The CVD health benefits of n-3 long-chain PUFA consumption have been demonstrated in experimental animal and human studies, resulting in approval by the US Food and Drug Administration of a qualified health claim for n-3 long-chain PUFA and coronary heart disease (USFDA, 2004). A high n-3 long-chain PUFA status is thought to be cardioprotective through reduction of risk factors such as dyslipidaemia, hypertension, thrombosis and arrhythmia (McLennan et al. Reference McLennan, Howe, Abeywardena, Muggli, Raederstorff, Mano, Rayner and Head1996; Hu & Willet, Reference Hu and Willett2002; Wijendran & Hayes, Reference Wijendran and Hayes2004) and improvement of arterial compliance, endothelial vasodilator function (Nestel et al. Reference Nestel, Shige, Pomeroy, Cehun, Abbey and Raederstorff2002) and heart rate variability (Christensen, Reference Christensen2003). In the GISSI-Prevenzione study, a large-scale intervention trial in post-myocardial infarction patients, supplementation with 850 mg/d n-3 long-chain PUFA reduced total mortality, cardiovascular mortality and, specifically, sudden cardiac death (GISSI-Prevenzione Investigators, 1999). To maintain cardiovascular health, the American Heart Association recommends eating at least two fatty-fish meals per week, equalling 400–500 mg/d n-3 long-chain PUFA. The International Society for the Study of Fatty Acids and Lipids recommends consuming at least 500 mg/d of EPA and DHA, and the Australian National Health and Medical Research Council recommends consuming 410 mg/d for women and 630 mg/d for men (Baghurst, Reference Baghurst2005). Countries with a high fish intake readily achieve this target, e.g. average n-3 long-chain PUFA consumption in Japan is 1·6 g/d (Dolecek, Reference Dolecek1992). However, these recommendations may be difficult to attain in countries with traditionally low fish intake such as the USA and Australia, where median n-3 long-chain PUFA intakes are only 100 and 140 mg/d, respectively (Meyer et al. Reference Meyer, Mann, Lewis, Milligan, Sinclair and Howe2003).
An alternative strategy to increase the n-3 long-chain PUFA status of the population is to expand the range of foods containing n-3 long-chain PUFA. A variety of foods including chicken meat (Lopez-Ferrer et al. Reference Lopez-Ferrer, Baucells, Barroeta and Grashorn2001), pork (Howe et al. Reference Howe, Downing, Grenyer, Grigonis-Deane and Bryden2002), eggs (Lewis et al. Reference Lewis, Seburg and Flanagan2000), bread (Yep et al. Reference Yep, Li, Mann, Bode and Sinclair2002), spreads (Roche & Gibney, Reference Roche and Gibney1994; Kolanowski et al. Reference Kolanowski, Swiderski, Lis and Berger2001) and other processed products (Lovegrove et al. Reference Lovegrove, Brooks, Murphy, Gould and Williams1997; Metcalf et al. Reference Metcalf, James, Mantzioris and Cleland2003) have now been enriched with n-3 long-chain PUFA. However, there has been little evaluation of the potential contribution of these foods to increased population n-3 intakes. We conducted a double-blind, parallel, dietary intervention trial of 6 months duration at two sites in Australia, Adelaide and Perth, to examine the feasibility and anticipated cardiovascular benefits of increasing habitual n-3 long-chain PUFA intake to 1 g/d through regular consumption of a variety of n-3 long-chain PUFA food sources.
Materials and methods
Subjects
Ninety-four men and women aged 20–65 years, who were overweight and had fasting plasma triacylglycerols (TG) >1·6 mmol/l, were recruited through local media advertisements in Perth and Adelaide to participate in a 6-month double-blind, randomized, parallel comparison of the effects of consuming enriched or control foods. Subjects were excluded if they reported one of the following: diagnosed diabetes mellitus, recent symptomatic heart disease including angina pectoris; history of myocardial infarction or stroke; peripheral vascular disease; major surgery within the last 3 months; blood pressure (BP) >160/100 mmHg; liver or renal disease (plasma creatinine >120 μmol/l); regular non-steroidal anti-inflammatory, antihypertensive or hypocholesterolaemic drug therapy; eating more than one fish meal per week or taking fish oil supplements; and inability to consume the test foods. Of ninety-four volunteers who attended screening, eighty-six were stratified according to baseline TG concentration and BMI, and randomly allocated to one of two groups. The study was approved by the Human Research Ethics Committees at the University of Western Australia, the University of Adelaide and the CSIRO. All participants gave written informed consent.
n-3-enriched foods
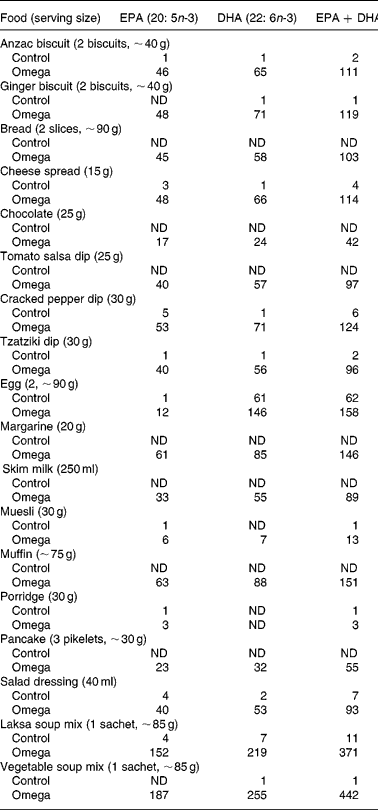
Foods enriched with n-3 long-chain PUFA and matching control foods, including cheese spread, chocolates, instant oats, milk, dips (pepper, tzatziki, salsa), biscuits (Anzac and ginger), pancakes, muffins, bread, salad dressing and dry soup mix (chicken laksa and vegetable) were provided by Goodman Fielder Ltd, Sydney, Australia. Processed foods were enriched with blended or microencapsulated cod fish oil provided by Maritex, Aarhus, Denmark. Regular eggs or Omega-3 Eggs were obtained from Pace Farm Pty Ltd, Sydney, Australia. The EPA and DHA contents of these foods are shown in Table 1.
Table 1 Content (mg/serving) of n-3 long-chain PUFA in n-3-enriched foods and control foods
Study design—food consumption and diet analysis
Participants were asked to consume eight servings of study foods per day for 6 months. Each serving of the n-3-enriched foods was intended to provide approximately 125 mg EPA + DHA, i.e. a total of 1 g of n-3 PUFA/day. Subjects were advised to replace customary foods with study food equivalents. Intervention foods (enriched with n-3 long-chain PUFA; omega-3 group) and equivalent control foods (not enriched; control group) were supplied to all subjects in unmarked packages. The identity of the study foods was blinded to the subjects as well as the researchers conducting the intervention. Subjects were provided with 2 weeks supply of study foods and instructed on how to incorporate eight portions of study foods into their ad libitum background diet.
Subjects were seen every second week to monitor body weight, collect study foods and to discuss any issues arising from the intervention. Daily logs of study food consumption were kept by each subject to monitor the number of servings and types of foods consumed. However, after the first month of the study, those subjects who gained weight were advised by a qualified dietitian on strategies to reduce their energy intake whilst accommodating the study foods. This was monitored throughout the study. Subjects completed a diet history questionnaire and 3 d weighed food records (two weekdays and one weekend day) at baseline, 3 months and 6 months. Details of the dietary collection methods have been described previously by Patch et al. (Reference Patch, Tapsell, Mori, Myer, Murphy and Mansour2005). Dietary data were entered into the Foodworks™ nutrient analysis software package (Xyris Software, Highgate Hill, Brisbane Australia, Professional Version 3.2, 2002), using the Australian nutrient database AusNut Rev 0.14 (AusNut 2000, Department of Human Services & Health, Canberra) and Australian Fatty Acids Rev 0·6 (Royal Melbourne Institute of Technology, 2002) to derive values for energy, macronutrient intakes and fatty acids. Data on consumption of all study foods (control and n-3-enriched) were entered into the Foodworks™ database. Nutrient content was analysed through recipe analysis using product information and analytical data from microencapsulated fish oil powder used in the products. Where foods reported were not found listed in the database, data on equivalent food items were used.
Study design—clinic visits
Subjects attended the clinic on two consecutive days at baseline and after 3 and 6 months of intervention, and the following assessments were made at each time point unless stated otherwise. Weight was recorded to calculate BMI (kg/m2; height was measured at baseline only). Waist circumference (midway between the lower rib and iliac crest) and hip circumference (at greater trochanter) were measured with a metric tape to calculate the waist to hip ratio (WHR). A fasted blood sample was taken on each of the two consecutive clinic days and a 24 h urine sample was collected between the two clinic days (see below).
Subjects were instructed to lie supine for 10 min in a quiet temperature-controlled (24°C) room prior to measurement of BP and arterial compliance using a PulseWave CR-2000 instrument (HDI, Egan, MN, USA). Arterial compliance calculates compliance from the spatial difference between the incident and reflected BP peaks, differentiating between the large and small arteries. It refers to the degree to which the internal diameter of the artery changes in response to alterations in intravascular pressure. Systolic and diastolic BP, heart rate and indices of compliance in proximal (large capacitance) arteries (C1) and distal (small resistance) arteries (C2) were derived from a series of three measurements conducted at 5 min intervals.
Erythrocyte fatty acids
Erythrocytes were isolated from whole blood, washed with isotonic saline (0·9 %), frozen, thawed then lysed in hypotonic 0·01-m Tris EDTA buffer, pH 7·4. A membrane pellet was obtained by ultracentrifugation (50 000 g for 30 min) from which lipids were extracted and transmethylated according to the method of Lepage & Roy (Reference Lepage and Roy1986). Fatty acid methyl esters were analysed using a Shimadzu gas chromatograph 20A (Shimadzu Corporation, Kyoto, Japan) fitted with a flame ionisation detector and a 50 m BPX70 column (0·32 mm id and 0·25 mm film thickness; SGE, Australia). Samples were injected at 150°C and held for 0·5 min. The oven temperature was increased at 4°C/min to 200°C, then at 2°C/min to a final temperature of 230°C which was held for 2 min. The injector and detector were maintained at 260°C and hydrogen was used as the carrier gas. Individual fatty acids were identified by comparison with known standards (Nuchek Prep Inc., Elysian, MN, USA) and expressed as a percentage of total fatty acids quantified from peak areas.
Urinary 11-dehydro thromboxane B2 and creatinine
The 24 h urine samples were collected with preservative (15 mg of indomethacin). An aliquot was assayed for the thromboxane A2 metabolite 11-dehydro-thromboxane B2 (TXB2) by the method of Perneby et al. (Reference Perneby, Granstrom, Beck, Fitzgerald, Harhen and Hjemdahl1999) with modifications, which reflects platelet activity in vivo. Briefly, urine (1 ml) was incubated at room temperature with 63-mm Ambic buffer pH 8·6 (2 ml) for 3 h to hydrolyse the δ-lactone ring of 11-dehydro-TXB2. The urine was applied to Bond Elute Certify II columns (Varian) conditioned with methanol (2 ml) and water (3 ml), and washed with 0·63-mm Ambic buffer (3 ml), water (3 ml) and methanol (6 ml). The 11-dehydro-TXB2 was eluted with 2 % formic acid in methanol (2 ml), dried, resuspended in 0·5 ml of immunoassay buffer and assayed in duplicate with one dilution, using a 11-dehydro-TXB2 enzyme immunoassay system (Cayman Chemical Co., Ann Arbor, MI, USA). Urinary thromboxane was expressed as ng/24 h or corrected for creatinine levels. Creatinine levels were measured using a commercially available kit on an autoanalyser (Roche Diagnostica, Basel, Switzerland).
Plasma lipids and high sensitive C-reactive protein (hsCRP)
Plasma total cholesterol, TG and HDL-cholesterol were determined enzymatically on the Cobas MIRA analyser (Roche Diagnostica, Basel, Switzerland) with reagents from Trace Scientific (Melbourne, Australia). In order to measure HDL-cholesterol, the non-HDL lipoproteins were precipitated using heparin–manganese (Warnick & Albers, Reference Warnick and Albers1978). LDL-cholesterol was calculated using the Friedewald formula (Friedewald et al. Reference Friedewald, Levy and Fredrickson1972). Samples were measured in a single assay to minimise inter-assay variation. Plasma hsCRP was measured by solid-phase chemiluminescent assay (Diagnostic Products Corporation, Los Angeles, CA, USA).
Plasma glucose and insulin
Plasma glucose concentrations were measured on a Cobas-Bio centrifugal analyser (Roche Diagnostica, Basel, Switzerland) using a commercial enzymatic kit (Roche Diagnostics, Australia). Plasma insulin concentrations were determined using an enzyme-linked immunosorbent assay kit (Merccolia, American Laboratory Products Company, New Hampshire, USA).
Statistical analysis
Based on previous determinations of the variance in a primary outcome measure, namely the change of plasma TG, we estimated that a total of ninety subjects would give 90 % power to observe a significant (P < 0·05) reduction of 0·2 mm in plasma TG. Data were analysed using repeated measures ANOVA with post hoc analyses where significance was seen. Analysis focused on changes in variables from baseline to 3 and 6 months. Correlations between dietary intakes of n-3 long-chain PUFA, erythrocyte levels of individual (EPA, docosapentaenoic acid and DHA) and total n-3 long-chain PUFA and cardiovascular risk factors were examined using SPSS 12·1 (SPSS, Chicago, IL, USA) or SAS 9.1 (SAS Institute, Cary, NC, USA). End-points were determined using Mixed Models (Proc Mixed, SAS) adjusting for study site, with subject as a random effect and treatment group as a fixed effect. Significance was set at P < 0·05 unless otherwise stated.
Results
Subject characteristics
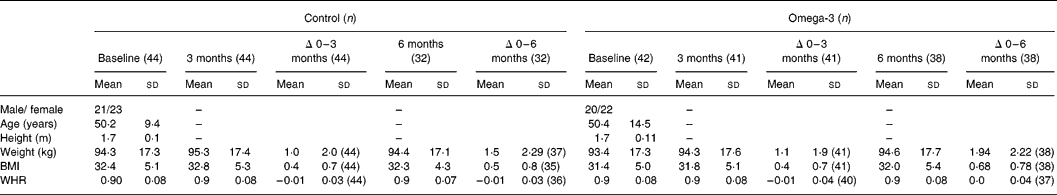
There were no significant differences in age, height, weight, BMI and WHR between the two treatment groups at baseline (Table 2). However, weight increased progressively during the intervention (1·1 % after 3 months and 1·8 % after 6 months; P < 0·001, paired t test), with no difference between treatment groups. Of the ninety-four subjects initially enrolled in the study, six withdrew prior to commencement and two withdrew at commencement, one due to dislike of the foods and the other for personal reasons. Thus eighty-six subjects (n 44, control group and n 42, omega-3 group) proceeded with the intervention, but twelve withdrew between 3 and 6 months, due to dislike of the study foods, changes in employment, health or lost to follow-up. Hence seventy-four subjects (n 36 control group and n 38 omega-3 group) completed the full 6-month intervention period.
Table 2 Subject characteristics (Mean values and standard deviation)
Food consumption and n-3 intake
There was no significant difference between treatment groups in the amounts of foods consumed or in the consumption of any type of food other than those allocated. No side effects from consumption of either n-3-enriched or control foods were reported. Daily logs and food compliance questionnaires revealed that both groups consumed between 6 and 7·5 servings/d of the study foods, slightly below the target of 8 servings/d, but sustained throughout the 6-month study period. However, as seen in Table 1, the actual EPA + DHA content of randomly sampled n-3-enriched foods varied markedly from the intended 125 mg/serving. These variations were taken into account in estimating individual intakes of fatty acids from weighed food records, as described elsewhere (Patch et al. Reference Patch, Tapsell, Mori, Myer, Murphy and Mansour2005).
There were no significant differences in EPA and DHA intakes between the two groups at baseline (Table 3). There was no significant change in the control group during the intervention, but n-3 long-chain PUFA intake increased from 0·2 to 1·0 g/d in the omega-3 group. After 6 months, the major sources of n-3 long-chain PUFA-enriched foods in the omega-3 group were milk, cereal and bread. In contrast, fish (both fatty and white) remained the major source of n-3 long-chain PUFA in the control group, averaging approximately one serving per fortnight (~0·1 g/d).
Table 3 Fatty acid intakes (g/d) (Mean values with their standard errors)
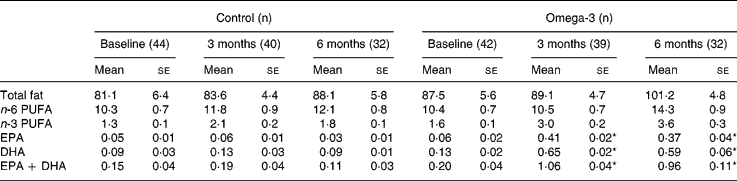
* Significant change over time (repeated measures ANOVA).
At the end of the study, participants rated the acceptability of the study foods on a 7-point Likert scale ranging from − 3 (extremely dislike) to +3 (extremely like) as described elsewhere (Patch et al. Reference Patch, Tapsell, Mori, Myer, Murphy and Mansour2005). The omega-3 group rated the oats and milk slightly favourably (1·4 and 0·5, respectively) whereas they rated the bread as quite unfavourable ( − 0·3), which was significantly different (P < 0·05) from the control group (1·1). The salad dressing was also rated unfavourably ( − 0·3 for both groups). All other food items were rated favourably with no reported significant differences between the groups. There were no major differences in macronutrient intakes between groups (Patch et al. Reference Patch, Tapsell, Mori, Myer, Murphy and Mansour2005).
Erythrocyte EPA and DHA levels
Total n-3 long-chain PUFA content of erythrocytes in the omega-3 group increased by 43 and 60 % at 3 and 6 months of the intervention, respectively (P < 0·05). EPA and DHA content of erythrocytes increased by 82 and 53 %, respectively, at 3 months and by 111 and 76 % at 6 months (P < 0·05) (Table 4). There were no changes in n-3 long-chain PUFA content of erythrocytes in the control group.
Table 4 Fatty acid composition of erythrocytes (% of total fatty acids) (Mean values with their standard errors)
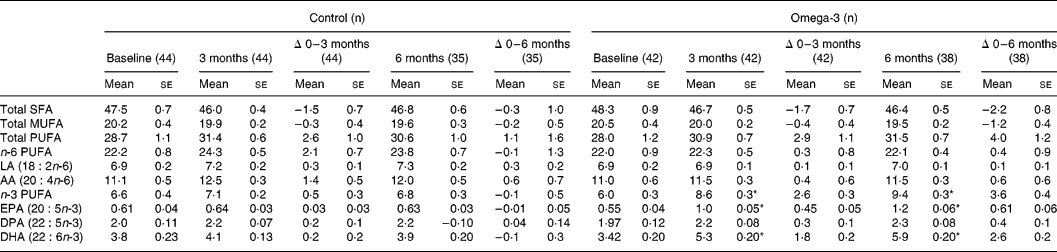
SFA, saturated fatty acids; LA, linoleic acid; AA, arachidonic acid; DPA, docosapentaenoic acid.
* Significant change over time (repeated measures ANOVA).
Erythrocyte EPA and DHA levels were not related to intakes at baseline in the whole group (n 80) perhaps due to the difficulties in dietary assessment methodology (Patch et al. Reference Patch, Tapsell, Mori, Myer, Murphy and Mansour2005). However, there was a significant correlation between the calculated intake of EPA + DHA from foods and erythrocyte levels of EPA + DHA in individuals at 3 months (r 0·35; P < 0·01), which became stronger after 6 months (r 0·69; P < 0·001), reflecting the progressive incorporation on PUFA from food into erythrocytes.
Cardiovascular risk factors
There was no significant difference between omega-3 and control groups in the effects of the intervention for 3 or 6 months on the following parameters: systolic and diastolic BP, compliance of small or large arteries, blood glucose, insulin, lipoprotein lipids (total, HDL and LDL cholesterol and TG), CRP or urinary 11-dehydro-TXB2 (Table 5). However, when using mixed model analyses that included all three time points (0, 3 and 6 months) and adjusted for weight changes and study site, there were significant positive associations between erythrocyte total n-3 long-chain PUFA (EPA + docosapentaenoic acid + DHA) and both small (C1) (log likelihood = 1162, P = 0·02) and large (C2) (log likelihood = 1380, P = 0·08) artery compliance, and negative associations between erythrocyte n-3 long-chain PUFA and serum CRP (log likelihood = 340, P = 0·006) and urinary 11-dehydro-TXB2 (creatinine adjusted) (log likelihood = 502, P = 0·08). Furthermore, erythrocyte DHA was positively associated with C1 (log likelihood = 1156, P = 0·04) and C2 (log likelihood = 1372, P = 0·07) and inversely associated with serum CRP (log likelihood = 339, P = 0·02). Erythrocyte EPA was positively associated with small artery compliance (log likelihood = 1153, P = 0·07) and LDL-cholesterol (log likelihood = 618, P = 0·07).
Table 5 Arterial compliance, blood pressure and biochemical indices (Mean values with their standard errors)
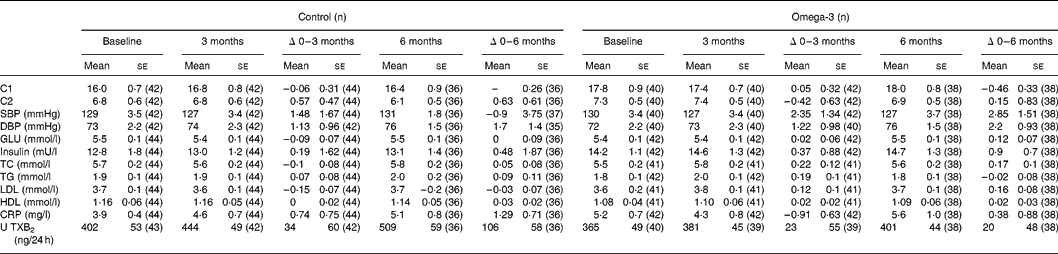
C1, small artery compliance; C2, large artery compliance; SBP, systolic blood pressure; DBP, diastolic blood pressure; GLU, glucose; TC, total cholesterol; TG, triacylglycerols; CRP, C-reactive protein; U TXB2, urinary 11-dehydro-TXB2.
Interestingly, there was a positive correlation between the change in weight and the change in plasma TG from 0 to 3 months in the control group (P < 0·05) but not the omega-3 group (P = 0·25); this pattern of results was also obtained between 3 and 6 months.
Discussion
Subjects in the present study had baseline intakes of n-3 long-chain PUFA (155–200 mg/d) typical of the Australian population (Ollis et al. Reference Ollis, Meyer and Howe1999; Meyer et al. Reference Meyer, Mann, Lewis, Milligan, Sinclair and Howe2003; Howe et al. Reference Howe, Meyer, Record and Baghurst2006). We were successful in encouraging those in the omega-3 group to increase their n-3 long-chain PUFA intake to 1 g/d by replacing habitual foods with a variety of n-3-enriched study foods. Moreover, we found that this 5-fold increase in n-3 long-chain PUFA intake could be maintained for a long duration (i.e. 6-month intervention period) by utilising n-3 food sources other than fish.
This increase in regular consumption of n-3 long-chain PUFA resulted in a progressive increase in the n-3 long-chain PUFA content of erythrocytes, as observed by others (Mantzioris et al. Reference Mantzioris, Cleland, Gibson, Neumann, Demasi and James2000; Wallace et al. Reference Wallace, McCabe, Robson, Keogh, Murray and Kelly2000; Metcalf et al. Reference Metcalf, James, Mantzioris and Cleland2003). The EPA + DHA content of erythrocytes rose from 4 to 7 % of total fatty acids, placing these subjects in a lower risk category for cardiac death, according to the Omega-3 Index suggested by Harris & Von Schacky (Reference Harris and Von Schacky2004). The Omega-3 Index relates to the level of EPA and DHA in erythrocytes and risk of sudden cardiac death; a high level of EPA + DHA is associated with greatest protection from sudden cardiac death. The Omega-3 Index is supported by a number of clinical studies that have shown the benefits of n-3 long-chain PUFA such as the GISSI-Prevenzione trial (GISSI-Prevenzione Investigators, 1999), in which an n-3 long-chain PUFA supplement of 1 g/d over 3 years reduced the risk of sudden cardiac death by 45 % in men with a previous myocardial infarction. The emergence of a correlation between intakes and erythrocyte levels of EPA + DHA after baseline could well reflect the high variability in habitual n-3 long-chain PUFA intakes prior to intervention and the greater consistency of n-3 long-chain PUFA intakes achieved subsequently from regular consumption of the study foods.
Weber & Raederstorff (Reference Weber and Raederstorff2000) suggested that a dose of approximately 1 g/d of n-3 long-chain PUFA is the smallest necessary for a TG-lowering effect. Consistent with this, the 0·8 g/d increase in n-3 long-chain PUFA intake achieved in the present study by consuming a variety of n-3-enriched foods failed to improve plasma TG or HDL-cholesterol levels. It is well known that high intakes of fish oils (~3 g/d) can lower plasma TG in hypertriglyceridaemic subjects (Calabresi et al. Reference Calabresi, Villa, Canavesi, Sirtori, James, Bernini and Franceschini2004). Reductions have been reported at much lower doses (~1 g/d) in both hypertriglyceridaemic subjects (Junker et al. Reference Junker, Pieke, Schulte, Nofer, Neufeld, Assmann and Wahrburg2001) and normotriglyceridaemic subjects (Roche & Gibney, Reference Roche and Gibney1996; Visioli et al. Reference Visioli, Rise, Plasmati, Pazzucconi, Sirtori and Galli2000). However, in another study with n-3-enriched foods (Lovegrove et al. Reference Lovegrove, Brooks, Murphy, Gould and Williams1997), healthy males were given foods providing ~1·4 g/d of n-3 long-chain PUFA for 22 d then crossed over to the equivalent unenriched foods for a further 22 d after a 5-month washout period. They reported an increase of HDL-cholesterol but no change in plasma TG. On the other hand, Visioli et al. (Reference Visioli, Rise, Plasmati, Pazzucconi, Sirtori and Galli2000) showed that consuming 500 ml of skim milk enriched with n-3 long-chain PUFA (300 mg of EPA + DHA) resulted in a 19 % reduction of plasma TG and a 19 % increase in HDL-cholesterol in normolipidaemic volunteers. The lack of effect on plasma TG in our study may be attributed to the marginal increase in n-3 long-chain PUFA intake, falling short of a 1 g/d threshold.
Any effect of n-3 long-chain PUFA on plasma TG in this study may have been masked by the weight gain observed in both groups during the 6-month intervention. Other studies have also failed to see a reduction in plasma TG when the subjects also gained weight (Lovegrove et al. Reference Lovegrove, Brooks, Murphy, Gould and Williams1997).
An HDL-raising effect of fish oils has been shown in some, but not all studies. A study by Laidlaw & Holub (Reference Laidlaw and Holub2003) used a 4 g dose of fish oil for 28 d, which resulted in a 7–10 % increase in HDL-cholesterol in healthy women. However, in subjects with combined hyperlipidaemia supplemented with 3·4 g EPA + DHA (Omacor), there was no HDL-cholesterol-raising effect.
Compared with the effects of fish oil supplementation, other studies where n-3 long-chain PUFA were provided in foods have shown mixed results. HDL-cholesterol did not increase significantly in a cross-over study in which hypertriglyceridaemic subjects consumed 1·6 g/d of n-3 long-chain PUFA for 3 weeks (Junker et al. Reference Junker, Pieke, Schulte, Nofer, Neufeld, Assmann and Wahrburg2001). However, in the cross-over study by Lovegrove (1997), consumption of n-3 long-chain PUFA-enriched foods for 22 d resulted in a significant increase in HDL-cholesterol. All these studies were of short duration and, as in our long-term study, no significant HDL-cholesterol-raising effect was seen. The effect of diet on HDL-cholesterol, however, is likely to be due to the overall composition of the diet, and not just one nutrient (or a few foods), so the apparent ambiguity seen in these studies could also be due to lack of control in the background diet.
The likely reason for the lack of effect of n-3 long-chain PUFA on either BP or arterial compliance is the relatively low intake of n-3 long-chain PUFA in the present study. In a meta analysis of studies of 3–24 weeks duration, intakes of 3–5·6 g/d were effective in reducing BP by between 3·4/2·0 and 5·5/3·5 mmHg in hypertensive subjects (Geleijnse et al. Reference Geleijnse, Giltay, Grobbee, Donders and Kok2002). Furthermore, our study population were mainly normotensive and the effects of fish oils on BP are usually seen in hypertensive individuals. Similarly, improvements in arterial compliance have been observed at intakes comparable with those that have reduced BP (Nestel et al. Reference Nestel, Shige, Pomeroy, Cehun, Abbey and Raederstorff2002). However, even with the lower intakes achieved in our study, there was a significant correlation between erythrocyte n-3 long-chain PUFA content and compliance in resistance arteries (C2), which was more closely associated with DHA than EPA.
Similarly, n-3 long-chain PUFA supplementation in the present study did not elicit any change in CRP, but erythrocyte n-3 long-chain PUFA levels, particularly DHA, were inversely associated with serum CRP. CRP is an acute phase protein marker of systemic inflammation and is a predictor of cardiovascular risk. Previous studies have also shown an inverse relationship between n-3 PUFA (α-linolenic acid, DHA and EPA) and CRP levels (Lopez-Garcia et al. Reference Lopez-Garcia, Schulze, Manson, Meigs, Albert, Rifai, Willett and Hu2004). The findings herein are similar to those of Geelen et al. (Reference Geelen, Brouwer, Schouten, Kluft, Katan and Zock2004) who showed no change in CRP following 12 weeks of supplementation with 3·5 g fish oil/d (1·5 g/d n-3 PUFA). Furthermore, Mori et al. (Reference Mori, Woodman, Burke, Puddey, Croft and Beilin2003) supplemented the diets of fifty-nine non-smoking, treated-hypertensive, type 2 diabetic subjects with 4 g of purified EPA or DHA for 6 weeks and showed no changed in CRP. Similarly, Madsen et al. (Reference Madsen, Christensen, Blom and Schmidt2003) showed that 12 weeks of supplementation with either a high dose (6·6 g) or low dose (2 g) of n-3 long-chain PUFA had no effect on CRP. However, our data support an anti-inflammatory effect of n-3 long-chain PUFA in subjects with cardiovascular risk factors which may decrease the risk of CVD.
We showed no significant change in urinary 11-dehydro-TXB2 following increased consumption of n-3 long-chain PUFA. However, we demonstrated that erythrocyte n-3 long-chain PUFA were significantly inversely associated with urinary 11-dehydro-TXB2. These findings are in accordance with the antithrombotic benefits of n-3 long-chain PUFA that are in part related to their antiplatelet effects (Knapp, Reference Knapp1997). Mori et al. (Reference Mori, Beilin, Burke, Morris and Ritchie1997) showed that n-3 long-chain PUFA reduced ex vivo platelet aggregation in response to collagen and platelet-activating factor stimulation. Others have shown reduced aggregatory responses to ADP, thrombin and adrenalin (Kristensen et al. Reference Kristensen, Schmidt and Dyerberg1989). Moreover, DHA and not EPA reduced collagen-induced platelet aggregation in type 2 diabetic patients (Woodman et al. Reference Woodman, Mori, Burke, Puddey, Barden, Watts and Beilin2003). The reduction in platelet aggregation was shown to be mediated largely by decreased platelet TXB2 release (Woodman et al. Reference Woodman, Mori, Burke, Puddey, Barden, Watts and Beilin2003). Others have demonstrated a reduction in urinary TXB2 following higher intakes of n-3 long-chain PUFA (von Schacky & Weber, Reference von Schacky and Weber1985).
In summary, regular consumption of foods enriched with n-3 long-chain PUFA for 6 months increased erythrocyte n-3 long-chain PUFA to levels consistent with a reduction in cardiovascular risk (Harris & Von Schacky, Reference Harris and Von Schacky2004), although there were no improvements in plasma lipids, BP or arterial compliance. Nevertheless, the level of n-3 long-chain PUFA, specifically DHA, in erythrocytes was positively associated with arterial compliance and negatively associated with both serum CRP and 11-dehydro-urinary TXB2. These potentially beneficial relationships warrant further investigation.
Acknowledgements
This study was funded by an Australian Research Council Linkage Grant with Goodman Fielder Pty Ltd. We thank Dr Geoffrey Annison, Geoff Weldon, Donna Ross and Coral Colyer (formerly of Goodman Fielder Foods Ltd, Sydney) for their contribution to the study design and development, and provision of study foods; Jennifer Keogh, Paul Foster, Jane Bowen, Lisa Moran for their assistance with diet consultations; laboratory staff at CSIRO (Adelaide) and the School of Medicine and Pharmacology (Western Australia).







