There is limited information on the Zn status of populations based on national survey data, in part because, until recently, there has been a lack of consensus on appropriate indicators of Zn status. Indeed, to date, only national surveys in the United States in 1984(Reference Pilch and Senti1), and more recently, the UK(Reference Gregory, Lowe and Bates2) and Mexico(Reference Villalpando, García-Guerra and Ramírez-Silva3), have published data that included a biomarker (i.e. serum Zn concentrations) of Zn status. This is unfortunate because adequate Zn nutriture is essential to support growth during childhood, reduce the risk of common infections, improve neurobehavioural function and prevent adverse pregnancy outcomes, as a result of the critical role of Zn in a wide range of biochemical, immunological and clinical functions(Reference Brown, Rivera and Bhutta4).
Recently, three indicators – a biochemical, dietary and functional indicator – have been recommended for assessing the risk of Zn deficiency in populations and for identifying subgroups at elevated risk. The biochemical indicator is the proportion of the population with serum Zn concentrations below the appropriate lower cut-offs(5), when the correct protocols are followed for the collection, separation and analysis of serum Zn(Reference Brown, Rivera and Bhutta4). The dietary indicator is the prevalence of usual Zn intakes below the estimated average requirements(5). The functional indicator is the percentage with length- or height-for-age < − 2·0 z scores (i.e. stunted) and applies to the children < 5 years(5). For each indicator, a cut-off for the prevalence considered indicative of public health concern has been set(5). At present, the 1999 nutrition survey in Mexico is the only national survey which has used these three indicators to assess the risk of Zn deficiency at the population level and to identify the subgroups at risk(Reference Villalpando, García-Guerra and Ramírez-Silva3).
In 2002, New Zealand (NZ) conducted a nationally representative survey of school children aged 5–15 years during which serum Zn and dietary Zn intakes were measured using the International Zinc Nutrition Consultative Group procedures(Reference Brown, Rivera and Bhutta4) in urban and semi-urban school children from three main ethnic groups of NZ. These data provide a unique opportunity to examine the Zn status of school-aged children of Māori, Pacific and NZ European and Other (NZEO) ethnicities and to investigate factors that might predispose them to suboptimal Zn status.
Subjects and methods
Study design and population
The 2002 Children's National Nutrition Survey (CNS02) was a cross-sectional survey of a national sample of NZ school-aged children aged 5–15 years, conducted during the school year from February to December 2002. A school-based sampling frame of children was used with over-sampling of Māori and Pacific children to allow for ethnic-specific analysis. Details of the sampling strategy are available elsewhere(Reference Parnell, Scragg and Wilson6). Briefly, a two-stage process was used to recruit the sample involving a random selection of 172 schools on Ministry of Education rolls. Children from the designated schools were selected randomly. Recruitment from each school was in proportion to the number of students on the school roll. The total number of children invited to participate in the survey was 4728, of whom 3275 agreed to participate.
Of the 3275 participating children, only 1927 provided a non-fasting blood sample; 1348 children did not provide a blood sample, because either they refused or they were not asked because they attended a rural school. Blood samples were not collected from any of the children attending the sixty-six rural schools because it was not logistically possible to transport them from the rural areas to a laboratory in a timely manner. Sampling weights were based on the provision of a blood sample and not on survey participation as there were differences in the proportions of the three ethnicities in the blood sample group compared with the CNS02 survey sample. Weights were based on the inverse probability of selection. The weights included adjustment for differential non-response and stratification by ethnicity, age and sex to reflect the national proportions.
In the CNS02 survey, no weighting was applied to account for the effects of time of day of the blood collection, region within NZ or the onset of puberty, all variables known to have a significant effect on serum Zn and/or serum Se concentrations(Reference Brown, Rivera and Bhutta4, Reference Rűkgauer, Klein and Kruse-Jarres7, Reference Thomson, McLachlan and Parnell8). Post hoc analysis showed that there were significant differences in these variables among the three ethnic groups. Therefore, we have explored the predictors of both serum Zn concentrations and the prevalence of Zn deficiency for each of the three ethnic groups independently, using multiple linear and logistic regression models. Further details of the anthropometry, serum Zn concentrations and dietary Zn data for the overall weighted population in the CNS02 survey are available in the study done by Parnell et al. (Reference Parnell, Scragg and Wilson6).
The survey received ethical approval from the Auckland Ethics Committee and thirteen regional health ethic committees. Informed written consent was obtained from both the children and their parents or guardians. Demographic data and 24 h recalls were recorded at children's homes in the presence of their parents or guardians from the last week of February 2002 to the second week of December 2002, whereas anthropometric measurements and blood samples were taken at school.
Classification by ethnicity, socio-economic status and anthropometry
Children were assigned to one of three ethnic groups: Pacific, NZEO or Māori based on self-reports by the children or their parents or guardians. The term ‘Pacific’ is used here to describe the children who were identified as Samoan (55 %), Tongan (24 %), Cook Island Māori (16 %), Niuean (10 %), Tokelauan (2 %), Fijian (3 %) and other Pacific ethnic groups (3 %). The NZEO group includes the children who were identified as NZ European (80 %), Asian (10 %), Other European (6 %), Indian (5 %) and other (4 %). For children who were reported as belonging to more than one ethnic group, unequivocal rules were established to assign the children to a single ethnic category. In particular, if Māori was one of the groups reported, the participant was assigned to ‘Māori’. In cases where Māori was not reported, but any of the Pacific groups were reported, then the participant was assigned to Pacific. All the remaining participants were assigned to NZEO; further details are given by Parnell et al. (Reference Parnell, Scragg and Wilson6). Children were also classified according to the 2001 NZ Index of Deprivation(Reference Salmond and Crampton9). Deprivation index scores were ranked into quintiles. In the present study, children ranked in the most deprived quintile were considered to be of low socio-economic status (SES).
Height and weight were measured by trained research assistants, with children wearing light clothing and no shoes. Measurements were taken using standardised procedures and calibrated equipment(Reference Lohman, Roche and Martorell10) and the mean of the two closest measurements was used. Accuracy of anthropometric measurements was monitored throughout the survey using cumulative summation charts, as described by Parnell et al. (Reference Parnell, Scragg and Wilson6). Of the children, six had unacceptably extreme anthropometric z scores (i.e. >4 sd or < − 4 sd), which were therefore omitted from the dataset(11). To compare the prevalence of overweight and obesity internationally, the age- and sex-specific cut-off points of Cole et al. (Reference Cole, Bellizzi and Flegal12) for BMI for children were used.
Collection and analyses of blood samples for serum zinc
Non-fasting venous blood samples were drawn into trace-element-free evacuated tubes (Becton Dickinson, Frankton Lakes, NJ, USA) from children in the sitting position, and the time of the blood collection was recorded. Blood was refrigerated immediately after collection(Reference Tamura, Johnston and Freeberg13) and then couriered chilled to a central laboratory for processing the next morning using appropriate trace-element-free techniques to avoid all sources of adventitious Zn contamination. After centrifugation and separation of the serum using trace-element-free techniques, the serum samples were stored at − 80°C until analysed. This protocol was developed after extensive pilot-testing which revealed that serum Zn concentrations analysed from blood samples using this protocol were not significantly different from those values obtained for serum from the same blood samples separated within 1 h of blood collection, provided the blood sample was kept cold at all times.
Serum Zn was analysed using flame atomic absorption spectrophotometry (Perkin Elmer AAnalyst 800; Perkin Elmer Corp., Norwalk, CT, USA) using a modified method of Smith et al. (Reference Smith, Butronvitz and Purdy14). Serial replicates of a pooled serum sample and quality-control sera were used to check the precision and accuracy of the analytical method. The CV for Zn in the pooled serum was 3·9 % (n 82). The value for the certified reference material, Bovine Serum Reference Material no. 1598 (National Institute of Standards and Technology), was 13·6 (sd 0·3) μmol/l (CV 2·5 %, n 21) compared with the certified value of 13·6 μmol/l. The presence of acute inflammation was assessed by serum C-reactive protein (CRP) >5 mg/l(Reference Thurnham, Mburu and Mwaniki15) using an immuno-turbimetric assay with a Roche Hitachi 917 automated analyser (Roche Diagnostics, Indianapolis, IN, USA). Values for the manufacturer's controls for serum CRP fell within the certified ranges.
Assessment of inadequate intakes of zinc
Trained research assistants recorded all foods and beverages (including dietary supplements) consumed by the participant in the previous 24 h using a computer-assisted, multiple-pass 24 h diet recall interview in the home. Bar-code scanners were used to improve the accuracy of the product-name information for branded items. Recall data were entered into the computer at the time of the interviews using direct-capture programmes. Interviews were conducted to ensure that all days of the week were represented and included input from a parent or adult caregiver for the children < 10 years of age. Repeated 24 h diet recalls were conducted on a subsample of the children (n 505) so that nutrient intakes could be adjusted for within-person variability to obtain usual intake distributions. All of the 3275 children who participated in the survey completed a reliable 24 h recall, resulting in an overall analytic response of 100 % for the diet recall component.
Intakes (as mg/d and per kg body weight (BW)) and major food sources of Zn (as percentage), but not phytate, were calculated from the 24 h recall data using the NZ Food Composition Database compiled and maintained by Crop and Food Research Limited in NZ(Reference Athar, Spriggs and Taptiklis16). The prevalence of inadequate Zn intakes was calculated for each of the age-, sex- and ethnic-specific subgroups using the Estimated Average Requirement cut-point method based on the Australian and NZ estimated average requirements(17), after adjusting for the distribution of observed intakes to partially remove the day-to-day variability in Zn intakes (within-person variation) using PC-SIDE (Department of Statistics, Iowa State University, Ames, IA, USA)(Reference Carriquiry18).
Data analysis
Anthropometric z scores were calculated from the US Center for Disease Control and Prevention 2000 growth reference data(Reference Kuczmarski, Ogden and Grummer-Strawn19). Multiple linear regression analyses were used to examine the independent predictors of serum Zn concentrations for each of the three ethnic groups. Sampling weights were used in all the analyses. Demographic characteristics of those participants who did and did not provide blood samples for serum Zn analysis were also examined. The explanatory variables included in the regression models were those that were known(Reference Pilch and Senti1, Reference Brown, Rivera and Bhutta4) or suspected(Reference Krittaphol, Bailey and Pongcharoen20, Reference Gibson, Ferguson and Smit Vanderkooy21) to be biologically important for Zn status. The variables investigated included sex, age category, SES, time of day of blood sampling (morning or afternoon), acute infection (serum CRP >5 mg/l), season (summer or winter) and serum Se concentrations. For the purpose of this analysis, the Southern Hemisphere winter was defined as the months of May to October and summer as November to April. There was no evidence in the multiple regression models of multiple co-linearity among the independent variables.
Adjusted mean (95 % CI) serum Zn concentrations were estimated for each ethnic group for three ages, stratified by sex, while accounting for survey weights and the variables in the regression model. In addition, the prevalence of serum Zn concentrations below the appropriate age-, sex-, and time of day-specific cut-offs were calculated by age category and sex for each ethnicity. Logistic regression was used to identify the predictors of low serum Zn concentrations.
Dietary variables were not included in the linear regression models because repeated 24 h diet recalls were only collected on a subsample of the survey participants. Hence, data on usual intakes at the individual level were not available(Reference Carriquiry18). Mean (95 % CI) adjusted intakes of Zn (per day; per MJ; per kg BW) by the age categories specified for the Nutrient Reference Values for Australia and NZ(17), and stratified by sex, were calculated and compared using the adjusted Wald test following regression. In addition, adjusted mean serum Se concentrations and adjusted dietary intakes of Zn, for those participants within each ethnic group with serum Zn concentrations below and above the appropriate age-, sex-, and time of day-specific lower cut-offs were calculated and compared using the adjusted Wald test.
All P values were two-sided and were not adjusted for multiple testing. Statistically significant differences were indicated by P < 0·05. All the statistical analyses were performed using STATA, version 11.0 (Stata Corporation, College Station, TX, USA), accounting for the complex survey design.
Results
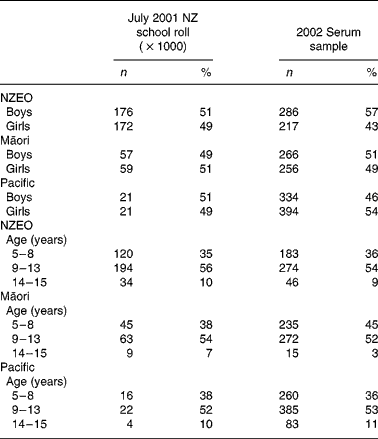
Table 1 shows the proportions by age and sex in the three ethnicities in relation to the 2001 National School Roll. Of the 1927 children providing a blood sample, data for stature, serum Zn and dietary Zn intakes were available for 1753 children, yielding an overall response rate of 37·1 % (1753 of the 4728 selected). There were no significant differences between the age, SES or BMI for those NZEO children who did or did not provide a blood sample for serum Zn analysis. However, the Māori and Pacific Island children for whom serum Zn concentrations were available tended to be 6 months younger and 3 months older, respectively, than their counterparts for whom no serum Zn values were available. Some data for serum Se concentrations (n 132) and SES (n 77) were also not available because of insufficient sample for serum Se analysis and lack of information on SES, respectively.
Table 1 Characteristics of the 2002 Children's Nutrition Survey serum sample relative to the 2001 New Zealand (NZ) school roll
(Numbers and percentages)
NZEO, NZ European and other.
Anthropometry
At ages 7, 11 and 14 years, children from the Pacific group had the highest positive weight-for-age z scores, height-for-age z scores (HAZ), and BMI z scores, and the highest prevalence of overweight and obesity, followed by the Māori; z scores for the NZEO group were the lowest. In the Māori and NZEO but not in the Pacific group, the z scores were lower among the older children, the difference being significant in some cases (Table 2). Sex differences in all the three ethnicities were small. About 2 % of the children were stunted (i.e. HAZ < − 2·0) and/or underweight (weight-for-age z score < − 2·0).
Table 2 Anthropometric z-scores for three ethnic groups of New Zealand children at three ages (7·0, 11·0 and 14·0 years)
(Adjusted mean values and 95 % confidence intervals)

NZEO, New Zealand European and other; CRP, C-reactive protein; SES, socio-economic status.
* Mean values were significantly different among ethnicities.
Menarche (based on self-reports) had been reached by more girls in the Pacific (36·5 %) and Māori (36·2 %) groups than those in the NZEO (27·8 %) group (P < 0·001) (Table 2). No assessment of pubertal development was made in the boys.
Predictors of serum zinc concentrations among the three ethnic groups
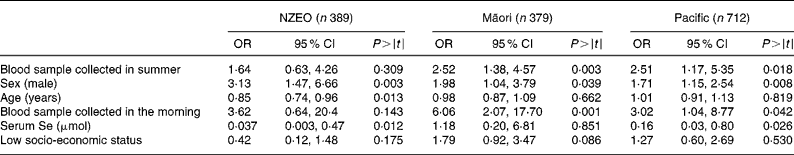
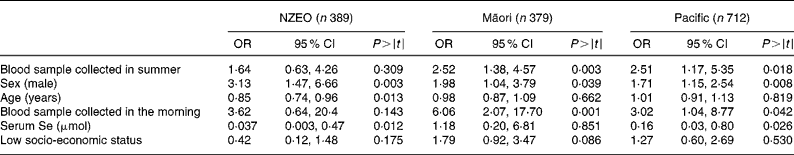
Significant predictors of serum Zn concentrations identified by regression analysis differed among the three ethnic groups (Table 3). In the NZEO group, sex, age and serum Se concentrations were all significant predictors. In contrast, for the Māori children, we were unable to identify any significant predictors, whereas for the Pacific children, sex, age, serum Se concentrations and season were significant. The interaction sex × age was significant in the Pacific group. Note that the indicator variable for infection (CRP>5·0 mg/l) was not a significant predictor of serum Zn concentrations in any of the three ethnic-specific models.
Table 3 Biological and technical factors associated with variations in serum zinc concentrations deduced from regression analysis with serum zinc as the dependent variable
(Coefficients and 95 % confidence intervals)

NZEO, New Zealand European and other; CRP, C-reactive protein.
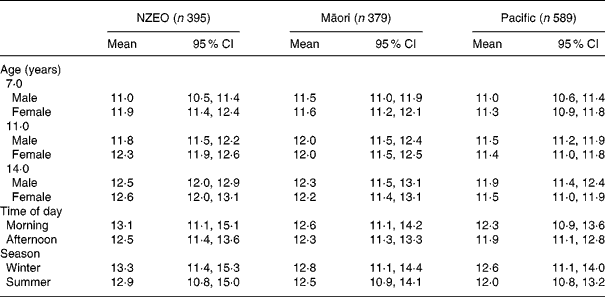
Mean serum Zn concentrations for males and females by age for each of the three ethnic groups are shown in Table 4. An age-related increase in mean serum Zn concentrations was evident in all the three ethnicities and significant for the NZEO and Pacific groups (Table 3).
Table 4 Serum zinc concentrations (μmol/l)* of three ethnic groups of New Zealand children at three ages (7·0, 11·0 and 14 years), sex, time of day of blood sampling (morning or afternoon)† and season (winter or summer)†
(Adjusted means and 95 % confidence intervals)
NZEO, New Zealand European and other.
* Data by age and sex account for survey design and the other variables in the regression models (Table 3).
† Data for time of day and season are for age 11 years.
Sex differences in mean serum Zn concentrations were significant in the NZEO group; concentrations in girls were higher than boys (P = 0·012). No sex differences were apparent in the Māori children (Table 4).
The time of day for the blood collection had an impact on mean serum Zn concentrations. Values tended to be higher in morning blood samples compared with the afternoon in all three ethnic groups. This difference was NS for each of the three ethnic groups (Table 3).
Seasonal differences were also apparent: serum Zn concentrations from winter blood samples were higher than the summer in all three ethnic groups, although this difference was only significant in the Pacific group (P = 0·016) (Table 3) and for the overall sample (P>|t| = 0·049; data not shown).
Acute inflammation, as indicated by elevated serum CRP levels, was generally absent; only 1·7 % of the overall sample had CRP concentrations >5 mg/l.
Prevalence of zinc deficiency and predictors of low serum zinc among the three ethnic groups
The prevalence of Zn deficiency was highest in boys of the two younger age groups and ranged from 18 to 28 % (Table 5), declining significantly with age in the NZEO group. The prevalence was significantly higher for boys compared with girls within each ethnicity and age category, although the number of subjects >14 years was small. The time of day for the blood collection and the season of the year were important factors in the prevalence of low serum Zn concentrations (Table 5), but neither obesity nor infection appeared to be significant factors (data not shown).
Table 5 Prevalence* of zinc deficiency based on low serum zinc concentrations by age and sex, time of sampling and season
(Mean values and 95 % confidence intervals)

NZEO, New Zealand European and other.
* Adjusted for survey weights.
Predictors of low serum Zn concentrations based on the logistic regression analysis varied among the three ethnic groups (Table 6). For the NZEO group, sex, age and serum Se concentrations were significant predictors, whereas for the Māori group, season, sex and time of day of blood sampling were significant. For the Pacific group, season, sex, time of day of blood sampling and serum Se concentrations were significant.
Intakes and major food sources of zinc among the three ethnic groups
For all the three ethnic groups, the oldest children (aged 14–15 years) had the highest mean intakes of Zn/d. Further, boys had significantly higher mean Zn intakes/d than girls in all the three ethnic groups. Mean Zn intakes expressed per kg BW decreased significantly with age and were significantly higher for boys than girls in all the three ethnic groups (Table 7). Intakes of both insoluble and soluble dietary fibre for three ethnic groups were not significantly different (P>0·05). Dietary Zn supplements, taken by only 1·3 % of the children, provided a negligible contribution to the overall total Zn intake (data not shown).
Table 7 Dietary zinc intakes expressed (mg/d) and mg/kg body weight (BW) of three ethnic groups of New Zealand children by age category and stratified by sex
(Mean values and 95 % confidence intervals)*

NZEO, New Zealand European and other.
* Adjusted for survey weights.
For the children overall, the major food sources of Zn were cereals (30 %), meat/poultry/fish (23 %), dairy products (15 %) and fruits/vegetables (10 %). However, ethnic differences in food sources of Zn were marked: dairy products provided significantly (P < 0·001) different proportions of dietary Zn for Pacific (8·4 %), Māori (12·9 %) and NZEO (17·0 %) children.
In general, the prevalence of inadequate Zn intakes was very low; < 1 % for both boys and girls 5–8 years and for boys aged 9–13 years in all the three ethnic groups. For girls aged 9–13 years, 2·3 % of NZEO, 1·9 % of Māori and 7·9 % of the Pacific had inadequate intakes of Zn. There were too few children aged 14–15 years to calculate the prevalence of inadequate Zn intakes in the separate ethnic groups.
Inter-relationships of dietary, anthropometric and biochemical indicators of zinc and selenium status
In addition to the inter-relationships, apparent in the regression analysis (Tables 3 and 6), we also examined the dietary and anthropometric indicators in relation to biochemical Zn status. Differences in mean Zn intakes/d for those children with low v. normal serum Zn concentrations were small and only significant (P = 0·029) in the NZEO group where lower Zn intakes (mg/d) were associated with low Zn status. In contrast, mean Zn intakes/kg BW and major food sources of Zn were not significantly different for those children with low v. normal serum Zn concentrations in any of the three ethnicities (data not shown). Nevertheless, it is of interest that the proportion of dietary Zn (as %) from meat, poultry and fish tended to be lower in the children with low v. normal serum Zn concentrations in each of the three ethnic groups.
The only anthropometric indicator that was related to low serum Zn concentrations was the mean HAZ score. In the Pacific group, boys, but not the girls with low serum Zn concentrations, had a significantly lower mean HAZ score than their counterparts with normal serum Zn concentrations (P for interaction = 0·007). There was a tendency for the same relationship in Māori (P for interaction = 0·096). No comparable relationship was observed in the NZEO group. No relationship was observed between mean BMI z scores in the boys or girls and low serum Zn concentrations in any of the three ethnic groups.
Mean serum Se concentrations for the NZEO and Pacific children (but not the Māori) with low serum Zn concentrations were significantly lower than those for their counterparts with normal serum Zn concentrations (NZEO: 0·88 v. 0·97 μmol/l; P = 0·022 and Pacific: 1·00 v. 1·04 μmol/l; P = 0·006) – a relationship supported by the regression analysis (Tables 3 and 6).
Discussion
Serum Zn concentrations are now the recommended biomarker of population Zn status(5, Reference Lowe, Fekete and Decsi22), even though levels are influenced by several factors independent of Zn status, including time of day of blood sampling, fasting status and infection. Here, we report for the first time, two additional variables – serum Se and season – as predictors of serum Zn concentrations.
Serum Zn concentrations for the morning blood samples were higher than afternoon samples, as noted by others(Reference Pilch and Senti1, Reference Brown, Rivera and Bhutta4, Reference King, Hambidge and Westcott23). Concentrations are generally also higher after a recent meal(Reference King, Hambidge and Westcott23). However, we collected only non-fasting blood samples, without information on the time of the most recent meal, to minimise respondent burden. Hence, we were unable to examine the impact of fasting status. Meal-induced changes may account for some of the unexplained variance in serum Zn concentrations. Very few children (1·7 %) had elevated serum CRP levels, probably because those with an acute infection on the blood-collection day were absent from school and thus not sampled. Hence, failure to demonstrate an inverse relationship between elevated concentrations of serum CRP and low serum Zn is not surprising(Reference Pilch and Senti1, Reference Brown, Rivera and Bhutta4).
Age and sex influence serum Zn concentrations(Reference Pilch and Senti1). Here, mean serum Zn concentrations for boys and girls increased with increasing age across the three ethnicities, a trend also observed in the US(Reference Pilch and Senti1) and Mexico(Reference Villalpando, García-Guerra and Ramírez-Silva3) national surveys and elsewhere(Reference Rűkgauer, Klein and Kruse-Jarres7). A parallel fall in the prevalence of low serum Zn concentrations was also observed (Table 5). The NZEO boys had significantly lower mean serum Zn concentrations than girls (Table 4), and in all three ethnicities, the prevalence of low serum Zn concentrations is higher in males than in females (Table 5). In general, we conclude that it is the younger male children who are particularly at risk of suboptimal Zn status. Lower serum Zn concentrations in boys have been reported elsewhere(Reference Gibson, Manger and Krittaphol24, Reference Ruz, Castillon-Duran and Lara25). Indeed, in some Zn-supplementation trials, boys have shown a greater response in linear growth than girls(Reference Ruz, Castillon-Duran and Lara25–Reference Walravens, Krebs and Hambidge27); possibly, their higher growth rates and greater proportion of muscle/kg BW(Reference Brown, Rivera and Bhutta4) generate higher Zn requirements. Other postulated mechanisms involve sex-related changes in growth hormone and testosterone concentrations which increase the metabolic requirements for Zn(Reference Nishi28).
Serum Se concentrations were a strong predictor of serum Zn in the NZEO and Pacific children, but not in the Māori (Table 3). The reason for this inconsistency is uncertain. It may be related to the greater range in serum Se concentrations in the Pacific (0·44–1·56 μmol/l) and NZEO (0·55–1·41 μmol/l) ethnicities compared with the Māori (0·55–1·41 μmol/l). Other studies in NZ(Reference de Jong, Gibson and Thomson29) and elsewhere(Reference Krittaphol, Bailey and Pongcharoen20) have reported positive associations between serum Se and serum Zn concentrations. Selenoproteins, specifically glutathione peroxidases, have the potential to have an impact on Zn status through their role in regulating the delivery of Zn from metallothionein to certain Zn enzymes, specifically Cu, Zn superoxide dismutase(Reference Maret30). According to Thomson et al. (Reference Thomson, McLachlan and Parnell8), serum Se concentrations of many of these NZ children are unlikely to be adequate for maximal glutathione peroxidase activity because this enzyme is the first selenoprotein to decline in Se deficiency. It is possible therefore, that low glutathione peroxidase activity induces low serum Zn concentrations and as a consequence, serum Zn and serum Se concentrations become positively correlated, as reported here among the Pacific and NZEO groups.
Serum Zn concentrations were higher in the winter than in the summer across all ethnic groups (Table 4), a pattern noted previously for hair Zn levels in children(Reference Gibson, Ferguson and Smit Vanderkooy21, Reference Hambidge, Chavez and Brown31). Such seasonal trends in Zn status may be associated with the well-documented seasonal changes in linear growth(Reference Xu, Wang and Guo32), which may result in depletion of body Zn reserves in the spring/summer, as a consequence of rapid growth.
The NZ children studied here had mean adjusted serum Zn values, when classified by sex and age, which were comparable with those reported in US National Health and Nutrition Examination Survey II(Reference Pilch and Senti1). Much higher serum Zn values have been reported(Reference Rűkgauer, Klein and Kruse-Jarres7, Reference Thane, Bates and Prentice33, Reference Malvy, Arnaud and Burtschy34), but not all of these studies followed the latest recommended protocols for serum Zn analysis(Reference Brown, Rivera and Bhutta4, Reference Tamura, Johnston and Freeberg13).
In these NZ children, risk of Zn deficiency, based on low serum Zn concentrations, varied significantly by ethnicity. Pacific children had the highest risk (21 %), with a prevalence indicative of public health concern (i.e. >20 %)(5), followed by Māori children (16 %), and NZEO children (15 %) had the lowest risk. We believe that these differences are probably, at least in part, due to an effect of ethnicity. Pacific and Māori children undergo puberty earlier and are taller for chronological age than Europeans(Reference Gordon, Ferguson and Toafa35), trends also observed here (Table 2). As a result, their Zn requirements are likely to be higher than that of their European counterparts. Pacific children also have a greater lean body mass compared with European children of similar height(Reference Grant, Gordon and Ferguson36) which, because of the higher Zn content of muscle compared with fat(Reference Brown, Rivera and Bhutta4), may further exacerbate the risk of Zn deficiency among this ethnic group.
It is noteworthy that data for all the three ethnicities showed a higher risk of Zn deficiency in morning v. afternoon blood samples (Table 5). This aberration may be related to uncertainties in the morning and afternoon serum Zn cut-offs(Reference Brown, Rivera and Bhutta4).
The present study provides some evidence of functional disturbances related to suboptimal Zn status. In the Pacific group, the mean HAZ score of the boys with low serum Zn concentrations was significantly lower than those with normal serum Zn concentrations, again emphasising the higher risk of Zn deficiency in male children. Observational(Reference Butrimovitz and Purdy37, Reference Buzina, Jusic and Sapunar38) and Zn supplementation(Reference Brown, Peerson and Baker39) trials have demonstrated comparable positive relationships between serum Zn and linear growth. No relationship was observed with BMI z scores. Morbidity data were not collected, so whether the lower Zn status of the Pacific children was associated with a higher prevalence of infectious illnesses is unknown. However, NZ Pacific children are known to have the highest hospitalisation rates for pneumonia, followed by Māori; NZEO children have the lowest(Reference Grant, Scragg and Tan40).
Interestingly, Zn intakes were lowest in the Pacific group (Table 7) when expressed in terms of mg/kg BW and may reflect differences in food selection patterns associated with ethnicity, real differences in energy intakes or bias, resulting from under- or over-reporting(Reference Thane, Bates and Prentice33), although the latter was not assessed(Reference Parnell, Scragg and Wilson6). The significant decrease in intake with age in all the three ethnic groups may reflect the existence of a non-linear relationship between Zn intake and BW.
Overall, in the NZ CNS02 survey, Pacific girls aged 9–13 years had the highest risk of inadequate intakes of Zn, although the prevalence was very low among all the children. However, the dietary requirements for Zn may be higher for the larger Pacific and, to a lesser extent, Māori children. Hence, the prevalence of inadequate intakes may be underestimated in these two ethnic groups, thus contributing, at least in part, to the lack of concordance between our predicted prevalence for risk of Zn deficiency based on dietary and biochemical indicators. Other contributing factors include the wide array of confounding factors influencing serum Zn concentrations, some identified for the first time in our NZ survey, and most importantly, uncertainties in the cut-offs for children used to define low serum Zn status and inadequate Zn intakes(Reference Hotz41). Certainly, in adults, the magnitude of the inhibitory effect of dietary phytate on Zn absorption is said to be much greater than previously recognised. Whether this is also the case for young children is less certain(Reference Hambidge, Miller and Westcott42).
Despite the apparent greater risk of population Zn deficiency in the Pacific children, very few were classified as stunted. Indeed, their mean HAZ scores for all three age categories tended to be higher than those for the Māori and NZEO groups. However, whether the US Center for Disease Control and Prevention 2000 growth reference data(Reference Kuczmarski, Ogden and Grummer-Strawn19) are appropriate for Pacific and Māori children, who are taller for chronological age than Europeans(Reference Grant, Gordon and Ferguson36), is questionable.
Some of the study limitations derive from the intentional over-sampling of the Māori, and particularly the Pacific group to permit these minority groups to be better characterised in the NZ CSN02. For this reason, we analysed the data for the three ethnicities separately, applying nationally based survey weights for age, sex and non-response for each ethnicity. The age- and sex-related associations were less marked among the Māori compared with the Pacific and NZEO ethnicities, probably at least in part, because of the very small number of Māori aged 14–15 years (i.e. n 15) for whom serum Zn values were available compared with the numbers in the other two groups. A second limitation relates to requesting serum samples only from children in urban and semi-urban areas. Specific national weights for this sampled fraction of the NZ CSN02 were used, and urban children make up more than approximately 90 % of NZ children. Nevertheless, the absence of serum Zn values from the rural school children, as well as the urban and semi-urban children who refused to provide a blood sample, limited the representativeness of our data. Finally, our data are observational, and therefore the present results do not imply causality.
In summary, notwithstanding the limitations, the present results indicate that, of the three ethnicities, the Pacific group had the highest prevalence of low serum Zn concentrations (21 %), considered indicative of population Zn deficiency, followed by Māori children (16 %); NZEO children had the lowest prevalence (15 %). Suboptimal Zn status among the Pacific children may have been exacerbated by their greater stature and lean body mass, and thus higher requirements for Zn than the NZEO children, coupled with a lower Zn intake, when expressed per kg BW. Consequently, the lack of concordance between risk of population Zn deficiency based on the prevalence of inadequate Zn intakes and low serum Zn concentrations may be partly associated with an underestimate of the prevalence of inadequate intakes of Zn in the larger Pacific and Māori children, together with the multiple factors influencing serum Zn concentrations. The relative importance of these various factors varied with ethnicity, but age, sex and time of the sample collection along with two previously unreported variables – serum Se and season – were significant determinants of serum Zn concentrations in one or more ethnic groups, and the risk of suboptimal Zn status appears to be particularly high among younger male children. Our findings highlight the necessity for more research to better define the cut-offs for both dietary and biochemical indicators used to define the risk of population Zn deficiency.
Acknowledgements
The present study was supported by the NZ Ministry of Health. We thank the children who participated in this survey, Andrew Gray for his invaluable statistical advice, Ian L. Gibson for performing the statistical analysis, Heather Walker for calculating the prevalence of inadequate intakes and the other principal investigators: Robert Scragg, David Schaff and Eljon D. Fitzgerald. W. R. P. and N. W. were involved in CNS02 data collection, E. L. F. and R. S. G. standardised the blood sample collection for serum Zn and K. B. B. performed the serum Zn analysis. R. S. G. supervised the analysis and interpretation of the serum Zn values. The manuscript was drafted by R. S. G. with contributions by K. B. B., W. R. P., N. W. and E. L. F. None of the authors had any financial or personal conflicts of interest in connection with the present study.