The systemic and mucosal immune systems are continuously in development, and their function is highly influenced by maternal, environmental, dietary and behavioural factors(Reference Pérez-Cano, Castellote and González-Castro1–Reference Pérez-Cano, Marín-Gallén and Castell3). In rats and man, before birth, IgG crosses the placenta and reaches the fetus; thereafter, milk constitutes the communication route between the maternal and infant immune systems. It represents an active mechanism directing and educating the immune system, metabolism and gut microflora within the infant, while conferring multiple means of protection from pathogens(Reference Lönnerdal4, Reference Field5). Breast milk has Ig and many other bioactive molecules, such as growth factors, cytokines, nucleotides, cellular components and lipids, which promote maturation of the developing immune system(Reference Field5–Reference Gil and Rueda7).
It has been suggested that PUFA, specifically DHA and arachidonic acid, which constitute a relatively low fraction of the total fatty acids in human breast milk, participate in neonate immune development(Reference Field, Clandinin and Van Aerde8). In parallel, conjugated linoleic acid (CLA), a PUFA found in breast milk, has been also suggested to contribute to immune development(Reference Mcguire, Park and Behre9–Reference Chin, Storkson and Albright12). The predominant CLA isomer in dairy products is cis-9, trans-11 (c9, t11)-CLA, also called rumenic acid, which ranges in human milk from 83 to 100 % of total CLA(Reference Mcguire, Park and Behre9, Reference Luna, Juarez and Angel de la Fuente11). The trans-10, cis-12 (t10, c12)-CLA isomer is also found in dairy products, but in lower proportions. In human milk, this isomer is in lower amounts than rumenic acid(Reference Luna, Juarez and Angel de la Fuente11, Reference Ha, Grimm and Pariza13). Even very low doses of the t10, c12-CLA isomer seem to have large biological effects(Reference Hayashi, de Medeiros and Carvalho14).
CLA has been reported to exert beneficial physiological effects on the development of cancer(Reference Kelley, Hubbard and Erickson15), atherosclerosis(Reference Nicolosi, Rogers and Kritchevsky16), diabetes(Reference Taylor and Zahradka17), and on body composition(Reference Park and Pariza18). The immunomodulatory properties of CLA in both rodents and human subjects show controversial results ranging from stimulation to inhibition(Reference Kelley, Warren and Simon19–Reference Bhattacharya, Banu and Rahman22). These discrepancies are mainly due to the different mixtures of CLA isomers used in the studies, since each isomer has specific biological effects. The t10, c12-CLA isomer is responsible for body fat reduction(Reference Pariza, Park and Cook23–Reference Moloney, Toomey and Noone25), although both CLA isomers have shown immunomodulatory effects(Reference Kelley, Warren and Simon19–Reference Bhattacharya, Banu and Rahman22).
Although many studies have been carried out with CLA isomer mixtures, most of them have used 50:50 mixtures of the c9, t11 and t10, c12 isomers and have been carried out in animals of at least 3 weeks old. Based on the predominance of the c9, t11-CLA isomer in breast milk and that CLA intake during developmental phases might have effects later in life(Reference Bassaganya-Riera, Reynolds and Martino-Catt26, Reference Frazier, Ryan and Rockett27), we hypothesised that CLA would be transferred from dams to pups and exert immune-enhancing effects. Thus, the aim of the present immunonutrition study was to investigate the effects of supplementing Wistar rats from gestation to the end of suckling with an 80:20 isomer mixture of c9, t11- and t10, c12-CLA on the systemic immune response in Wistar rats. We quantified CLA transfer to pups, serum and milk Ig concentrations, in vitro splenocyte Ig production and spleen cell proliferation and cytokine secretion ability as biomarkers of immune development.
Materials and methods
Animals
Pregnant Wistar rats at 7 d gestation were obtained from Harlan (Barcelona, Spain). The animals were housed in individual cages under controlled temperature and humidity conditions in a 12 h light–12 h dark cycle, and had access to food and water ad libitum. The rats were monitored daily and allowed to deliver at term. The day of birth was registered as day 1 of life. Litters were unified to ten pups per lactating dam; pups had free access to the nipples and rat diet. Animals were daily identified and weighed, and handling was done in the same time range to avoid the influence of biological rhythms. Body weight and body length (nose–anus length) were used to determine the following morphometrical parameters: BMI, calculated as body weight/length2 (g/cm2); the Lee index, calculated as 3√weight/length (g/cm), as markers of obesity in rats and other mammals(Reference Novelli, Diniz and Galhardi28).
At the day of weaning (day 21), rats were anaesthetised with ketamine (90 mg/kg rat weight) and xylazine (10 mg/kg rat weight) to obtain spleen and blood for serum and plasma samples, which were immediately frozen at − 80°C until processing. Studies were performed in accordance with the institutional guidelines for the care and use of laboratory animals established by the Ethical Committee for Animal Experimentation of the University of Barcelona and approved by the Catalonian Government (CEEA 303/05, UB/DMA 3242).
Pregnant rats were randomly assigned to one of the following four dietary groups, according to the total period of CLA supplementation and administration route used in the pups (Fig. 1):
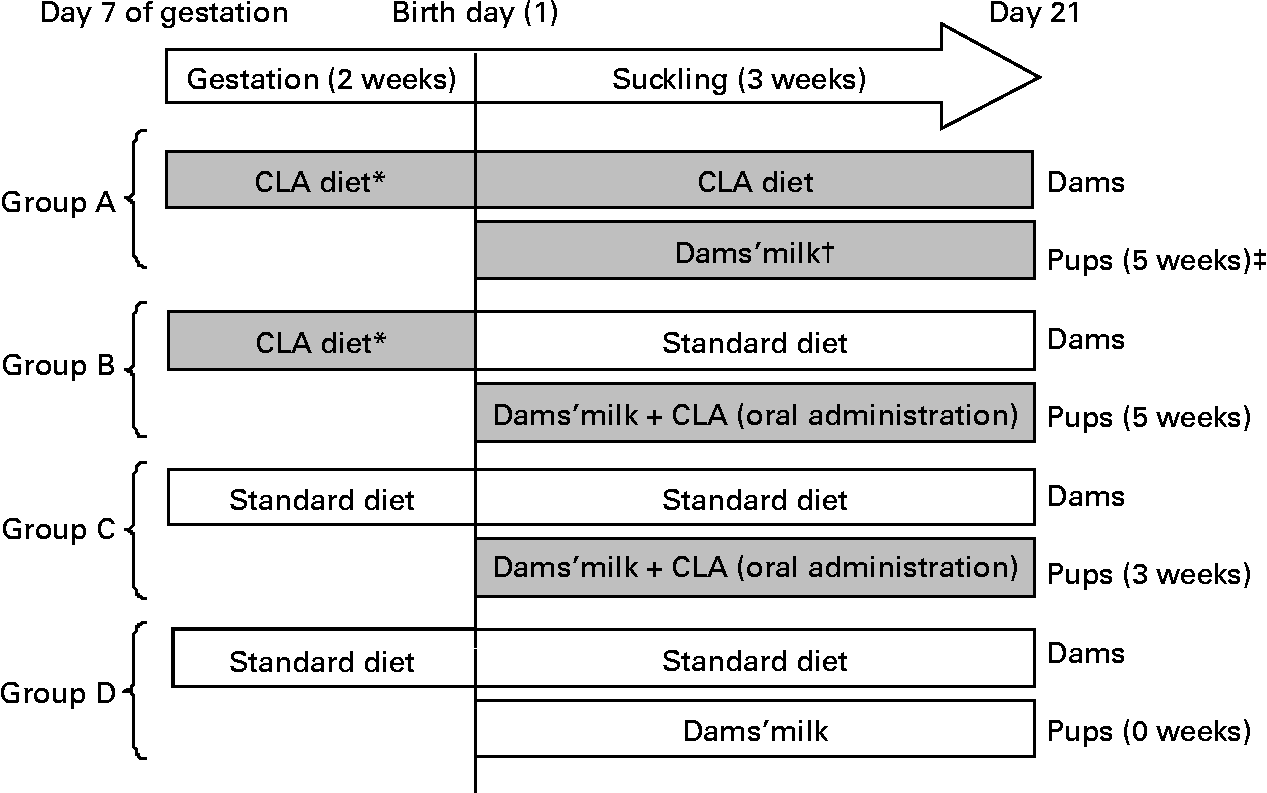
Fig. 1 Diagram of the experimental design beginning on day 7 of gestation until day 21 of suckling. * Conjugated linoleic acid (CLA) arrives at the fetus by transplacental transfer. † CLA arrives at pups through the milk of dams. ‡ Indicates total period of CLA supplementation from gestation until the end of suckling. (![]() ), CLA-supplemented animals; (□), non-supplemented animals.
), CLA-supplemented animals; (□), non-supplemented animals.
Group A
Group A were pups from dams fed a 1 % CLA diet (see below) during the last 2 weeks of gestation and throughout the suckling period. During suckling, the pups received CLA through the dams' milk. The total period of supplementation was 5 weeks.
Group B
Group B were pups from dams fed during gestation the 1 % CLA diet and during suckling a standard diet (AIN-93G(Reference Reeves, Nielsen and Fahey29)). The pups were CLA supplemented daily during suckling by oral administration. The total period of supplementation was 5 weeks.
Group C
Group C were pups from dams fed the standard diet during gestation and suckling. The pups received CLA by daily oral administration throughout the suckling period. The total period of supplementation was 3 weeks.
Group D
Group D were pups from dams fed the standard diet throughout the study. These animals constitute the reference diet group. The total period of supplementation was 0 weeks.
Diets
The standard diet corresponded to the AIN-93G formulation(Reference Reeves, Nielsen and Fahey29), containing 7 % soyabean oil. The 1 % CLA diet was obtained from modified standard flour (AIN-513; Harlan) containing 10 g CLA/kg (Table 1). Thus, the supplemented diet contained 6 % soyabean oil plus 1 % CLA oil. The CLA isomer mixture used was approximately 80 % c9, t11 and 20 % t10, c12 from the total CLA isomers in oil. The CLA mixture had 0·69 % NEFA as oleic acid, a peroxide value of 0·2 mEq/kg, 5·6 % SFA and less than 5 % of minor CLA isomers. CLA oil was kindly supplied by Loders Croklaan (Lipid Nutrition, Wormerveer, The Netherlands).
Table 1 Composition of experimental dams' diets (g/kg diet)
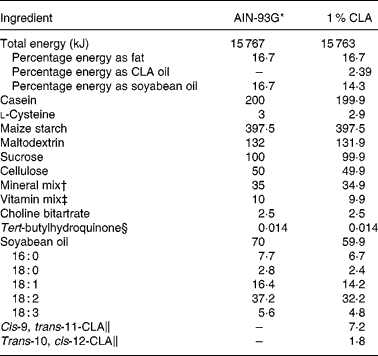
CLA, conjugated linoleic acid.
* The diet was prepared according to AIN guidelines(Reference Reeves, Nielsen and Fahey29).
† Supplied (per kg diet): 357 g calcium carbonate, 196 g potassium phosphate monobasic, 70·78 g potassium citrate, 74 g sodium chloride, 46·6 g potassium sulfate, 24·3 g magnesium oxide, 6·06 g ferric citrate, 1·65 g zinc carbonate, 0·63 g manganous carbonate, 0·31 g cupric carbonate, 0·01 g potassium iodate, 0·01 025 g sodium selenate, 0·00 795 g ammonium paramolybdate, 1·45 g sodium meta-silicate, 0·275 g chromium potassium sulfate, 0·0174 g lithium chloride, 0·0815 g boric acid, 0·0635 sodium fluoride, 0·0318 g nickel carbonate hydroxide, tetrahydrate, 0·0066 g ammonium vanadate and 220·716 g sucrose.
‡ Supplied (per kg diet): 3 g nicotinic acid, 1·6 g calcium pantothenate, 0·7 g pyridoxine HCl, 0·6 g thiamin HCl, 0·6 g riboflavin, 0·2 g folic acid, 0·02 g d-biotin, 2·5 g vitamin B12 (0·1 % in mannitol), 15 g dl-α tocopheryl acetate (500 IU/g), 0·8 g vitamin A palmitate (5 000 000 IU/g), 0·2 g vitamin D3 (cholecalciferol, 5 000 000 IU/g), 0·075 g vitamin K (phylloquinone) and 974·705 g sucrose.
§ Antioxidant.
∥ From total oil, CLA isomers are present in TAG form containing an 80:20 isomer ratio of cis-9, trans-11 and trans-10, cis-12, respectively. The remaining 10 % of oil is composed of oleic acid (0·7 %), SFA (5·6 %) and other CLA isomers (3·7 %).
The 1 % CLA diet in suckling animals corresponded to a daily administration of 1·5 mg CLA oil provided/g rat from day 1 to 21. Low-capacity syringes (Hamilton Bonaduz AG, Bonaduz, Switzerland) adapted to oral twenty-five-gauge or twenty-three-gauge gavage tubes, 27 mm in length (ASICO, Westment, IL, USA) were used for oral administration before and after day 5, respectively. To allow gastric emptying, litters were separated from dams 1 h before oral supplementation.
Collection and processing of dam milk
Milk was collected from dams on day 21 post-partum. Pups were separated from dams 1 h before milk extraction to allow the milk to accumulate in the mammary glands. Then females were anaesthetised intramusculary (i.m) with ketamine (90 mg/kg rat) and then treated i.m with 2 IU oxytocin (Novartis, Barcelona, Spain) 10 min before milking. By gentle hand stripping of teats, milk droplets were collected into a test-tube using silastic tubing connected to a gentle suction. Total milk was used for CLA quantification, whereas Ig determination was performed in milk whey supernatant fractions after centrifugation (600 g, 30 min, 4°C) and fat layer discarding.
Quantification of conjugated linoleic acid isomers in pup plasma and in dam milk
The content of c9, t11- and t10, c12-CLA isomers in the plasma of pups and fatty acid composition in the milk of dams were quantified by fast GC using a capillary column (40 m × 0·18 mm × 0·20 μm), coated with RTX-2330 non-bonded stationary phase (poly 90 % biscyanopropyl–10 % cyanopropylphenyl) siloxane from Thames Restek UK (High Wycombe, Bucks, UK) as previously described(Reference Bondía-Pons, Moltó-Puigmartí and Castellote30). The identities of sample methyl ester peaks were determined by comparison of their relative retention times with those of well-known fatty acid methyl ester standards. Quantification was based on the amount of the internal standard recovered. The fatty acid composition of dams' milk was also evaluated. The results were expressed in relative amounts (% total fatty acids). The CLA isomer content was also evaluated in the 1 % enriched diet after the manufacturing process.
Isolation and culture of spleen cells
Spleen cell suspensions were obtained by passing the tissue through a steel mesh (Cellector™; Bellco, Vertieb, Austria) in sterile conditions. Cells were then centrifuged and re-suspended in PBS. Erythrocytes were lysed by adding distilled water to the cell suspension, and tonicity was restored by adding PBS 10 × . Afterwards, cells were centrifuged, washed, and finally re-suspended in Roswell Park Memorial Institute (RPMI)–10 % fetal bovine serum containing 0·05 mm-2-mercaptoethanol (Merck, Darmstadt, Germany), streptomycin–penicillin (100 IU/ml; Sigma Chemical Co., St Louis, MO, USA) and 2 mm-l-glutamine (Sigma). Cell viability was determined by double staining with acridine orange and ethidium bromide (Sigma). Cells were plated and cultured in different conditions according to the assay.
Lymphocyte proliferation
Spleen cells were cultured at 1 × 105 cells/100 μl in a ninety-six-well plate and stimulated with phorbol myristate acetate plus ionomycin both at 250 ng/ml (Sigma). Lymphocyte proliferation was determined by a modified ELISA technique using Cell Proliferation Biotrak™ (Amersham Biosciences, Munich, Germany) after 72 h incubation. This assay is based on the measurement of 5-bromo-2′-deoxyuridine incorporation into proliferating cells during DNA synthesis. Absorbance (Ab) values correlate directly to the amount of DNA synthesised and, therefore, to the number of proliferating cells in culture. The proliferation rate (%) was expressed considering 100 % for the reference diet group, as follows:
A parallel plate was cultured with the same samples and conditions to determine cell viability.
In vitro cytokine production
Splenocytes were cultured at 3 × 106 cells/ml in a twenty-four-well flat-bottom plate (TPP, Trasadingen, Switzerland) and stimulated with phorbol myristate acetate plus ionomycin (250 ng/ml) for 24 h. The concentrations of IL-2, interferon-γ, IL-4 and IL-10 in the supernatant fractions were quantified using rat ELISA sets from Biosource (Nivelles, Belgium) and BD Pharmingen (Erembodegem, Belgium), following the manufacturers' instructions.
Splenocyte in vitro immunoglobulin production and immunoglobulin concentrations in serum and milk whey
Concentrations of IgG and IgM secreted during 7 d by non-stimulated spleen cells and serum and milk IgA, IgM and IgG concentrations were quantified by ELISA. Briefly, ninety-six-well polystyrene plates (Nunc Maxisorp, Wiesbaden, Germany) were coated with anti-rat IgA, anti-rat IgM or anti-rat IgG monoclonal antibodies (mAbs; BD Pharmingen) at 2, 2·5 and 10 μg/ml in PBS, respectively (overnight in a humidified chamber). The remaining binding sites were then blocked with PBS–1 % bovine serum albumin during 1 h at room temperature. Plates were washed (3 × with PBS–0·05 % Tween-20 and once with PBS), and the supernatant fractions, milk whey, serum and standard Ig dilutions (BD Pharmingen) in PBS–Tween 1 % bovine serum albumin were then added and incubated (3 h; room temperature). Plates were washed again and incubated (2 h; room temperature) with biotinylated anti-rat IgA or IgM (BD Pharmingen) at 0·625 and 1 μg/ml, respectively, Subsequently, extravidin–peroxidase conjugate (4 μg/ml in PBS–Tween 1 % bovine serum albumin) was added to the plates for 30 min at room temperature. A purified peroxidase anti-rat Ig antibody (Sigma) was used for IgG detection. Ig were detected by the addition of the substrate solution (o-phenylenediamine dihydrochloride plus H2O2 in 0·2 m-phosphate–0·1 m-citrate buffer, pH 5). The enzyme reaction was stopped with H2SO4 (3 mol/l) and Ab was measured at 492 nm.
Statistical analysis
SPSS 14.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. Conventional one-way ANOVA was performed considering the experimental group based on CLA supplementation as the independent variable. When CLA supplementation had a significant effect on the dependent variable, Bonferroni's and Scheffe's tests were applied. The content of Ig in rat milk whey was analysed by the Student's t test. Significant differences were accepted at P < 0·05.
Results
Body weight
The body weight of dams and pups was monitored daily throughout the study. The pregnant dams' starting body weight at 7 d gestation was 227·4 (sem 4·2) g. The 1 % CLA diet did not modify the dams' body weight increase during the gestation period: dams fed the 1 % CLA or standard diet gained similar weight up to day 21 of gestation (308·7 (sem 8·4) and 309·2 (sem 10·4) g, respectively). Moreover, from day 7 to 21 of gestation, dams from both dietary groups had similar chow intake (5·9 (sem 0·3) g/100 g rat per d for the 1 % CLA diet group; 5·5 (sem 0·5) g/100 g rat per d for the standard diet group). To assess the effect of CLA supplementation during gestation, pups from dams in groups A and B were taken together and compared on the day of birth with pups from dams fed the standard diet (groups C and D). The weight but not BMI and Lee index of neonates from the CLA-supplemented dams (5·7 (sem 0·1) g, 0·2 (sem 0·1) g/cm2, and 0·3 (sem 0·1) g/cm, respectively) was lower than that from neonates whose dams received the standard diet during gestation (6·7 (sem 0·1) g, 0·2 (sem 0·1) g/cm2 and 0·4 (sem 0·1) g/cm, respectively) (P < 0·05).
Despite the slight differences among groups during week 1 of life, CLA-supplemented animals exhibited a similar body weight pattern when compared with pups from group D throughout the suckling period (Fig. 2), and at the end of the suckling period (day 21), weight, BMI and Lee index were similar among the groups (49·1 (sem 0·6) g, 0·4 (sem 0·1) g/cm2 and 0·3 (sem 0·1) g/cm, respectively). As we have described in other previous experimental nutrition designs investigating this early life period(Reference Pérez-Cano, Marín-Gallén and Castell3), there were no deaths in any of the groups in the present study. Animals' behaviour and organ weight and appearance were recorded as markers of CLA side effects and there was no evidence of abnormality.
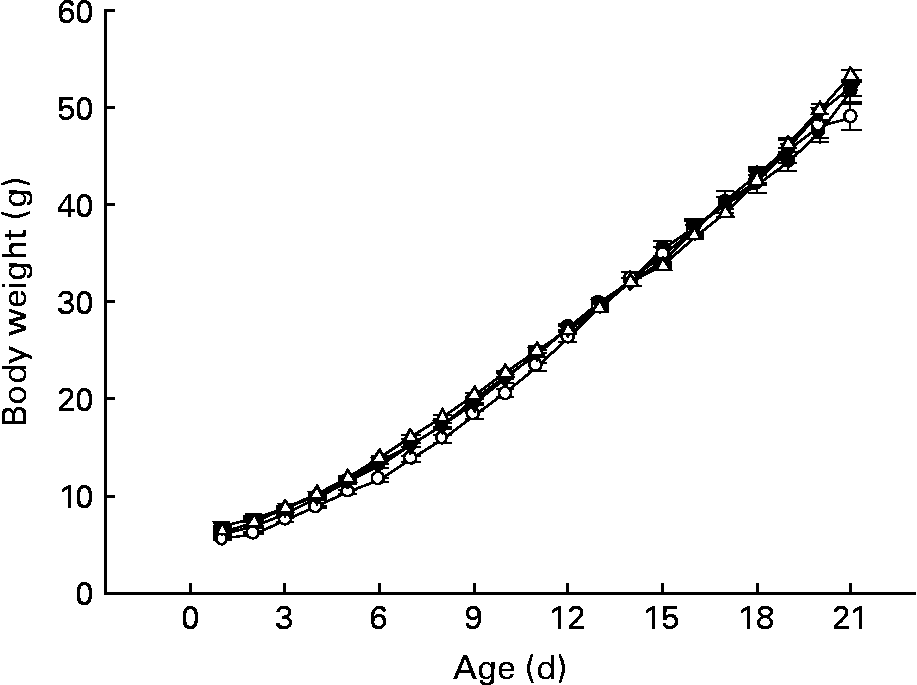
Fig. 2 Influence of conjugated linoleic acid (CLA) supplementation on body weight from day 1 to 21. (–●–), Group A (CLA supplementation during gestation and suckling through dams (5 weeks)); (–○–), group B (CLA supplementation during gestation and suckling by oral administration (5 weeks)); (–▾–), group C (CLA supplementation during suckling by oral administration (3 weeks)); (–△–), group D (non-supplemented animals (0 weeks)). Values are means (n 15–20 pups per group), with standard errors represented by vertical bars. There were no significant differences among the groups based on a general linear model (ANOVA) with Bonferroni's adjustment for multiple comparisons.
Conjugated linoleic acid content and fatty acid composition of dam milk
At the end of suckling (day 21), milk from dams fed the CLA diet during gestation and suckling showed higher concentrations of the c9, t11- and t10, c12-CLA isomers than rats fed the standard diet (P < 0·001) (Table 2). The proportion of these two isomers in milk, 86:14, varied from that supplemented to dams, 80:20. Moreover, the n-6:n-3 proportion present in the milk of dams fed CLA was 8·04, whereas it was 8·21 in milk from dams fed the standard diet. CLA induced changes in the fatty acid profile of milk from supplemented rats (Table 2).
Table 2 Milk fatty acid composition of rats fed the standard or conjugated linoleic acid (CLA) diet (g/100 g total fatty acids)†
(Mean values with their standard errors for four to seven dams per group)

* Mean value was significantly different from that of the standard diet group (P < 0·001; Student's t test).
† The composition of milk was evaluated on day 21 of suckling.
Conjugated linoleic acid content in pup plasma
The concentration of CLA in the plasma of pups was determined on the day of weaning, following dietary supplementation either through gestation and suckling from the dam or by oral administration. Pups from group D showed a low plasma content of c9, t11-CLA and no t10, c12-CLA. Groups A, B and C had approximately nine, twelve and six times higher levels of c9, t11-CLA than group D, respectively (P < 0·05) (Table 3). Moreover, groups A and B (both receiving CLA for 5 weeks) had higher concentrations of both CLA isomers than group C (fed CLA for 3 weeks; P < 0·05). Although the supplemented proportion to dams and pups of c9, t11- and t10, c12-CLA was 80:20, the CLA content of the plasma of pups did not show this proportion in any group. The proportion of CLA isomers was 86:14, 93:7 and 94:6 for groups A, B and C, respectively. Hence, group B presented a higher content of c9, t11-CLA, but a lower content of t10, c12-CLA than group A (P < 0·05).
Table 3 Relative content of cis-9, trans-11- and trans-10, cis-12-conjugated linoleic acid (CLA) isomers in the plasma of 21-d-old pups (% total fatty acids)*
(Mean values with their standard errors for ten pups per group)
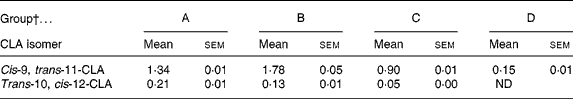
ND, non-detectable.
* The content of both CLA isomers in pups' plasma was significantly different among all groups (P < 0·001; one-way ANOVA).
† Group A were pups supplemented with 1 % CLA during gestation and suckling through dams (5 weeks). Group B were pups supplemented with 1 % CLA during gestation through dams and during suckling by oral administration (5 weeks). Group C were pups supplemented with 1 % CLA only during suckling by oral administration (3 weeks). Group D were non-supplemented pups (0 weeks).
Immunoglobulin concentration in milk whey
Concentrations of IgG, IgA and IgM were quantified in dams' milk whey at the end of the suckling period (21 d postpartum) (Fig. 3). The predominant Ig present in rat milk was IgG (about 280 μg/ml), followed by IgA (about 30 μg/ml) and finally IgM (about 5 μg/ml). Dams fed the CLA diet during gestation and suckling increased the concentration of the main Ig isotypes in rat milk, IgG and IgA, about 6- and 2-fold, respectively (P < 0·05).

Fig. 3 Effects of conjugated linoleic acid (CLA) on IgA (a), IgG (b) and IgM (c) concentrations of rat milk collected on day 21 of the suckling period. (■), CLA diet; (□), standard diet. Values are means (n 4–7 dams per group), with standard errors represented by vertical bars. * Mean value was significantly different from that of milk whey from dams fed the standard diet (P < 0·05; Student's t test).
Serum immunoglobulin concentration
Serum IgG, IgM and IgA concentrations were quantified in 21-d-old animals (Fig. 4). Animals from group D showed about 5 mg IgG/ml (Fig. 4(a)), about 95 μg IgM/ml (Fig. 4(b)) and about 2·7 μg IgA/ml (Fig. 4(c)). CLA supplementation for 5 weeks, 2 weeks during gestation and 3 weeks during suckling through the dams' milk (group A), increased the total Ig serum concentration almost 4-fold, mainly by enhancement of IgG (Fig. 4(a)) (P < 0·05). At this age, there were no differences in serum IgM concentration among the groups (Fig. 4(b)). However, group A exhibited a lower IgA serum concentration than those of the other groups (Fig. 4(c)) (P < 0·05).
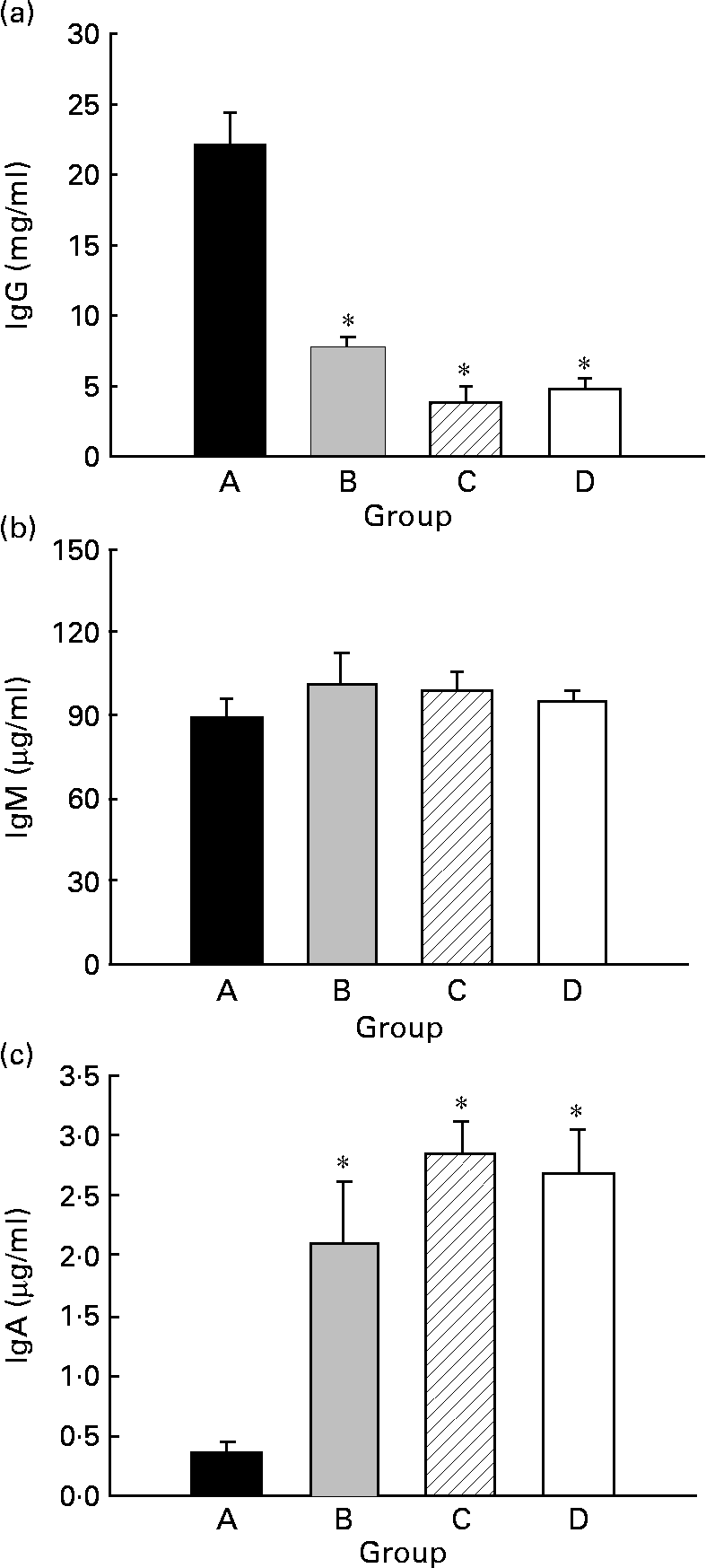
Fig. 4 Effects of conjugated linoleic acid (CLA) supplementation on serum IgG (a), IgM (b) and IgA (c) concentrations. Group A were pups supplemented with 1 % CLA during gestation and suckling through dams (5 weeks). Group B were pups supplemented with 1 % CLA during gestation through dams and during suckling by oral administration (5 weeks). Group C were pups supplemented with 1 % CLA only during suckling by oral administration (3 weeks). Group D were non-supplemented pups (0 weeks). Values are means (n 15–20 pups per group), with standard errors represented by vertical bars. * Mean value was significantly different from that of group A (P < 0·05; one-way ANOVA).
Spleen lymphocyte proliferation, viability, and cytokine secretion
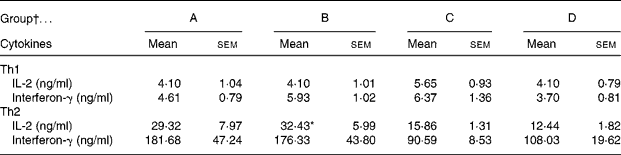
The CLA diet did not modify the ex vivo lymphoproliferative capacity in any group, measured 72 h after phorbol myristate acetate plus ionomycin stimulation (Fig. 5(a)). To evaluate the effect of CLA and the relationship between proliferation and cell viability, we assessed splenocyte starting viability, which was about 95 % for all groups, an optimum level for development of the assay. Cell viability was slightly reduced after phorbol myristate acetate plus ionomycin stimulation, but this decrease did not differ among groups, indicating that CLA dietary supplementation had no effect on splenocyte viability in 21-d-old animals. IL-2 production, the main proliferative signal for lymphocytes, was measured in supernatant fractions obtained after 24 h of spleen cell stimulation and was not modified by CLA supplementation (Table 4). Moreover, interferon-γ was also secreted in similar amounts in all groups after mitogen stimulation. T helper 2 (Th2) cytokines, IL-4 and IL-10, were also quantified in the same splenocyte supernatant fractions and although no statistical differences were found among groups, due to the large intra-group variability, rats from groups A and B showed almost 2-fold higher values than those observed in groups C and D (Table 4).
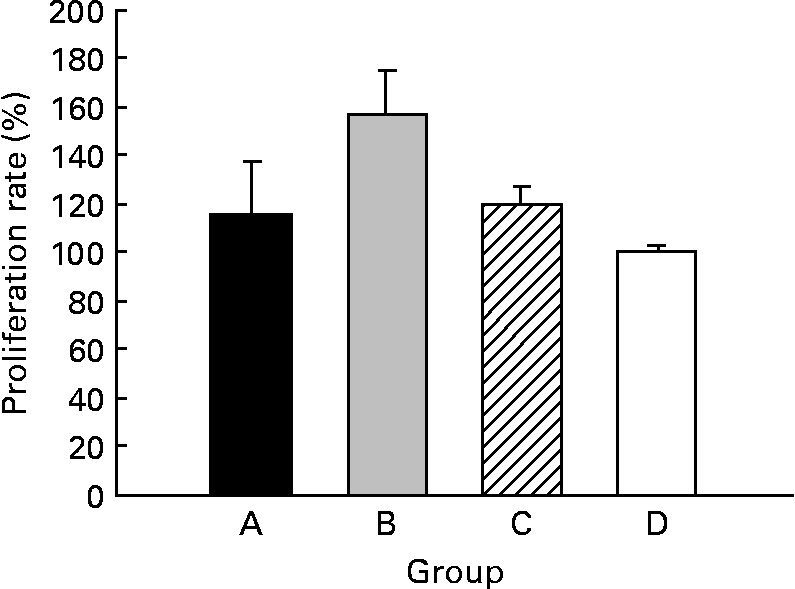
Fig. 5 Effects of conjugated linoleic acid (CLA) on proliferation rate in splenocytes stimulated with phorbol myristate acetate plus ionomycin. Group A were pups supplemented with 1 % CLA during gestation and suckling through dams (5 weeks). Group B were pups supplemented with 1 % CLA during gestation through dams and during suckling by oral administration (5 weeks). Group C were pups supplemented with 1 % CLA only during suckling by oral administration (3 weeks). Group D were non-supplemented pups (0 weeks). Data are shown as percentage of controls (set at 100 %). Values are means (n 5–20 pups per group), with standard errors represented by vertical bars. CLA dietary supplementation did not modify splenocyte proliferation, regardless of period of supplementation or supplementation duration.
Table 4 Content of cytokines in 24 h splenocyte supernatant fractions stimulated with phorbol myristate acetate plus ionomycin (250 ng/ml)
(Mean values with their standard errors for ten pups per group)
Th, T helper.
* Mean value was significantly different from that of group D (P = 0·06; one-way ANOVA).
† Group A were pups supplemented with 1 % CLA during gestation and suckling through dams (5 weeks). Group B were pups supplemented with 1 % CLA during gestation through dams and during suckling by oral administration (5 weeks). Group C were pups supplemented with 1 % CLA only during suckling by oral administration (3 weeks). Group D were non-supplemented pups (0 weeks).
Splenocyte in vitro immunoglobulin production
Spontaneous IgM and IgG production by splenocytes from weaning rats was quantified, and IgM was the main isotype found in supernatant fractions, being forty times higher than IgG (P < 0·05) (Fig. 6). Regarding CLA supplementation, IgM production from both groups supplemented for 5 weeks (groups A and B, about 900–1200 ng/ml) was higher than that of the groups supplemented for 3 weeks (group C) and 0 weeks (group D) (both approximately, about 500 ng/ml). However, this increase was only significant when pups received CLA during gestation and suckling by oral administration (group B) (P < 0·05) (Fig. 6(a)). Otherwise, IgG production was very low (about 10 ng/ml) and no differences were found among groups (Fig. 6(b)). Thus, continuous CLA supplementation during gestation and suckling enhances splenocyte IgM production, increasing total Ig concentration by almost two-fold that of non-supplemented animals.
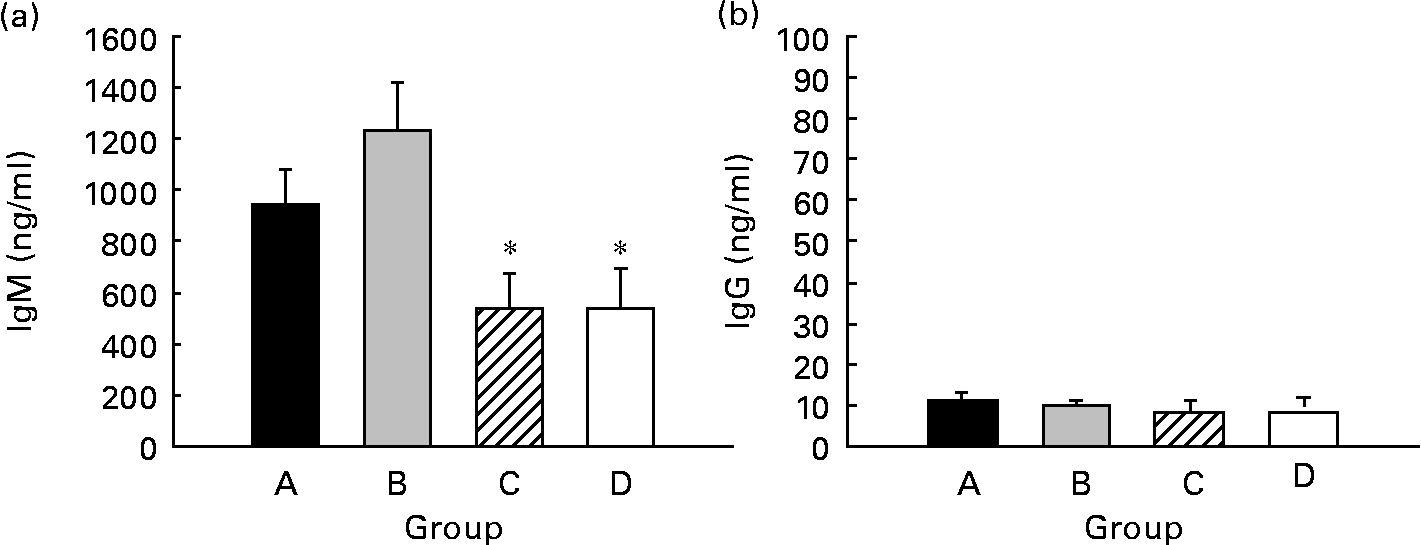
Fig. 6 Effects of conjugated linoleic acid (CLA) on spleen cell Ig secretion. IgM (a) and IgG (b) concentrations in supernatant fractions after 7 d of spleen cell culture. Group A were pups supplemented with 1 % CLA during gestation and suckling through dams (5 weeks). Group B were pups supplemented with 1 % CLA during gestation through dams and during suckling by oral administration (5 weeks). Group C were pups supplemented with 1 % CLA only during suckling by oral administration (3 weeks). Group D were non-supplemented pups (0 weeks). Values are means (n 15–20 pups per group), with standard errors represented by vertical bars. * Mean value was significantly different from that of group B (P < 0·05; one-way ANOVA).
Discussion
Evidence suggests that CLA has immunomodulatory properties(Reference Kelley, Warren and Simon19–Reference Bhattacharya, Banu and Rahman22), but there are no conclusive results in this regard either in animal or in human early life. We are not aware of any previous studies reporting the effects of CLA on immune function in suckling rats. Thus, this is the first study describing the influence of length (3, 5 weeks), life period (gestation, suckling) and route of supplementation (placenta, breast milk, oral administration) on the immune effects of CLA.
Dietary supplementation with 1 % of an 80:20 mix of c9, t11- and t10, c12-CLA isomers during gestation did not modify body weight or food intake of dams, in agreement with other studies of dams fed different CLA isomer mixtures during gestation and lactation(Reference Chin, Storkson and Albright12, Reference Hayashi, de Medeiros and Carvalho14, Reference Ringseis, Saal and Muller31). Body weight on the day of birth was lower in pups from the CLA-fed dams than those from dams fed the standard diet. Thus, the effect of CLA supplementation during gestation was already evident on the delivery day, probably due to the body fat reduction effects of the t10, c12-CLA isomer(Reference Park and Pariza18, Reference Terpstra32). Nevertheless, the effect of this isomer was not evidenced in older animals. This finding contrasts with results obtained in other species after feeding other CLA isomer mixtures(Reference Poulos, Sisk and Hausman33, Reference Schmid, Collomb and Bee34). Chin et al. (Reference Chin, Storkson and Albright12) reported a moderately enhanced body weight gain in rat pups after CLA administration during gestation and suckling. However, the present results are consistent with other studies that supplemented rats with a 50:50 isomer mixture during the same life period(Reference Hayashi, de Medeiros and Carvalho14, Reference Ringseis, Saal and Muller31). It is likely that these discrepancies are mainly due to differences in the isomers used in the related studies. Moreover, in the present study there were no negative side effects, as evidenced by the organs' weight and appearance, and the animals' behaviour. This is in keeping with CLA safety assessments carried out in animals(Reference Chin, Storkson and Albright12, Reference Hayashi, de Medeiros and Carvalho14, Reference Scimeca35, Reference Park, Albright and Pariza36) even when CLA comprised 5 % of the diet(Reference Scimeca35), and in human subjects consuming a maximum of 6 g/d(Reference Pariza37). Moreover, evidence from studies using pure isomers has suggested that the adverse effects attributed to CLA may be due to the t10, c12-CLA isomer(Reference Yamasaki, Nishida and Nou38). In this sense, in the present study the rat CLA intake based on a consumption of 10 g of a 1 % CLA diet per 100 g of rat per d is similar to the CLA doses used in human studies, which ranged from 0·7 to 6·8 g/d(Reference Tricon, Burdge and Williams39). However, the CLA intake of the rats in the present study is slightly higher than the average CLA intake of humans consuming occidental diets, which oscillates between 0·3 and 2·6 g/d(Reference Gómez-Candela40).
The results regarding the c9, t11- and t10, c12-CLA content in the plasma of all supplemented pups indicated that CLA was transferred from dams to pups during gestation and/or suckling. The plasma CLA content in the groups supplemented with the 80:20 CLA mix during gestation and suckling (groups A and B; 5 weeks) was about 1·5 and 2 times higher, respectively, than that of animals supplemented only during suckling (group C; 3 weeks). However, these results cannot demonstrate that CLA transfers through the placenta, since the plasma of newborns was not analysed. Nevertheless, Chin et al. (Reference Chin, Storkson and Albright12) found a 20-fold increase of CLA in the liver of fetuses (at day 20 of gestation) from dams fed a 0·5 % CLA diet during gestation than in fetal livers coming from dams fed a control diet. This, together with our findings of a higher content of c9, t11- and t10, c12-CLA in the milk of rats fed CLA than in those fed the standard diet and the higher content of this isomer in group B plasma than in group C, confirms that CLA is transferred through the placenta to the fetus, as well as through the milk to the pup. The highest plasma CLA values in pups from group B may be due to CLA transfer during suckling from the maternal stores accumulated during gestation, besides supplementation by oral administration. Moreover, differences between groups A and B in the proportion of CLA isomers in pups' plasma may be due to the influence of the food matrix in group A, as milk is one of the major factors that affect PUFA bioavailability(Reference Schram, Nielsen and Porsgaard41).
Despite the fact that an 80:20 isomer mix was used in all supplemented animals, the c9, t11- and t10, c12-CLA proportion was maintained neither in dams' milk (86:14), nor in pups' plasma (group A, 86:14; group B, 93:7; group C, 94:6). The lower proportion of t10, c12-CLA than that initially supplemented is probably due to the faster metabolism of this isomer. It has been reported that t10, c12-CLA activates the β-oxidation system more strongly than c9, t11-CLA in rats; therefore, the former could easily become oxidised(Reference Bowen and Clandinin42). In addition, the immune effects of CLA seen in the present study occurred in the groups supplemented during gestation and suckling, perhaps because of incorporation of c9, t11-CLA to cell membranes and tissue since gestation, while no effects were observed when CLA supplementation was restricted to the suckling period. This might be explained by the fact that c9, t11-CLA has been seen to accumulate to a greater extent than t10, c12-CLA in tissue phospholipids of liver(Reference Macarulla, Fernandez-Quintela and Zabala43), skin and bone of experimental animals(Reference Kavanaugh, Liu and Belury44). Interestingly, the CLA isomer proportion in pups' plasma from group A maintains the proportion found in dams' milk after consuming the CLA diet.
The presence of a small quantity of c9, t11- and not of t10, c12-CLA in the milk and plasma of non-supplemented rats supports the concept that rats produce rumenic acid (c9, t11-CLA), as has been suggested by other authors, by conversion of free linoleic acid by the intestinal bacterial flora(Reference Chin, Storkson and Liu45) or by the endogenous conversion of trans-vaccenic acid, present in vegetable oils contained in standard diets(Reference Santora, Palmquist and Roehrig46).
Rat milk not only transfers CLA to pups, but also antibodies, such as IgG, IgA and IgM. Dams fed the standard diet had a milk concentration pattern of IgG > IgA > IgM, which agrees with that described by Dahlgren et al. (Reference Dahlgren, Ahlstedt and Hanson47). This pattern was maintained in dams fed the CLA diet, although IgG and IgA concentrations were much higher in these rats. It has been described that IgA and IgG present in rat milk have a local mammary gland production(Reference Dahlgren, Ahlstedt and Hanson47), which could be advantageous for rat pups since they absorb these Ig from the ingested milk through the intestinal mucosa and may extend beyond this compartment.
The present study also evaluated the effect of feeding an 80:20 CLA isomer mixture during gestation and suckling in the incipient antibody production of weaning rats. As rats can transmit Ig across the placenta, the rat neonatal model seems more appropriate to investigate the role of CLA on Ig production during pregnancy than the piglet model, due to the six-layered structure of the pig placenta which does not allow the transfer of Ab from the mother to the fetus(Reference Redman48). Results in the rat model show the enhancing properties of CLA on the main in vivo serum Ig isotype, IgG. However, this effect was not observed in all the supplemented groups, a fact that underlines the importance of continuous CLA supplementation during gestation and suckling. This immune-enhancing effect has already been reported in older animals, as by Sugano et al. (Reference Sugano, Tsujita and Yamasaki49) in 7-week-old rats receiving 1 % CLA (50:50 isomer mix), showing an increase in serum IgA, IgG and IgM concentrations and a decrease in IgE. Song et al. (Reference Song, Grant and Rotondo50) reported a similar effect in human subjects following supplementation with a 50:50 isomer mixture for 12 weeks. Nevertheless, Yamasaki et al. (Reference Yamasaki, Kishihara and Mansho51) found no significant effect on serum IgA, IgG or IgM concentrations after feeding 5-week-old rats for 3 weeks with a 50:50 CLA isomer mixture at doses ranging from 0·05 to 0·5 %(Reference Yamasaki, Kishihara and Mansho51). Discrepancies with the study reported by Yamasaki et al. (Reference Yamasaki, Kishihara and Mansho51) might be due to the low doses of CLA tested in that study. Studies carried out in other species during gestation and lactation periods have also reported serum IgG increases(Reference Bontempo, Sciannimanico and Pastorelli52, Reference Rossi, Pastorelli and Bontempo53), in keeping with the present results.
With respect to the serum IgA decrease after CLA supplementation seen in the present study, Turpeinen et al. (Reference Turpeinen, Ylonen and von Willebrand54) also detected an IgA reduction in subjects with birch pollen allergy supplemented for 12 weeks with a CLA mixture containing 63·5 % c9, t11. Yamasaki et al. (Reference Yamasaki, Kishihara and Mansho51) found a slightly lower concentration of serum IgA after feeding the 0·5 % CLA dose, although the reduction was not significant. Considering that IgA is the main Ig in the gut surface (80–90 %) and the fact that systemic IgA-plasma cells continuously migrate to the intestinal wall(Reference Brandtzaeg and Johansen55), the serum IgA decrease should not be interpreted as harmful. In fact, this serum IgA decrease is accompanied by an increase in intestinal IgA in CLA-supplemented weaned rats(Reference Pérez-Cano, Ramírez-Santana and Molero-Luís56). We also have reported an increase in mucosal IgA after a specific challenge in adult rats following this CLA diet through life(Reference Ramirez-Santana, Castellote and Castell57). Moreover, in the present study, CLA supplementation during gestation and suckling enhanced spleen IgM production. These results agree with other studies carried out in older rats, which reported enhancement of splenocyte Ig production after feeding 50:50 CLA isomer mixtures(Reference Sugano, Tsujita and Yamasaki49, Reference Yamasaki, Kishihara and Mansho51). In addition, some authors have reported this increase in old mice after feeding the pure t10, c12-CLA isomer(Reference Yamasaki, Chujo and Hirao58). Although the present results confirmed the immune-enhancing effect of the c9, t11 isomer, we cannot rule out immune functions for t10, c12-CLA.
In the present study, splenocyte proliferation rate and viability did not differ among the groups, partly due to the limited capacity of neonatal spleen lymphocytes to proliferate; at weaning this functional capacity is far less than that of adult rats(Reference Pérez-Cano, Castellote and Marín-Gallén59). Despite the present results, a wide range of PUFA, including CLA, have been found to reduce the mitogen-stimulated proliferation of lymphocytes isolated from several species(Reference Calder, Yaqoob and Thies60, Reference Nunes, Bonatto and de Oliveira61). A lack of effect of dietary CLA on lymphocyte proliferation has also been reported by Kelley et al. (Reference Kelley, Warren and Simon19) after feeding 8-week-old mice with pure isomers for 56 d, and in human subjects either by ingestion of pure isomers(Reference Tricon, Burdge and Kew62) or 50:50 and 80:20 isomer mixtures(Reference Kelley, Warren and Simon19, Reference Albers, van der Wielen and Brink63). Since IL-2 plays a central role in the cell-mediated immune response by regulating proliferative abilities, it could be expected that if CLA does not modify splenocyte proliferation, it will not affect IL-2 production. The present results are consistent with most other studies in animals and human subjects, which have found no significant effects on IL-2 and interferon-γ splenocyte secretion among the dietary groups(Reference Yamasaki, Chujo and Hirao58, Reference Tricon, Burdge and Kew62–Reference Kelley and Erickson64). Nevertheless, some other studies have reported enhancement of IL-2 splenocyte production after CLA supplementation(Reference Yang and Cook65–Reference Turpeinen, von Willebrand and Salminen67). Once again, the assay conditions, particularly the isomer mixtures used, might hold the key to the differing impact of CLA in these studies. Although no significant effects were found on IL-4 and IL-10 splenocyte production, groups A and B exhibited a tendency towards higher values than groups C and D. This IL-10 increase is in line with recent studies showing higher IL-10 production by dendritic cells incubated with c9, t11-CLA after stimulating with lipopolysaccharide(Reference Loscher, Draper and Leavy68). This effect may be related to the anti-inflammatory properties attributed to CLA(Reference Bassaganya-Riera, Reynolds and Martino-Catt26, Reference Bergamo, Maurano and D'Arienzo69). Moreover, by increasing IL-4, CLA might be promoting T helper 2 (Th2) responses, such as modulating antibody production and inhibiting several cellular functions, which is in agreement with the present results regarding CLA enhancement of the principal in vivo and in vitro Ig, whereas CLA did not modify splenocyte proliferation.
In summary, the present study is the first to investigate immune effects after supplementation with an 80:20 c9, t11–t10, c12 CLA isomer mixture during gestation and the entire suckling period. CLA supplementation increased milk IgG and IgA concentrations, serum IgG concentration and spleen IgM production. These effects were only observed in the CLA-supplemented groups from gestation on, and for the longer time period of 5 weeks, a fact that underscores the importance of this supplementation during gestation. Moreover, these data contribute to the scientific evidence pointing to the potential impact of lipid nutrition on immune system development during early life, particularly the effect of the c9, t11-CLA isomer. To better delineate the importance of dietary supplementation during the early stages of life, further studies should focus on the effects of CLA mixtures in early suckling, when the immune system is even more immature, as well as in older weaning animals, when the immune system bears a high antigenic load with ingestion of the first solid diet.
Acknowledgements
The present study was supported in part by the Generalitat de Catalunya (SGCR-2005-00833). C. C. and A. F. acknowledge partial funding for this research from CIBER Epidemiología y Salud Pública (CIBERESP), Spain. C. R.-S. has a grant from the Spanish Agency for International Development Cooperation (Agencia Española de Cooperación Internacional; AECI). The oil used in the study was a gift from Loders Croklaan (Lipid Nutrition, Wormerveer, The Netherlands).
C. C., F. J. P.-C., M. R., M. R.-P. and A. F. designed the study and supervised the experimental work. C. R.-S., F. J. P.-C., C. C., M. C. and A. F. performed the experimental work. C. R.-S. and F. J. P.-C. analysed the data. C. R.-S. and F. J. P.-C. wrote the manuscript, with input from all authors. C. R.-S. and F. J. P.-C. contributed equally to the present study.
The authors have declared no conflict of interest.












