratified analysis for birth weight by maternal baseline Hb concentration in Chinese womenFootnote † (Mean values and standard deviations; β coefficients and 95 % confidence intervals)

FA, folic acid; IFA, Fe–FA; MMN, multiple micronutrients, Ref. referent values.
* P<0·05.
† Interaction test shows significant interaction of micronutrient supplementation with baseline Hb level on birth weight.
‡ With adjustment for week of enrolment and gestational age at delivery.
Discussion
Although concerns have been raised about the benefits and risks associated with Fe supplementation among women with high Hb levels, in the present study, prenatal supplements containing Fe were not associated with lower birth weight in this pool of women. Among women with baseline Hb concentrations of 100–132 or 133–145 g/l, the consumption of Fe-containing supplements (IFA or MMN) was not associated with reduced birth weight. Among women with very high (>145 g/l) baseline Hb levels, a significantly higher birth weight in the IFA and MMN groups was found compared with the FA group. In the FA group, an inverse correlation between baseline Hb and birth weight was found among women with high or very high Hb levels, which was not observed in the IFA and MMN groups.
Previous observational studies examining the relationship between birth weight and Hb concentration during pregnancy found that, compared with normal maternal Hb levels, high Hb concentrations (cut-off value ranged from 130 to 145 g/l) are associated with a lower birth weight and an increased risk of LBW( Reference Murphy, O’Riordan and Newcombe 16 – Reference Steer, Alam and Wadsworth 18 , Reference Zhou, Yang and Hua 22 – Reference Scanlon, Yip and Schieve 24 ). However, the effect of Fe-containing prenatal supplements in women with high or very high Hb levels has not been examined in a large clinical trial. In the present study, we observed that, among women with high Hb levels of 133–145 g/l, birth weight did not significantly differ among women who took MMN, IFA or FA supplements. Among women with very high baseline Hb concentrations (>145 g/l), birth weight was significantly higher by 91·4–107·6 g for women who took MMN or IFA compared with FA alone. The clinical significance of this increase is unknown among infants with normal birth weight. In the FA group, 91 % of infants were born with normal birth weight among women with very high Hb concentrations. Consistent with the RCT conducted by Ziaei et al.( Reference Ziaei, Norrozi and Faghihzadeh 12 ), our trial shows that providing Fe-containing supplements to women with high and very high Hb levels is not associated with reduced birth weight.
Some studies have shown that nutrient supplements such as Fe, Se, Ca and vitamin D can decrease blood viscosity, improve blood fluidity( Reference Abdulah, Koyama and Miyazaki 25 , Reference Ma, Schouten and Sun 26 ) and prevent preeclampsia( Reference Chen, Tong and Wu 27 , Reference Haugen, Brantsaeter and Trogstad 28 ). The mechanism underlying the benefits of IFA and MMN supplements may be related to low blood viscosity, adequate plasma volume expansion and efficient blood flow within the placenta, which improve maternal vascular and placenta function. Some researchers postulate that more attention should be paid to high rather than low Hb concentrations because an elevated Hb level might be an indicator for possible pregnancy complications( Reference Ziaei, Norrozi and Faghihzadeh 12 , Reference Scanlon, Yip and Schieve 24 ). Our findings provide support for prophylactic supplementation with Fe-containing supplements to improve birth weight among women with high Hb levels, including women with high Hb concentrations.
A limitation of our RCT is that there was no placebo group, because at the time the trial was conducted the Chinese government already had a FA supplementation policy during pregnancy in place; it was considered unethical to have a placebo group in our study. Another limitation is the somewhat arbitrary cut-offs for high and very high Hb levels; these cut-offs were based primarily on studies conducted in European( Reference Murphy, O’Riordan and Newcombe 16 , Reference Steer, Alam and Wadsworth 18 ) and Iranian populations( Reference Ziaei, Norrozi and Faghihzadeh 12 ). There are no Chinese Han-specific cut-offs in published studies( Reference Ziaei, Norrozi and Faghihzadeh 12 , Reference Murphy, O’Riordan and Newcombe 16 , Reference Steer, Alam and Wadsworth 18 ). The third limitation is that our study focused on birth weight, which is only one of the pregnancy outcomes associated with high Hb levels. Our study did not collect information on passive smoking during pregnancy; thus, we cannot assess the impact of passive smoking on Hb levels or birth weight. However, we found that among the 17 705 women in the study, only eleven of them smoked; the mean Hb level in the smoker was 129 (sd 12) g/l, whereas it was 124 (sd 10) g/l for non-smokers. Our findings are generalisable only to well-nourished populations with good medical care. Our participants received regular prenatal visits, and all the women delivered in a healthcare facility. Additional studies are needed in women without good medical care, such as populations from some developing countries.
Our study has several strengths. The double-blind, individually randomised controlled design of the trial is one of the strengths of this study. Although the findings presented are from post hoc, subgroup analyses by maternal Hb concentration, the baseline characteristics among the supplement groups were balanced across each Hb stratum. Finally, birth weight was assessed by trained staff using standardised and accurate equipment within 60 min of delivery. Hb levels were also measured by trained staff with standardised equipment.
In conclusion, prenatal supplementation with IFA and MMN was associated with improved birth weight among Chinese women with Hb concentrations >145 g/l before 20 weeks of gestation and had no effect on birth weight in women with Hb concentrations of 100–145 g/l. We highlighted the differential response to prophylactic supplementation by various baseline Hb concentrations. Our findings provide additional evidence for prophylactic supplementation with Fe-containing supplements to improve birth weight in well-nourished pregnant women, particularly in women with very high Hb concentrations. However, confirmation of these findings in other populations and additional studies on the use of Fe-containing supplements at conception and its effect on other pregnancy outcomes are needed.
Acknowledgements
This study was supported by a cooperative agreement between Peking University Health Science Center and the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The sponsor had a role in the study design, data collection, data analysis and interpretation and writing of the report.
We thank all of the study participants and health care workers from Yuanshi, Mancheng, Xianghe, Fengrun, and Laoting counties in China.
The authors’ contributions are as follows: Z. M., M. K. S., J. L. and L. W. conceived and designed the study; L. W. posed the hypothesis, analysed the data and drafted the manuscript; H. L. and Y. Z. contributed to acquisition of data; Z. M. and M. K. S. contributed to reviewing the manuscript; J. L. had the primary responsibility for the final content. All the authors had full access to the data in the study, and take responsibility for the integrity of the data and accuracy of the data analysis.
The authors declare that there are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114515004870





