Probiotics or probiotic bacteria generally refer to live micro-organisms pre-existing in human bodies that are beneficial to intestinal tract health, or micro-organisms administered from external sources that favour any aspect of human health( Reference Gerritsen, Smidt and Rijkers 1 , Reference Dwivedi, Kumar and Laddha 2 ). Various food products that are made from probiotics are regarded as functional foods( Reference Nagpal, Kumar and Kumar 3 , Reference Govender, Choonara and Kumar 4 ). Lactobacilli are being widely used in the development of novel bio-therapeutic probiotic formulations for managing various diseases. Indeed, the effects of probiotics on various diseases have been widely studied in both animal experiments and human clinical trials( Reference Matsuzaki, Nagata and Kado 5 – Reference Laitinen, Poussa and Isolauri 8 ). Evidence has demonstrated that Lactobacillus casei Shirota differentially the inflammatory cytokine responses of macrophages and T cells in either Peyer’s patches or the spleen( Reference Shida, Nanno and Nagata 9 ). A recent study indicated that Lactobacillus jensenii TL2937 can interact with intestinal epithelial cells and immune cells through Toll-like receptors (TLR) and improve animal and human health( Reference Villena and Kitazawa 10 ). In another study, probiotic L. casei Zhang reduced the lipopolysaccharide/D-galactosamine N-induced expression of IL-1β and inducible nitric oxide synthase (iNOS) in the liver by modulating the TLR–mitogen-activated protein kinase (MAPK)–PPAR-γ signalling pathways and intestinal microbiota in a rat model of acute liver failure( Reference Wang, Xie and Li 11 ). These findings reveal that certain probiotics potentially have beneficial effects on both animals and humans by regulating the immune system.
Systemic lupus erythematosus (SLE) is an autoimmune inflammatory disorder that affects various organs, including the liver( Reference Hahn 12 ). Notably, an increasing body of evidence reveals that liver abnormality is more common in cases of SLE than has typically been reported. Significantly increased liver abnormalities, such as elevated aspartate aminotransferase, alanine aminotransferase, fatty liver, portal inflammation, histopathological change and apoptosis, have been observed in the livers of SLE patients and lupus-prone mice, relative to controls( Reference Abraham, Begum and Isenberg 13 – Reference Tzang, Chiang and Lai 17 ). Interestingly, the intake of dietary supplements has recently been demonstrated to be strongly associated with disease activity and intestinal microbiota in both lupus-prone animals and SLE patients( Reference Cuervo, Hevia and López 18 , Reference Johnson, Gaudreau and Al-Gadban 19 ). Therefore, supplementation with certain probiotics could be beneficial to immune function and gastrointestinal health in cases of SLE.
Growing evidence reveals that a damaged intestinal barrier, also known as ‘leaky gut’, enhances interactions between intestinal bacteria and liver receptors, such as toll-like receptors, causing various hepatic disorders, such as liver cirrhosis, non-alcoholic fatty liver diseases and hepatic encephalopathy. However, the alteration of gut microbiota by administrating probiotics has been demonstrated to have a potentially beneficial effect on hepatic disorders( Reference Lo, Austin and Freeman 20 , Reference Paolella, Mandato and Pierri 21 ). Our recent study revealed that heat-killed Lactobacillus reuteri GMNL-263 (GMNL-263) reduces liver and heart fibrosis in hamsters on a high-fat diet by suppressing transforming growth factor-β ( Reference Ting, Kuo and Hsieh 22 ). Both live and heat-killed GMNL-263 significantly reduce obesity-induced metabolic abnormalities by reducing insulin resistance and hepatic steatosis formation( Reference Hsieh, Lan and Huang 23 ). However, no investigation of the functions of Lactobacillus paracasei GMNL-32 (GMNL-32) and L. reuteri GMNL-89 (GMNL-89) has been performed. As hepatic abnormality has been strongly associated with the pathogenesis of SLE( Reference Hahn 12 ), this study investigates whether orally administering GMNL-32, GMNL-89 and GMNL-263 reduces hepatic inflammation and apoptosis in NZB/W F1 mice.
Methods
Preparation of Lactobacillus strains
GMNL-32, GMNL-89 and GMNL-263 were obtained from GenMont Biotech, Inc., and the doses of these Lactobacillus strains (109 colony-forming units (CFU)/mouse per d) that were used in this study were based on our previous publications( Reference Ting, Kuo and Hsieh 22 , Reference Liao, Kuo and Kuo 24 ). The catalogue numbers at the Bioresource Collection and Research Center in Taiwan are ‘BCRC 910220’, ‘BCRC 910340’ and ‘BCRC 910452’, respectively. Powders of three Lactobacillus strains were prepared in PBS for use in the oral gavage treatment of mice.
Animals and treatments
This study was approved by the Institutional Animal Care and Use Committee at Chung Shan Medical University (approval no. 847). A total of thirty-two female NZB/W F1 mice of 6 weeks of age were purchased from Jackson Laboratory and housed under the supervision of the Institutional Animal Care and Use Committee at Chung Shan Medical University, Taichung, Taiwan. All animals were kept in a 12 h light–12 h dark cycle and ambient temperature was maintained at 25°C. Animals had free access to water and standard laboratory chow (LabDiet 5001; PMI Nutrition International Inc.). Animal welfare was maintained and experimental procedures were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animals at an age of 8 weeks were randomly divided into four groups (each of eight mice) – control, GMNL-32, GMNL-89 and GMNL-263 groups, which are treated with PBS, 109 CFU/mouse per d of GMNL-32, GMNL-89 and GMNL-263, respectively, by oral gavage. The experimental period was 12 weeks long and the mice were killed by asphyxiation using CO2 at an age of 20 weeks. Liver tissues were collected and stored at –80°C until analysis.
Gel zymography
Matrix metalloproteinase (MMP)-2 and MMP-9 activities were analysed by gelatin zymography assays as previously described( Reference Hsu, Chiu and Chang 25 ). Protein lysates amounting to 25 μg from the liver tissue of NZB/W F1 mice were separated onto an 8 % SDS-PAGE gel containing 0·1 % gelatin. Gels were washed for 30 min in 2·5 % Triton X-100 to remove SDS and then soaked in a reaction buffer containing 40 mm TRIS-HCl (pH 8.0), 10 mm CaCl2 and 0·02 % NaN3 for 30 min. The gels were incubated at 37°C for 24 h after transferring to a fresh reaction buffer. Gelatinolytic activity was visualised by staining the gels with 0·5 % Coomassie Brilliant Blue R-250; they were then destained with methanol–acetic acid water, and relative MMP levels were quantified by a gel documentation and analysis system (Appraise; Beckman Coulter).
Haematoxylin–eosin staining
Haematoxylin–eosin (H–E) staining was performed as described elsewhere( Reference Lin, Chiu and Chen 26 ). Liver samples from the animals were excised and soaked in formalin and covered with wax. The waxed tissue blocks were cut into 5-μm-thick sections and prepared by deparaffinisation and dehydration. The sections were passed through a series of graded alcohols (100, 95 and 75 %) for 15 min each and then dyed with haematoxylin. Photomicrographs were obtained using Zeiss Axiophot microscopes.
Detection of terminal deoxynucleotidyl transferase dUTP nick end labelling-positive cells
Apoptotic cells were detected with terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay (Roche Applied Science) as described elsewhere( Reference Hsu, Chiu and Lin 27 ). Under florescence (excitation wavelength of 460 nm and detection in the range of 515–565 nm), TUNEL-positive nuclei with fragmented DNA were illuminated in bright green. To visualise the nuclei, the tissue sections were reacted with 0·1 μg/ml 4, 6-diamidino-2-phenylindole, and the nuclei were detected and photographed at 454 nm using a Zeiss Axiophot microscope.
Protein preparation and immunoblotting
All procedures were performed at 4°C in a cold room as previously described( Reference Tzang, Lin and Tsai 28 , Reference Chiu, Shi and Yang 29 ). The liver tissues obtained from NZB/W F1 mice were homogenised in 600 μl PRO-PREP solution (iNtRON Biotech) by thirty strokes using a Dounce Homogenizer (Kontes Glass). The homogenates were centrifuged at 13 000 rpm for 10 min at 4°C and the supernatant was then stored at –80°C until use. The protein content in the supernatant was measured according to the procedures of a Bio-Rad protein assay kit (Bio-Rad Laboratories, Inc.). Immunoblotting was performed as described in our previous study. In brief, protein samples were separated in 10 or 12·5 % SDS-PAGE and electrophoretically transferred to a nitrocellulose membrane (Amersham Biosciences). After blocking with 5 % non-fat dry milk in 1× PBS, antibodies against C-reactive protein (CRP), iNOS, IL-1β, TNF-α, IL-6, caspase-3, phosphorylated extracellular signal-regulated kinase (p-ERK), ERK, phosphorylated P38 (p-P38), P38, phosphorylated c-Jun N-terminal kinase (p-JNK), JNK, IκB kinase (IKK) (Santa Cruz Biotechnology), NF-κB (Merck Millipore) and β-actin (Upstates) were diluted in PBS with 2·5 % bovine serum albumin and incubated for 1·5 h with gentle agitation at room temperature. The membranes were washed twice with PBS–Tween for 1 h and secondary antibody conjugated with horseradish peroxidase (HRP) (Santa Cruz Biotechnology) was added. Pierce’s SuperSignal West Dura HRP Detection Kit (Pierce Biotechnology Inc.) was used to detect antigen–antibody complexes. Quantified results were performed by densitometry (Appraise).
Statistical analysis
Sample size was calculated using free sample size calculating software G*Power version 3.1.9.2 (Franz, Universitat Kiel). With a power of 80 %, 0·05 level of statistical significance and an effect size of 0·8, sample size for each test was calculated to be eight. A total of thirty-two mice were randomly assigned into one of the four experimental groups. All values are expressed as means and standard deviations. The comparisons in gel zymography, TUNEL and immunoblot among groups were performed using GraphPad Prism 5 software (GraphPad Software, Inc.) by one-way ANOVA followed by Tukey’s multiple comparisons test. P<0·05 was considered to indicate a statistically significant difference. The significant differences were stressed with symbols as shown in figures.
Results
Supplementation with Lactobacillus strains reduces hepatic matrix metalloproteinase-9 activities and C-reactive protein and inducible nitric oxide synthase expressions in lupus-prone mice
To clarify the anti-inflammatory effects of various Lactobacillus strains on lupus-prone mice, the activity of MMP-9 and expressions of CRP and iNOS proteins in the livers of NZB/W F1 mice that were given a control, GMNL-32, GMNL-89 or a GMNL-263 diet were determined. Notably, significantly lower MMP-9:MMP-2 activity ratios were detected in the livers of NZB/W F1 mice that were given a GMNL-32, GMNL-89 or a GMNL-263 diet, respectively, than in the livers of those that were given the control diet (Fig. 1(a)). In addition, significantly lower expressions of CRP and iNOS proteins were observed in the livers of NZB/W F1 mice that were given a GMNL-32, GMNL-89 or a GMNL-263 diet, respectively, than in the livers of those that were given the control diet (Fig. 1(c)). Fig. 1(d) and (e) present quantitative results concerning CRP and iNOS levels, respectively, relative to the β-actin level.

Fig. 1 Detection of matrix metalloproteinase (MMP)-9 activity and expressions of C-reactive protein (CRP) and inducible nitric oxide synthase (iNOS). Liver lysates were obtained from NZB/W F1 mice receiving control, Lactobacillus paracasei GMNL-32 (GMNL-32), Lactobacillus reuteri GMNL-89 (GMNL-89) or L. reuteri GMNL-263 (GMNL-263) diet, respectively. (a) Hepatic MMP-9 and MMP-2 activities. (b) Signal intensity of MMP-9 and MMP-2 activities were quantitated using a Phosphoimager and the ratio of MMP-9:MMP-2 is also presented. (c) Expression of CRP and iNOS proteins were detected by zymorgraphy and probed with antibodies against CRP and iNOS. Relative protein quantification of (d) CRP and (e) iNOS are represented by vertical bars on the basis of β-actin. Similar results were observed in three repeated experiments. Mean value was significantly different from that of the control group: * P<0·05.
Supplementation with Lactobacillus strains reduces hepatic lymphocyte infiltration and IL-1β, IL-6 and TNF-α expressions in lupus-prone mice
To further investigate the effects of various Lactobacillus strains on hepatic histopathological changes and expressions of pro-inflammatory cytokines, IL-1β, IL-6 and TNF-α were detected by H–E staining and immunoblotting. Considerably greater lymphocyte infiltration was observed in the liver sections of NZB/W F1 mice that were given a GMNL-32, GMNL-89 or a GMNL-263 diet, respectively, than in the liver sections of mice that were given the control diet (Fig. 2(a)). Significantly lower expressions of IL-1β, IL-6 and TNF-α proteins were detected in the livers of NZB/W F1 mice that were given a GMNL-32, GMNL-89 or GMNL-263 diet than in the livers of mice that were given the control diet (Fig. 2(b)). Fig. 2(c)–(e) present quantitative results concerning IL-1β, IL-6 and TNF-α levels, respectively, relative to β-actin level.

Fig. 2 Histopathological changes and expressions of IL-1β, IL-6 and TNF-α. (a) Liver sections obtained from NZB/W F1 mice receiving control, Lactobacillus paracasei GMNL-32 (GMNL-32), Lactobacillus reuteri GMNL-89 (GMNL-89) or L. reuteri GMNL-263 (GMNL-263) diet, respectively, were stained with haematoxylin–eosin. Images of hepatic architecture were 400× magnified. Liver lysates obtained from NZB/W F1 mice receiving control, GMNL-32, GMNL-89 or GMNL-263 diet, respectively, were probed with antibodies against (b) IL-1β, IL-6 and TNF-α. Relative protein quantification of (c) IL-1β, (d) IL-6 and (e) TNF-α are represented by vertical bars on the basis of β-actin. Similar results were observed in three repeated experiments. Mean value was significantly different from that of the control group: * P<0·05.
Supplementation with Lactobacillus strains reduces hepatic apoptosis in lupus-prone mice
To investigate the effects of various Lactobacillus strains on hepatic apoptosis, TUNEL-positive cells and cleaved caspase-3 protein were detected. Significantly fewer TUNEL-positive cells were detected in the livers of NZB/W F1 mice that were given the GMNL-32, GMNL-89 or the GMNL-263 diet, than in those of mice that were given the control diet (Fig. 3(a)). Fig. 3(b) presents the percentage of cells that were TUNEL-positive. Significantly less cleaved caspase-3 protein was detected in the livers of NZB/W F1 mice that were given the GMNL-32, GMNL-89 or GMNL-263 diet, respectively, than in the livers of mice that were given the control diet (Fig. 3(c)). Fig. 3(d) presents quantitative results concerning the cleaved caspase-3 level relative to the β-actin level.

Fig. 3 Detection of terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)-positive cells and expression of caspase-3. Liver sections and liver lysates were obtained from NZB/W F1 mice receiving control, Lactobacillus paracasei GMNL-32 (GMNL-32), Lactobacillus reuteri GMNL-89 (GMNL-89) or L. reuteri GMNL-263 (GMNL-263) diet, respectively. (a) TUNEL assay was performed with liver sections. Fluorescein isothiocyanate (FITC)-labelled terminal deoxytransferase was bound to nicked end of DNA. 4, 6-diamidino-2-phenylindole (DAPI) staining was used as control. (b) The percentage of TUNEL-positive cells in liver sections are represented by vertical bars. (c) Expression of caspase-3 was detected with antibodies against caspase-3. Relative protein quantification of (d) cleaved caspase-3 are represented by vertical bars on the basis of β-actin. Similar results were observed in three repeated experiments. Mean value was significantly different from that of the control group: * P<0·05.
Signalling molecules involved in reduction of hepatic injury in lupus-prone mice by Lactobacillus strains
To identify the signalling molecules that may be involved in the reduction of liver injury in lupus-prone mice by Lactobacillus strains, the signalling molecules of the MAPK and NF-κB pathways were examined. Significantly lower ratios of p-ERK:ERK, p-P38:P38 and p-JNK:JNK were observed in the livers of NZB/W F1 mice that had been given a GMNL-32, GMNL-89 or a GMNL-263 diet, respectively, than in the livers of mice that had been given the control diet (Fig. 4(a)). Fig. 4(b)–(d) present the ratios of p-ERK:ERK, p-P38:P38 and p-JNK:JNK, respectively. The expressions of both IKK and NF-κB proteins were significantly lower in the livers of NZB/W F1 mice that had been given a GMNL-32, GMNL-89 or a GMNL-263 diet, respectively, than in the livers of mice that had been given the control diet (Fig. 5(a)). Fig. 5(b) and (c) present quantitative results concerning IKK and NF-κB levels, respectively, relative to the β-actin level.

Fig. 4 Expression of extracellular signal-regulated kinase (ERK), P38 and c-Jun N-terminal kinase (JNK). Liver lysates obtained from NZB/W F1 mice receiving control, Lactobacillus paracasei GMNL-32 (GMNL-32), Lactobacillus reuteri GMNL-89 (GMNL-89) or L. reuteri GMNL-263 (GMNL-263) diet, respectively, were probed with antibodies against (a) ERK, phosphorylated ERK (p-ERK), P38, phosphorylated P38 (p-P38), JNK and phosphorylated JNK (p-JNK). Relative protein quantification of (b) p-ERK, (c) p-P38 and (d) p-JNK are represented by vertical bars on the basis of ERK, P38 and JNK, respectively. Similar results were observed in three repeated experiments. Mean value was significantly different from that of the control group: * P<0·05.
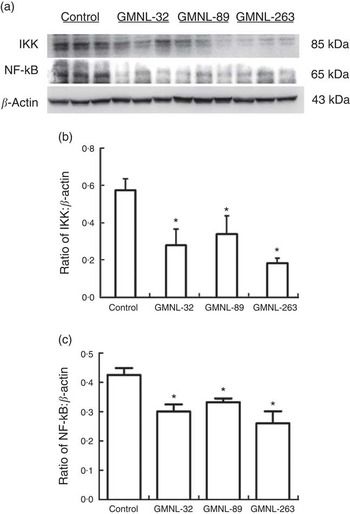
Fig. 5 Expression of IκB kinase (IKK) and NF-κB. Liver lysates obtained from NZB/W F1 mice receiving control, Lactobacillus paracasei GMNL-32 (GMNL-32), Lactobacillus reuteri GMNL-89 (GMNL-89) or L. reuteri GMNL-263 (GMNL-263) diet, respectively, were probed with antibodies against (a) IKK and NF-κB. Relative protein quantification of (b) IKK and (c) NF-κB are represented by vertical bars on the basis of β-actin. Similar results were observed in three repeated experiments. Mean value was significantly different from that of the control group: * P<0·05.
Discussion
Certain probiotics are known to exhibit diverse functions, such as modulation of the immune system and homoeostasis of intestinal microbiota against various diseases( Reference Gerritsen, Smidt and Rijkers 1 , Reference Dwivedi, Kumar and Laddha 2 ). This study is the first to reveal that oral gavage of Lactobacillus strains, including GMNL-32, GMNL-89 and GMNL-263, mitigates hepatic apoptosis and inflammatory indicators, such as MMP-9 activity and CRP and iNOS expressions in lupus-prone mice. Supplementation with GMNL-32, GMNL-89 or GMNL-263 in NZB/W F1 mice also reduces the expressions of hepatic IL-1β, IL-6 and TNF-α proteins by reducing MAPK/NF-κB inflammatory signalling. These findings reveal the beneficial effects of GMNL-32, GMNL-89 and GMNL-263 on hepatic inflammation and apoptosis in lupus-prone mice and suggest an alternative treatment for liver disorders in cases of SLE.
Although microbes and diet have been suggested to play critical roles in initiating autoimmunity( Reference Cuervo, Hevia and López 18 , Reference Johnson, Gaudreau and Al-Gadban 19 , Reference Tian and Zhang 30 ), the effect of gut microflora on the initiation and progression of SLE remains unclear. Gastrointestinal symptoms are common in SLE patients, and more than half of them are caused by adverse reactions to medications and microbial infections( Reference Tian and Zhang 30 ). These findings suggest that an imbalance of gastrointestinal microflora is involved in the pathogenesis of SLE. Recent studies have paved the way to treatments that involve probiotics( Reference Dwivedi, Kumar and Laddha 2 , Reference Dongarrà, Rizzello and Muccio 31 ). Evidence has shown that certain Lactobacillus strains can promote the production of anti-inflammatory cytokines such as IL-10 and TNF-α, and thus can be used to treat autoimmune diseases such as SLE( Reference Dwivedi, Kumar and Laddha 2 , Reference Honad and Littman 32 , Reference Lescheid 33 ). However, few studies have investigated whether Lactobacillus is effective in treating liver injuries that are associated with autoimmune diseases such as SLE. This study reveals that supplementation with GMNL-32, GMNL-89 or GMNL-263 mitigates hepatic apoptosis and inflammation in NZB/W F1 mice by reducing MMP-9 activity and the expressions of CRP and iNOS proteins. Furthermore, supplementation with GMNL-32, GMNL-89 or GMNL-263 reduced the expressions of inflammatory cytokines IL-1β, IL-6 and TNF-α in the livers of NZB/W F1 mice. These results suggest that GMNL-32, GMNL-89 and GMNL-263 have beneficial effects in the treatment of hepatic inflammation and apoptosis in cases of SLE.
The major output of MAPK signalling downstream of inflammatory cytokines is a pro-inflammatory response. The TNF superfamily ligands subsequently cause a cascade and then phosphorylate JNK and P38. Activated JNK and P38 translocate to the nucleus and activate multiple transcription factors such as NF-κB, which leads to the expression of pro-inflammatory cytokines( Reference Arthur and Ley 34 ). Indeed, special attention has been paid to the specific role of the autoantibody-induced activation of P38 MAPK-mediated immunopathology in the pathogenesis of autoimmune diseases( Reference Mavropoulos, Orfanidou and Liaskos 35 ). SLE is a highly complex autoimmune disorder with unknown aetiology in which a diffuse, chronic inflammatory reaction plays an important aetiological role( Reference Hahn 12 , Reference Gottschalk, Tsantikos and Hibbs 36 ). Clinical trials in SLE are focusing on the development of such agents as belimumab to control the activation of T- and B-lymphocytes by targeting the B-cell survival factor BAFF (B-cell activating factor)( Reference Gottschalk, Tsantikos and Hibbs 36 ). However, the efficacy of belimumab is limited. Recently, emphasis has been placed on elucidating the association between inflammasome and pro-inflammatory cell death (pyroptosis), which results in the modification of autoantigens and the generation of autoimmunity in lupus( Reference Buyon, Cohen and Merrill 37 ). Therefore, the potential effectiveness of the modulation of MAPK pathways in treating autoimmune and inflammatory diseases such as SLE should be considered( Reference Gottschalk, Tsantikos and Hibbs 36 ). Notably, this study is the first to identify the reduction of hepatic IL-1β, IL-6 and TNF-α protein expression through reducting the MAPK/NF-κB signalling. Although the interplay of GMNL-32, GMNL-89 and GMNL-263 with the gastrointestinal microbiota and immune cells requires further investigation, this study suggests that certain probiotics mitigate hepatic inflammation in cases of SLE.
Conclusions
Although the precise mechanism by which GMNL-32, GMNL-89 and GMNL-263 act on liver injuries in cases of SLE requires further investigation, we postulate that the anti-apoptosis and anti-inflammation effects of GMNL-32, GMNL-89 and GMNL-263 probably involve their regulatory effects on gastrointestinal microbiota, intestinal epithelial cells and immune cells in gut-associated lymphoid tissue. As displayed in Fig. 6, GMNL-32, GMNL-89 and GMNL-263 significantly mitigate liver apoptosis in NZB/W F1 mice by reducing IKK/NF-κB signalling. GMNL-32, GMNL-89 and GMNL-263 also significantly mitigate liver inflammation by reducing the activity of MMP-9 and the expressions of CRP, iNOS, IL-1β, IL-6 and TNF-α proteins by inhibiting MAPK/NF-κB inflammatory signalling. These findings reveal the inhibition of liver injuries in lupus-prone mice by GMNL-32, GMNL-89 and GMNL-263, and suggest the therapeutic potential of GMNL-32, GMNL-89 and GMNL-263 against hepatic injuries in SLE.

Fig. 6 Schematic illustration of the possible mechanism of Lactobacillus paracasei GMNL-32 (GMNL-32), Lactobacillus reuteri GMNL-89 (GMNL-89) and L. reuteri GMNL-263 (GMNL-263) involved in systemic lupus erythematosus (SLE)-associated liver injuries. Lactobacillus strains of GMNL-32, GMNL-89 and GMNL-263 ameliorate hepatic apoptosis and inflammation through mitogen-activated protein kinase/NF-κB signalling in NZB/W F1 mice. ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinase; IKK, IκB kinase; MMP, matrix metalloproteinase; iNOS, inducible nitric oxide synthase.
Acknowledgements
This work was supported by GenMont Biotech, Inc., Tainan, Taiwan (E0150100). GenMont Biotech had no role in the design, analysis or writing of this article.
T.-C. H., C.-Y. H., Y.-H. C. and B.-S. T. conceived and designed the experiments; T.-C. H., C.-Y. H., C.-H. L., K.-C. H., Y.-H. C. and B.-S. T. performed the experiments and analysed the data; and T.-C. H., Y.-H. C. and B.-S. T. wrote the paper.
None of the authors has any conflicts of interest to declare.









