Breast cancer is the most frequently diagnosed female cancer worldwide, and its incidence rates have been increasing globally, including in Japan, in recent years( Reference Torre, Siegel and Ward 1 , Reference Katanoda, Matsuda and Matsuda 2 ). A higher circulating level of oestrogens is an important risk factor for breast cancer( Reference Key, Appleby and Barnes 3 , Reference Missmer, Eliassen and Barbieri 4 ) because oestrogens stimulate the growth of cancer cells in breast tissue( Reference Gruber, Tschugguel and Schneeberger 5 ). After menopause, the production of endogenous oestrogen ceases in the ovaries. However, extragonadal sites such as peripheral adipose tissue continue to synthesise oestrogens through aromatisation of androgens by the cytochrome P450 enzyme( Reference Al-Azzawi and Palacios 6 ). The level of oestrogen synthesis in those tissues increases as a function of age and body weight( Reference Gruber, Tschugguel and Schneeberger 5 ). In terms of dietary factors, it has been hypothesised that fat intake increases the risk of postmenopausal breast cancer. However, findings from many studies are inconsistent with each other and an international review by the World Cancer Research Fund/American Institute for Cancer Research concluded that a direct relationship between fat intake and the development of postmenopausal breast cancer is ‘limited-suggestive’( 7 ).
Serum oestrogen concentration is a good biomarker for the evaluation of breast cancer risk. Dietary habits may modify breast cancer risk by changing the oestrogen level, and therefore a diet that leads to a lower serum oestrogen level might contribute to reducing the risk of postmenopausal breast cancer. Although many prior studies have reported associations of foods or nutrients with endogenous sex hormones, the findings are inconsistent. Intakes of red meat and dairy products were shown to affect sex hormone levels, whereas total fat or protein was not associated with levels of oestrogens among a Western population( Reference Brinkman, Baglietto and Krishnan 8 ). Another study reported that higher fat intake was inversely associated with plasma oestradiol (E2) levels( Reference Holmes, Spiegelman and Willett 9 ). However, a meta-analysis of dietary interventions showed that a low-fat diet reduced serum E2 levels( Reference Wu, Pike and Stram 10 ). In addition to such dietary factors, genetic factors have been suggested to play an important role in the risk of hormone-dependent cancer through the biosynthesis of oestrogens( Reference Gruber, Tschugguel and Schneeberger 5 , Reference Olson, Bandera and Orlow 11 ). Polymorphisms in the CYP19A1 gene that encodes aromatase, an enzyme that converts androgens to oestrogens, were shown to be associated with serum sex hormone levels in Japanese people( Reference Olson, Bandera and Orlow 11 – Reference Kidokoro, Ino and Hirose 13 ). The rs605059 polymorphism in the HSD17B1 gene that encodes 17β-hydroxysteroid dehydrogenase type 1 (17β-HSD1), which catalyses the synthesis of E2 from oestrone (E1), has been investigated regarding its association with multiethnic breast cancer risk( Reference Shi, Yang and Dong 14 , Reference Feigelson, Cox and Cann 15 ).
Currently, information is limited regarding the relationships between macronutrient intakes and blood oestrogen concentrations in postmenopausal Asians. Although higher intakes of total, MUFA and PUFA were shown to be associated with higher E1 levels( Reference Nagata, Nagao and Shibuya 16 ), these findings have not been widely endorsed. Furthermore, as far as we know, no study has shown that polymorphisms of hormone-related genes modify associations between macronutrient intakes and serum oestrogen concentrations. We therefore conducted a cross-sectional study to examine the association of dietary intake, especially intakes of macronutrients, with serum oestrogen levels in postmenopausal Japanese women. At the same time, we tested for effects of interactions between polymorphisms in CYP19A1 and HSD17B1 genes and these dietary intakes on the circulating oestrogen concentration.
Methods
Study subjects
The Japan Multi-Institutional Collaborative Cohort Study was launched in 2005 to investigate gene–environment interactions in lifestyle-related diseases, especially in cancer. The details of this cohort study have been described elsewhere( Reference Hamajima 17 ). More than 90 000 participants were enroled in the baseline survey from 14 study areas throughout Japan. Participants completed a questionnaire on lifestyles and medical conditions and donated blood samples.
To examine associations of lifestyle, genetic and medical factors by using a subset of this population, 500–600 consecutive subjects were selected in ten study areas and 4519 participants (2124 men and 2395 women) were eventually included in the cross-sectional study( Reference Wakai, Hamajima and Okada 18 ). Of these subjects, 799 naturally menopaused women aged 47–69 years were selected in nine study areas for the present study( Reference Hosono, Ito and Oze 12 ); one study area was dropped because serum samples were not available for the current study. Natural menopause was defined as no menstruation for at least 12 consecutive months, except for menopause caused by surgeries of the uterus or ovaries or other reasons. We excluded subjects who underwent any hormone therapy when they participated in this study (n 7) and participants whose estimated intakes of total energy were outside the mean (±3 sd) (n 8). Thus, this analysis was performed in 784 postmenopausal women. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the ethics committees of Nagoya University Graduate School of Medicine, Aichi Cancer Center and other participating institutions. Written informed consent was obtained from all subjects.
Lifestyle data and serum hormone measurements
Participants completed a self-administered questionnaire that included questions on anthropometric factors and lifestyles such as dietary intakes, alcohol drinking and smoking habits, physical activity and female reproductive factors. Regarding the validity of self-reported height and weight, the Pearson’s correlation coefficients between self-reported and actually measured values were 0·96 (n 268) for height and 0·97 (n 270) for weight.
For recording dietary intakes, we used a FFQ comprising forty-seven food items( Reference Tokudome, Goto and Imaeda 19 – Reference Imaeda, Goto and Tokudome 21 ). Regarding staple foods (rice, bread and noodles), subjects were asked about daily intake frequency (six possible categories) and portion size for each of breakfast, lunch and supper. Intake frequencies of other food items, averaged over the previous 1 year, were reported within eight categories. We estimated total energy and macronutrient intakes based on intake frequencies of each food with female standard portion sizes by using the Standard Tables of Food Composition, Version 5( 22 ). For alcohol drinking, we asked about drinking habits in the previous 1 year with three potential responses (current drinkers, former drinkers or never drinkers). The amount of alcohol consumption was calculated based on the type of alcoholic beverage (Japanese sake, shochu, shochu highball, beer, whiskey or wine) and the frequency and usual amount of drinking in 1 d for each beverage. Alcohol consumption was considered energy intake. We also calculated consumption of staple foods and fish; the latter is rich in n-3 highly unsaturated fatty acids (n-3 HUFA) that are suggested to be protective against breast cancer( Reference Wakai, Tamakoshi and Date 23 – Reference Zheng, Hu and Zhao 25 ).
The validity of this FFQ was previously evaluated by referring 3-d weighed diet records in middle-aged Japanese( Reference Tokudome, Goto and Imaeda 20 ). The de-attenuated, log-transformed and energy-adjusted Pearson’s correlation coefficients of daily intakes of total energy, protein, fat, carbohydrate, SFA, MUFA, PUFA and n-3 HUFA were 0·44, 0·36, 0·48, 0·64, 0·42, 0·34, 0·25 and 0·35, respectively.
For smoking status, subjects answered whether they smoked with three potential responses (never smokers, former smokers or current smokers). Physical activity was evaluated by computing metabolic equivalent (MET)-h/week from the multiplication of intensity, frequency and times of daily and leisure-time activities. We further asked about female reproductive factors including menstrual status (regular, irregular or ceased), age at menopause and its reason (natural, surgical or others) and history of any hormone therapy.
Serum samples were frozen and stored at −80°C until analysis. Both serum E1 and E2 levels were measured using liquid chromatography-tandem MS (LC-MS/MS), and serum sex hormone-binding globulin (SHBG) was measured using an immunoradiometric assay (IRMA). These analyses were performed at ASKA Pharmaceutical Co., Ltd. The sensitivity of LC-MS/MS was 5·0 pg/ml for both E1 and E2, and that of IRMA was 0·2 nmol/l for SHBG. The intra-assay CV were 0·4–3·3 % for E1, 3·2–6·6 % for E2 and 3·5–6·5 % for SHBG. The inter-assay CV for E1, E2 and SHBG were 3·0–3·2, 4·8–9·5 and 1·5–8·2 %, respectively.
SNP selection and genotyping
To examine the interaction between dietary intakes and genetic factors, we selected two SNP of the CYP19A1 (rs4441215 and rs936306) gene based on our previous report with the same subjects of the present study( Reference Hosono, Ito and Oze 12 ); both E1 and E2 concentrations were associated with these SNP. In addition, the rs605059 polymorphism in the HSD17B1 gene was included in the study because several reports have investigated its influence on breast cancer risk( Reference Shi, Yang and Dong 14 , Reference Feigelson, Cox and Cann 15 ). These selected polymorphisms were genotyped by the multiplex PCR-based Invader assay (Third Wave Technologies) at the Laboratory for Genotyping Development, Center for Genomic Medicine, RIKEN( Reference Ohnishi, Tanaka and Ozaki 26 ).
Statistical analysis
BMI was calculated based on self-reported height and weight. Values of serum E1 and E2 concentrations were logarithmically transformed for all statistical analyses to approximate to the normal distribution. We excluded subjects whose serum E1 or E2 concentration was ten times or higher than the respective median (E1, n 1; E2, n 12). The associations between serum oestrogen levels and potential confounders (age, current BMI, total energy intake, alcohol drinking, physical activity and menopause period) were examined by using Pearson’s correlation coefficients. The differences in oestrogen levels among categories of smoking status (never smokers, former smokers, current smokers) and history of hormone therapy use (yes, no) were examined by one-way ANOVA (ANOVA) and t test, respectively.
To examine associations between serum E1 or E2 level and dietary intakes, we used linear regression models regressing serum E1 or E2 concentration on quartiles of each nutrient or food group intake. We used the energy density method to adjust daily intakes of all nutrients and foods for total energy intake (per 4184 kJ (1000 kcal)). The regression coefficients were adjusted for age at study enrolment (years) and total energy intake (kJ/d (kcal/d)) in model 1. In model 2, we further adjusted for current BMI (kg/m2), smoking status (never smokers, former smokers, current smokers), alcohol drinking (g/d), physical activity (MET-h/week), menopause period (years) and history of any hormone therapy in addition to covariates of model 1. Tests for trend were performed by assigning medians of nutrient or food group intakes in each quartile. We calculated ratios of serum oestrogen concentrations of the second, third and highest quartiles with 95 % CI to those of the lowest quartile.
In the analysis stratified by genotypes, linear regression analysis for serum oestrogen concentration was performed as in the above-mentioned model 2. The P values for interactions between dietary intakes and genetic polymorphisms were computed in the linear regression models that included products of the number of minor alleles (0, 1 or 2) and the median of nutrient or food group intake in each quartile as an independent variable. Genotype frequencies were tested for deviation from the Hardy–Weinberg equilibrium with the genhwi command of Stata version 14 (Stata Corp.). All P values were two-sided and P values<0·05 were considered statistically significant. All analyses were performed using Stata version 14.
Results
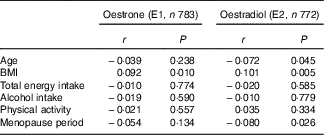
The background characteristics, dietary intake and serum oestrogen concentrations of the 784 women are shown in Table 1. The mean age was 60·5 years, and the median total energy intake was estimated as 6406 kJ/d (1531 kcal/d). Table 2 shows Pearson’s correlation coefficients between serum oestrogen levels and background characteristics. BMI was weakly correlated with both serum E1 and E2 concentrations. Age and menopause period were inversely correlated with serum E2, but the correlations were very weak. No significant association was found between either the smoking status or the history of hormone therapy use and serum oestrogens (data not shown).
Table 1 Characteristics, dietary intake and serum oestrogen levels of the subjects (n 784) (Mean values and standard deviations; numbers and percentages; medians and interquartile ranges (IQR))

MET, metabolic equivalent; %E, percentage of total energy.
* Menopause period was defined as the difference between age at enrolment and age at menopause.
Table 2 Correlation coefficients between serum oestrogen levels and characteristics of the subjects
Tables 3 and 4 summarise the ratios of serum oestrogen concentrations of the second to the highest quartiles (Q2–Q4) of dietary intake to those of the lowest quartile (Q1). None of the macronutrients, including groups of fatty acids, was significantly associated with serum E1 level in models 1 and 2 (Table 3).
Table 3 Serum oestrone concentration by quartile (Q) of dietary intake (n 783) (Ratios to the lowest quartile and 95 % confidence intervals)

* Model 1: adjusted for age and total energy intake.
† Model 2: adjusted for age, current BMI, smoking status, alcohol drinking, physical activity, total energy intake, menopause period and history of any hormone therapy.
Table 4 Serum oestradiol concentration by quartile (Q) of dietary intake (n 772) (Ratios to the lowest quartile and 95 % confidence intervals)

* Model 1: adjusted for age and total energy intake.
† Model 2: adjusted for age, current BMI, smoking status, alcohol drinking, physical activity, total energy intake, menopause period and history of any hormone therapy.
Serum E2 concentration was 15 % higher for Q4 of carbohydrate intake than for Q1 in model 2 (ratio: 1·15; 95 % CI 1·04, 1·28; P trend=0·017) (Table 4). For staple foods, noodle intake was positively associated with serum E2 (ratio for Q4 v. Q1: 1·15; 95 % CI 1·04, 1·26; P trend=0·004 (model 2)). Only in model 2, Q4 of total energy intake was significantly related to a 10 % lower serum E2 level than Q1 (ratio: 0·90; 95 % CI 0·82, 0·99; P trend=0·038). For groups of fatty acids in the model 2, SFA intake was inversely correlated with serum E2; the ratio of the highest quartile of SFA intake to that of the lowest quartile was 0·89 (95 % CI 0·81, 0·98; P trend=0·024). n-3 HUFA intake was also inversely associated with serum E2 concentration with significance. We further compared the serum E2 level between the quartiles of fish consumption, as fish contains abundant n-3 HUFA. The level for Q4 was 9 % lower than that for Q1. However, this association was not significant. No significant correlation of serum E2 level with total protein, total fat or fatty acids except for SFA and n-3 HUFA was observed. Furthermore, adjustment for serum SHBG level in model 2 scarcely changed the associations of dietary intakes with serum oestrogen levels (data not shown).
Both the polymorphisms (rs4441215 and rs936306) in the CYP19A1 gene and one polymorphism (rs605059) in the HSD17B1 gene were consistent with the Hardy–Weinberg equilibrium; P values for these polymorphisms were 0·400, 0·603 and 0·053, respectively. The minor allele frequencies of rs4441215, rs936306 and rs605059 were 0·37, 0·40 and 0·48, respectively. Table 5 shows assessment of the interaction between each SNP and nutrients or food groups, for dietary factors that were found to have statistically significant or marginally significant associations with serum E2 concentration in the analysis in Table 4. The combination of high carbohydrate intake and the heterozygote (CG) of CYP19A1 rs4441215 showed a higher serum E2 level (ratio of Q4 v. Q1: 1·24; 95 % CI 1·06, 1·45; P for trend=0·015). On the other hand, positive associations between noodle intake and serum E2 level were observed in the homozygotes (CC or GG). In addition, the highest quartile of fish consumption with the CC genotype was linked to 17 % lower serum E2 than the lowest quartile (ratio: 0·83; 95 % CI 0·72, 0·96; P for trend=0·015). However, the interactions of all these associations for this SNP were not statistically significant. Stratification by the rs936306 genotype in CYP19A1 showed neither significant associations nor interactions for serum E2 concentrations. Among women who carried the CC genotype of rs605059 in the HSD17B1 gene, carbohydrate intake was positively associated with serum E2 level (ratio of Q4 v. Q1: 1·24; 95 % CI 1·03, 1·48). On the other hand, intakes of n-3 HUFA and fish were inversely associated with serum E2; E2 concentrations of the highest quartile of both n-3 HUFA and fish were 24 % lower than those for the lowest quartile. We found statistically significant, or marginally significant, interactions between this SNP and these three nutrients or foods for serum E2 concentration (P for interaction<0·05). Although the interaction was not significant, SFA intake was also inversely associated with serum E2 in women with the CC genotype (ratio of Q4 v. Q1: 0·81; 95 % CI 0·68, 0·96). We found no substantial modification in the association between total energy intake and serum E2 level by any of the three polymorphisms.
Table 5 Serum oestradiol concentration by quartile of dietary intake: analysis stratified by CYP19A1 rs4441215 and rs936306 and HSD17B1 rs605059 genotypes (n 772)Footnote * (Ratios to the lowest quartile and 95 % confidence intervals)

n-3 HUFA, n-3 highly unsaturated fatty acids.
* Multivariate models adjusted for age, current BMI, smoking status, alcohol drinking, physical activity, total energy intake, menopause period and history of any hormone therapy.
Discussion
In this cross-sectional study of postmenopausal Japanese women, serum E2 concentration was significantly associated with intakes of carbohydrate, especially of noodles. In contrast, the E2 level was inversely correlated with intakes of total energy, SFA and n-3 HUFA. In stratified analyses by genetic polymorphisms, we found significant interactions between the rs605059 genotype of the HSD17B1 gene and intakes of n-3 HUFA and fish; the associations of serum E2 concentration with these nutrients or foods were limited to those with the CC genotype.
We found statistically significant but weak associations between dietary intakes and serum E2 concentrations in apparently healthy postmenopausal women; compared with the lowest quartiles of intakes of carbohydrate and n-3 HUFA, serum E2 levels were only 15 % higher and 9 % lower in the highest quartiles of the respective nutrient intake. However, in three studies on associations of endogenous sex hormone levels with breast cancer risk in postmenopausal women, in which serum E2 was measured by using MS, the median levels of serum E2 were only 1·5–15·6 % higher in the breast cancer patients than in the controls( Reference Dallal, Tice and Buist 27 – Reference Fuhrman, Schairer and Gail 29 ). All three of those studies concluded that higher serum E2 was significantly associated with an increase in the risk of postmenopausal breast cancer( Reference Key, Appleby and Reeves 30 ). We therefore consider that even small differences in E2 levels observed in the present study may be relevant to the health of postmenopausal women.
Although serum oestrogen concentrations in our population were relatively low, they are similar to the oestrogen concentrations of postmenopausal Japanese women reported in another study in which serum oestrogen was measured by using the same method( Reference Nagata, Nagao and Yamamoto 31 ). In addition, median serum values were 14·5 (interdecile ranges 7·0–24·1) pg/ml for E1 and 2·55 (interdecile ranges 0·4–5·4) pg/ml for E2 in our study; 90th percentiles were more than 3 and 13 times higher than 10th percentiles for E1 and E2, respectively. These ranges of serum oestrogen levels were comparable with those in previous studies of postmenopausal women that used MS( Reference Dallal, Tice and Buist 27 – Reference Fuhrman, Schairer and Gail 29 ). Although we cannot conclude that the measurement of serum oestrogen levels using MS resolves the previous contradictory findings, the current study provided sufficient dynamic range in serum oestrogen concentrations.
Our finding of an inverse association between total energy intake and serum E2 concentration is not in line with previous reports. In two weight-loss intervention studies among postmenopausal women, restriction of dietary energy reduced serum E2( Reference Campbell, Foster-Schubert and Alfano 32 , Reference van Gemert, Schuit and van der Palen 33 ). In particular, combinations of dietary energy restriction and exercise had stronger effects on serum sex hormone levels through greater weight loss than dietary interventions alone( Reference Campbell, Foster-Schubert and Alfano 32 ). The discordances between these results and the results of our study may arise from differences between interventional and cross-sectional studies.
As mentioned above, we observed positive and significant associations of the serum E2 concentration and intake of carbohydrate, especially of noodles. A previous study reported that substitution of fat intake for carbohydrate intake of equivalent energy reduced plasma E2 levels( Reference Holmes, Spiegelman and Willett 9 ). Previous results from some prospective cohort studies that were focused on postmenopausal breast cancer risk were inconsistent; higher carbohydrate intake tended to increase overall breast cancer risk, but this association was limited to oestrogen receptor (ER)− or ER−/progesterone receptor (PR)− breast cancer that is independent of endogenous hormone concentrations( Reference Murtaugh, Herrick and Sweeney 34 , Reference Lajous, Boutron-Ruault and Fabre 35 ). As far as we know, direct associations between noodle intake and serum sex hormone concentration or breast cancer have never been reported. Elderly Japanese people with dietary patterns characterised by higher noodle intake have been reported to tend to consume more processed meat, non-alcoholic soft drinks and beer( Reference Sugawara, Yasui-Furukori and Umeda 36 ). However, this tendency was not reproduced in our population.
Dietary SFA were inversely associated with serum E2 in our study, whereas two other studies have reported no association among postmenopausal women( Reference Brinkman, Baglietto and Krishnan 8 , Reference Wu, Stanczyk and Seow 37 ). No effects of SFA intake on breast cancer risk were shown in an international pooled analysis( Reference Smith-Warner, Spiegelman and Adami 38 ). It is unlikely that SFA increases breast cancer risk mediated by endogenous oestrogen. Intake of SFA was derived mostly from chicken eggs, whole milk, well-milled rice, pork and beef in the Japanese diet( Reference Tokudome, Imaeda and Ikeda 39 ). No association was reported between serum E2 levels and intakes of eggs or red meat, whereas a positive association was found with dairy products among a European population( Reference Brinkman, Baglietto and Krishnan 8 ). These disagreements may arise from the difference in SFA intake and sources between Japanese and Western people.
We found that the serum E2 level was reduced among women with a higher intake of n-3 HUFA. Our findings are inconsistent with the results of another study that showed a null association of serum oestrogen concentration with fish consumption( Reference Brinkman, Baglietto and Krishnan 8 ). However, a different study reported an inverse association between intake of n-3 fatty acids from fish and E2 levels( Reference Holmes, Spiegelman and Willett 9 ). Furthermore, our results may be consistent with the finding that higher intakes of n-3 HUFA or fish fat tend to decrease breast cancer risk, and that high erythrocyte compositions of these fatty acids were inversely correlated with this risk among Japanese women( Reference Wakai, Tamakoshi and Date 23 , Reference Kuriki, Hirose and Wakai 24 ). A meta-analysis also indicated that higher intake of marine n-3 PUFA was associated with decreased risk of breast cancer and that this association was stronger in Asia than in other areas( Reference Zheng, Hu and Zhao 25 ). Plasma concentrations of total n-3 HUFA, EPA and DHA, which are derived from marine foods, were moderately and positively correlated with intake of fatty acids( Reference Kuriki, Nagaya and Tokudome 40 , Reference Goto, Tokudome and Imaeda 41 ). These fatty acids, especially EPA, would alter oestrogen metabolism. PGE2, which is an inflammatory eicosanoid that is synthesised from arachidonic acid (AA), has been shown to stimulate the expression and activity of aromatase P450 that converts androgens to oestrogens. In contrast, PGE3 that is derived from EPA does not affect aromatase P450 activity and has been predicted to inhibit synthesis of PGE2 from AA( Reference Young, Kurzer and Thomas 42 – Reference Richards and Brueggemeier 44 ). Therefore, higher intake of EPA may lead to an increase in PGE3 production and a decline in PGE2 production, and thereby a decrease in oestrogen-stimulated cell growth through a reduction in oestrogen synthesis.
In the analyses for interaction between dietary intake and genotypes of oestrogen-synthesis-related genes, we focused on the CYP19A1 and HSD17B1 genes. The CYP19A1 gene encodes aromatase, which is a cytochrome P450 enzyme that converts androgens to oestrogens( Reference Olson, Bandera and Orlow 11 ). A previous study of the same population of our investigation reported that both serum E1 and E2 concentrations were higher in those with the C allele of rs4441215 or the T allele of rs936306 of the CYP19A1 gene than postmenopausal women without these alleles( Reference Hosono, Ito and Oze 12 ). To our knowledge, this is the first study to explore interactions between intakes of nutrients or foods and these polymorphisms. However, no significant associations were observed.
The HSD17B1 gene is located on chromosome 17q12-q21, and it produces 17β-HSD1 that increases oestrogen bioactivity by converting E1 to E2. The rs605059 polymorphism of this gene leads to an amino acid change from serine (T allele) to glycine (C allele) at position 312, although this change alters neither the catalytic nor the immunological properties of the enzyme( Reference Puranen, Poutanen and Peltoketo 45 ). According to the SNP database of the National Center for Biotechnology Information, the allele frequency of this polymorphism varies between races; the C allele is the major allele among Asians, whereas the T allele is dominant among Europeans( 46 ). Previous studies of this polymorphism with reference to breast cancer risk or blood sex hormone concentrations yielded mixed results. In Asia, postmenopausal Chinese women who carried the AA (or TT) genotype were at an increased risk of breast cancer( Reference Wu, Seow and Arakawa 47 ). However, there has been no study that indicated any associations among Japanese women( Reference Feigelson, Cox and Cann 15 , Reference Iwasaki, Hamada and Nishimoto 48 ). In America, higher E2 levels were observed among postmenopausal lean women with the AA (or TT) genotype, whereas no overall effect of the genotype was reported on breast cancer risk( Reference Setiawan, Hankinson and Colditz 49 ). Furthermore, two meta-analyses of multiethnic subjects showed no association of rs605059 with breast cancer risk( Reference Shi, Yang and Dong 14 , Reference Yao, Cao and Qiu 50 ). To our knowledge, we are the first to explore the interaction between macronutrient intake and the HSD17B1 rs605059 polymorphism with serum sex hormone concentrations. Given the established evidence of the positive association between serum E2 and postmenopausal breast cancer risk( Reference Key, Appleby and Barnes 3 , Reference Missmer, Eliassen and Barbieri 4 ), we suggest that breast cancer risk among postmenopausal women with the CC genotype may be decreased by taking less carbohydrate and more n-3 HUFA or fish. Further research may elucidate the interaction between genetic background and dietary factors for the effect on serum oestrogen level and breast cancer risk.
The main strength of our study was that serum oestrogen concentrations were measured by using LC-MS/MS. This method detects oestrogens with higher sensitivity than other methods usually used to measure blood sex hormone concentrations of postmenopausal women, which meant that we could exactly assess very low serum oestrogen concentrations in postmenopausal women. In addition, we could consider many potential confounders that have been suggested to affect sex hormone concentrations and examine interactions between dietary factors and genetic polymorphisms.
Several limitations of this study also need to be mentioned. First, we estimated dietary intakes by using a FFQ with moderate validity( Reference Tokudome, Goto and Imaeda 20 ). Although the validity of FFQ in the present study is comparable to that in other large prospective cohort studies in Japan( Reference Date, Fukui and Yamamoto 51 , Reference Tsugane, Kobayashi and Sasaki 52 ), an error in dietary assessment is likely to lead to a non-differential misclassification and to attenuate associations between dietary intakes and serum oestrogen levels. Second, we could not clearly show cause–effect relationships owing to the nature of a cross-sectional study. Indeed, a previous study showed that serum levels of oestrogens were positively associated with leptin, which influences appetite regardless of obesity status, and that low-level oestrogens may influence feeding behaviour in postmenopausal women( Reference Karim, Stanczyk and Brinton 53 ). Therefore, further longitudinal or intervention studies are required to reveal causal relationships.
In conclusion, we found significant and positive associations between serum E2 levels and intakes of carbohydrate or noodles, whereas inverse correlations were observed between E2 levels and intakes of total energy, saturated fatty acids or n-3 HUFA among postmenopausal Japanese women. A polymorphism of HSD17B1, which encodes one of the genes involved in oestrogen synthesis, modified the relationship between dietary intakes and circulating E2 levels. These findings are of significance in terms of discussing the role of diet in the risk of hormone-dependent cancer.
Acknowledgements
The authors thank Shinkan Tokudome at the National Institute of Health and Nutrition (formerly Nagoya City University); Chiho Goto at Nagoya Bunri University; Nahomi Imaeda at Shigakkan University; Yuko Tokudome at Nagoya University of Arts and Sciences; Masato Ikeda at the University of Occupational and Environmental Health; Shinzo Maki at the Aichi Prefectural Dietetic Association for providing the FFQ and programmes for calculation of dietary intakes; the assistant staff at the Laboratory for Genotyping Development, Center for Genomic Medicine, RIKEN for support with genotyping; and all of the participants and staff who took part in the Japan Multi-Institutional Collaborative Cohort Study for cooperation.
This study was supported by Grants-in-Aid for Scientific Research for Priority Areas of Cancer (no. 17015018) and Innovative Areas (no. 221S0001) and by JSPS KAKENHI Grants (nos 16H06277 and 15H02524) from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
S. T., S. H. and K. W.: formulating the research questions; S. T., C. N., S. H. and K. W.: designing the study; Y. N., N. K., S. S., I. S., H. M., S. H., M. N., S. K., R. O., H. U., N. K., M. K., N. H. and H. T.: carrying out the study; S. T. and K. W.: analysing the data; S. T., C. N., H. U., H. T., K. W.: writing the article.
The authors declare that there are no conflicts of interest.







