Weight gain and tissue deposition occur in a state of positive energy balance when energy intake exceeds energy expenditure. Predicting the magnitude and rate of weight gain for a given increase of energy intake requires a model of whole-body energy expenditure that includes the energy cost of tissue deposition. Here, I present a mathematical framework for investigating energy expenditure dynamics during tissue deposition that elucidates conceptual errors of the classical Kielanowski method(Reference Kielanowski and Blaxter1) for determining the efficiencies of protein and fat deposition and I also demonstrate why the efficiency values determined using this method vary widely across experiments and differ significantly from their theoretical biochemical efficiencies.
I propose an alternative mathematical method that considers the energy costs of protein and fat synthesis as determined by their biochemical ATP requirements which are presumably constant across species and can be used in combination with various models of energy expenditure that include body fat and protein turnover costs. I illustrate this approach by presenting a simple mathematical model applied to previously published data from growing rats and human infants. The main purpose of the present study is not to compare the predictive capability of various modelling approaches, but rather to illustrate how conceptual difficulties regarding the physiological interpretation of classical approaches can be avoided by using a new modelling framework.
Theory and methods
The classic equation of Kielanowski(Reference Kielanowski and Blaxter1) has been widely used for estimating the energy efficiencies of fat and protein deposition:
where I is the energy intake rate and E m is the so-called maintenance energy expenditure which is typically assumed to be a power law function of body weight. ![]() is the rate of change of body fat,
is the rate of change of body fat, ![]() is the rate of change of body protein, and ρF and ρP are the metabolisable energy densities of fat and protein, respectively. The parameters k F and k P are intended to represent the efficiencies of fat and protein deposition, respectively, which are typically fit using linear regression to measurements of I,
is the rate of change of body protein, and ρF and ρP are the metabolisable energy densities of fat and protein, respectively. The parameters k F and k P are intended to represent the efficiencies of fat and protein deposition, respectively, which are typically fit using linear regression to measurements of I, ![]() and
and ![]() during growth.
during growth.
Reported values for k F and k P determined by the Kielanowski method vary widely(Reference Birkett and de Lange2), but typical values are k F about 0·78 and k P about 0·56. These values are in marked contrast with the theoretical biochemical efficiencies k F = 0·98 and k P = 0·84, which is often explained by assuming that the efficiencies include the energy cost of steady-state turnover of fat and protein(Reference Blaxter3, Reference Whittemore, Green and Knap4). Another potential contributor to these discrepancies is the fact that the Kielanowski calculation of k F and k P depends sensitively on the functional form of maintenance energy expenditure, E m, which itself is an ill-defined quantity known to depend on diet and body composition(Reference Birkett and de Lange2, Reference Whittemore, Green and Knap4). Yet another difficulty is that the energy cost of body protein and fat turnover is distributed between E m, k P and k F in an arbitrary manner(Reference Hall5, Reference Roux6). Finally, the statistical regression procedure is complicated by the existence of correlations between ![]() and
and ![]() during growth which introduces large uncertainties in the determination of k P and k F by the Kielanowski method(Reference Birkett and de Lange2). Thus, if it were possible to reconcile the animal growth data using the theoretical constant biochemical efficiencies in place of k P and k F, then the many problems with the Kielanowski approach could be avoided.
during growth which introduces large uncertainties in the determination of k P and k F by the Kielanowski method(Reference Birkett and de Lange2). Thus, if it were possible to reconcile the animal growth data using the theoretical constant biochemical efficiencies in place of k P and k F, then the many problems with the Kielanowski approach could be avoided.
To recast the problem of modelling energy expenditure during tissue deposition, I begin with the energy balance equation:
where the total energy expenditure rate, E, is a function of energy intake, body protein, body fat, as well as a collection of parameters denoted by ![]() .
.
Part of the total energy expenditure, E, is devoted to the energetic cost of tissue deposition which includes synthesis of TAG and protein, S F and S P, respectively:
where ηF and ηP account for the ATP costs for TAG and protein synthesis from their NEFA and amino acid (AA) precursors, respectively(Reference Blaxter3). Note that the energy costs for synthesising the required precursors can be included in the function f and need not be included in ηF and ηP. The values of ηF and ηP can be estimated by assuming that five ATP molecules are required per peptide bond and eight ATP molecules are required per TAG molecule synthesised(Reference Blaxter3, Reference Elia, Zed and Neale7). For typical levels of mitochondrial coupling, AA oxidation produces ATP at a cost of about 90 kJ/mol ATP whereas carbohydrate and fat generate ATP at a cost of about 80 kJ/mol ATP(Reference Elia, Kinney and Tucker8, Reference Livesey9). Therefore, using an approximate value of 80 kJ/mol ATP results in a variation of less than 5 % even if the protein oxidation fraction varies from 0 % to 30 % of the total energy expenditure rate. Under these assumptions, the biochemical costs for TAG and protein synthesis are ηF = 8 mol ATP/mol TAG × 80 kJ/mol ATP÷860 g per mol TAG = 0·75 kJ/g and ηP = 5 mol ATP/mol AA × 80 kJ/mol ATP÷110 g per mol AA = 3·6 kJ/g, respectively.
Since the rates of body protein and fat deposition are given by the difference between synthesis and degradation rates, I rewrite equation (3) as:
where the degradation rates of body fat and protein, D F and D P, may depend on energy intake, body composition, as well as several other possible parameters. Note that at steady state, D F and D P are equivalent to the fat and protein turnover rates, respectively. Substitution of equation (4) into the energy balance equation (2) gives the following equation:
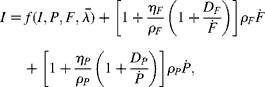
where I have included the influence of the steady-state turnover rates, D F and D P, within the square brackets to facilitate comparison with the Kielanowski equation (1). Thus, as previously suggested, k F and k P do indeed depend on turnover rates. But also notice that k F and k P depend on the independent variables ![]() and
and ![]() . Thus, the Kielanowski regression procedure is ill-posed.
. Thus, the Kielanowski regression procedure is ill-posed.
These difficulties with the Kielanowski approach can be avoided if all terms that depend on the steady-state turnover rates, D F and D P, can be grouped together separately from independent variables ![]() and
and ![]() . Thus, I rewrite equation (4) as:
. Thus, I rewrite equation (4) as:
where the function g represents the overall energy expenditure costs including the steady-state turnover costs of fat and protein as well as the cost of any variations of protein and fat turnover as a function of diet, body composition, or other parameters:
Note that the energy expenditure equation (6) is valid regardless of the sign of ![]() and
and ![]() . In other words, equation (6) can be used in cases of weight loss as well as weight gain.
. In other words, equation (6) can be used in cases of weight loss as well as weight gain.
Substituting the energy expenditure equation (6) into the energy balance equation (2) gives:
Note that unlike equation (5), all of the unknowns in equation (8) are grouped in the function g which may be a highly complex function accounting for the effects of diet, physical activity and body composition on various whole-body metabolic fluxes that have an impact on energy expenditure. Furthermore, when absorbing into the function g all of the energy costs for steady-state turnover of protein and fat, comparison of equation (8) with the Kielanowski equation (1) reveals that k F and k P are given by their constant theoretical biochemical efficiencies that are presumably applicable across species:

Since equation (8) uses the theoretical biochemical efficiencies, modelling the energy costs of tissue deposition becomes a problem of modelling the function g. Given measurements of I, ![]() and
and ![]() , the values for the function g can be calculated from these data as follows:
, the values for the function g can be calculated from these data as follows:
Such calculated values for the function g can be fit using any mathematical model of this function; a simple example would be:
where the first term represents the thermic effect of feeding as well as the impact of energy intake on various energy requiring metabolic fluxes such as de novo lipogenesis, fat and protein turnover. The second term represents the BMR as well as physical activity costs in proportion to body weight, BW. A more detailed model could replace the second term with a linear combination of body fat and fat-free masses(Reference Hall and Jordan10). A significantly more detailed model could explicitly represent the energy costs of various metabolic fluxes and their regulation by diet and body composition as proposed in a recent computational model of human metabolism(Reference Hall11, Reference Hall12).
Results
I have calculated values for the function g by processing real-life data on I, F and P of growing rats(Reference Donato and Hegsted13, Reference Pullar and Webster14) and human infants(Reference Roberts and Young15, Reference Towers, Schulze and Ramakrishnan16). Fig. 1 shows the results of fitting these values of g to the regression model of equation (11). Because equation (8) reduces modelling the energy costs of tissue deposition to modelling the function g (as argued above), this approach quantifies the predictive quality of equation (8). I plotted g v. I with both divided by BW such that the slope gave the dimensionless parameter β while the intercept gave the value for γ in kJ/kg per d. Fig. 1(a) shows the results of Pullar & Webster who investigated growth in lean and fatty Zucker rats(Reference Pullar and Webster14) and Donato & Hegsted who studied Charles River rats(Reference Donato and Hegsted13). The regression lines demonstrate that the simple model explained more than 87 % of the variability of the rat data. The values for β were consistent across the rat strains with a value of β = 0·4 (SE 0·05) but the values for γ were more variable and may have resulted from differences of physical activity or thermogenesis (γ = 620 (se 80) kJ/kg for the Donato & Hegsted(Reference Donato and Hegsted13) data, γ = 300 (se 30) kJ/kg for the lean rats and γ = 180 (se 30) kJ/kg for the fatty rats studied by Pullar & Webster(Reference Pullar and Webster14)). Fig. 1(b) shows pooled data for human infants(Reference Roberts and Young15, Reference Towers, Schulze and Ramakrishnan16) with the simple model explaining about 67 % of the data variability with β = 0·25 (se 0·04) and γ = 122 (se 19) kJ/kg per d.

Fig. 1 Modelling tissue deposition in (a) growing rats and (b) human infants using theoretical biochemical efficiencies of fat and protein synthesis along with a simple model of how energy expenditure depends on energy intake and body weight (BW). (○), Charles River rats (y = 0·4215x+615·7, R 2 0·8777; (■), lean Zucker rats (y = 0·4398x+301·19, R 2 0·9925); (△), fatty Zucker rats (y = 0·384x+176·8, R 2 0·992); (□), human infants (y = 0·2482x+122·05, R 2 0·6731).
Applying the Kielanowski equation (1) with E m = b × BW to the Donato & Hegsted data(Reference Donato and Hegsted13) gave values of b = 1130 (se 85) kJ/kg, k F = 0·62 (se 0·16) and k P = 0·61 (se 0·33), and the model explained about 90 % of the energy intake variability. The lean rats measured by Pullar & Webster(Reference Pullar and Webster14) had best-fit values of b = 530 (se 30) kJ/kg, k F = 2·6 (se 10) and k P = 0·18 (se 0·1) and the fatty rats had values of b = 450 (se 80) kJ/kg, k F = 1·8 (se 2) and k P = 0·13 (se 0·04), with the models explaining about 99 % of the energy intake variability. The human infants had best-fit values of b = 160 (se 30) kJ/kg, k F = 0·86 (se 0·08) and k P = 0·4 (se 0·09), and the model explained about 90 % of the energy intake variability.
Discussion
The classical method of Kielanowski for estimating tissue deposition efficiencies has been used for decades to accurately describe growth data in a variety of species. The fact that such methods adequately fit these data is not in question. Indeed, the Kielanowski model described the example data in growing rats and infants very well. However, some of the Kielanowski efficiency parameters were found to be greater than 1, which poses dire problems for interpreting these results physiologically. Furthermore, the precision of the estimated model parameters was low and their values varied substantially across experiments.
There are two main reasons for these problems. First, equation (5) shows that the underlying assumption of the Kielanowski method that k P and k F are constants is false. Rather, k P and k F depend on the turnover rates of fat and protein as well as the independent variables ![]() and
and ![]() . Second, the high degree of correlation between the independent variables makes it difficult to disentangle the contributions from body fat and protein changes, especially since these variables also have an impact on k P and k F using the Kielanowski approach.
. Second, the high degree of correlation between the independent variables makes it difficult to disentangle the contributions from body fat and protein changes, especially since these variables also have an impact on k P and k F using the Kielanowski approach.
The mathematical framework introduced here elucidates the conceptual problems with the Kielanowski method, recasts the problem of modelling energy expenditure during tissue deposition, helps clarify the physiological interpretation of the data, and points a way forward for future modelling efforts. I illustrated an alternative modelling approach using a simple mathematical model that assumed the constant theoretical biochemical efficiencies for k P and k F and provided a reasonably good fit to the data with one less free parameter than the Kielanowski method. Furthermore, the simple model revealed similar model parameter values for β across different rat strains and the value of β for growing human infants was similar to the value of β = 0·24 (se 0·13) obtained independently using adult underfeeding studies(Reference Hall and Jordan10). While it is interesting that similar values for β are found in the cases of both weight gain and loss in humans, it is unlikely that these situations involve similar physiological mechanisms.
The present analysis is not intended to represent a validation of the simple mathematical model since this would require comparison with more extensive datasets. Rather, the simple model was merely used to illustrate the approach and more comprehensive mechanistic models can be developed that explicitly represent protein and fat degradation rates and their dependence on diet and body composition(Reference Hall11, Reference Hall12). The complexity and choice of mathematical model should be dictated by the questions that the model is intended to address.
Another advantage of the new modelling approach is that the values of k P and k F are given by their theoretical biochemical efficiencies which can be used across species and across models. In contrast, the Kielanowski method for determining k P and k F gives values that depend on the choice of the functional form of E m. This means that it is erroneous to interpret values of k P and k F as the protein and fat deposition efficiencies without reference to the associated model for E m and the values cannot be used across species. Nevertheless, several mathematical models of human weight gain have used Kielanowski regression values for k P and k F derived from rats(Reference Pullar and Webster14), pigs(Reference Noblet, Karege and Dubois17) and infants(Reference Roberts and Young15) and have erroneously combined these values with equations for basal metabolism and physical activity modelled for human adults(Reference Christiansen, Garby and Sorensen18, Reference Payne and Dugdale19) and adolescents(Reference Butte, Christiansen and Sorensen20). Only the theoretical biochemical values for k P and k F can be combined with independent models of energy expenditure that either explicitly or implicitly include the energy cost of protein and fat turnover. The mathematical framework presented here shows how such modelling can be done.
Acknowledgements
I (K. D. H.) thank Jan B. van Klinken for helpful comments on the manuscript.
The present study was supported by the Intramural Research Program of the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK).
There are no conflicts of interest.



