Vitamin C (ascorbic acid, AA) is required for mammalian cell growth and normal cell function, and plays an essential role among the mechanisms of defence against free radicals and as an enzyme cofactor(Reference Wilson1). Together with glutathione, AA is the most abundant reducing agent in cells, and its antioxidant ability is especially important in the liver(Reference Wilson1).
Although the active form of vitamin C is AA, the oxidised form dehydroascorbic acid (DHAA) is also useful because it can be recycled into AA by several cellular mechanisms. Therefore, the limiting factors determining the availability of vitamin C for cells are the abundance of AA+DHAA in the extracellular medium and the ability of the cells to take them up. Since simple diffusion of AA and DHAA across plasma membrane is very poor, carrier-mediated uptake is required(Reference Rose2). This is performed by proteins belonging to two families of transporters: SLC2A (also known as GLUT) and SLC23A (also known as sodium-dependent vitamin C transporter, SVCT)(Reference Wilson1). DHAA is taken up through facilitative GLUT(Reference Rumsey, Kwon and Xu3, Reference Rumsey, Daruwala and Al-Hasani4), namely GLUT1, GLUT3 and GLUT4(Reference Rivas, Zuniga and Salas-Burgos5), whereas AA uptake is carried out by the SVCT; SLC23A1 and SLC23A2 also named SVCT1 and SVCT2 respectively(Reference Daruwala, Song and Koh6, Reference Tsukaguchi, Tokui and Mackenzie7). SLC23A1 is highly expressed in intestine, kidney and liver(Reference Tsukaguchi, Tokui and Mackenzie7), whereas SLC23A2 is essential for AA uptake in metabolically very active cells and in specialised tissues, such as brain, retina, bone and cartilage. In some tissues, such as lung, intestine and placenta, both SLC23A1 and SLC23A2 are highly expressed (for a review see Marin et al. (Reference Marin, Serrano, Perez and Columbus8)). In some polarised cells, such as enterocytes, these transporters have been localised on both apical and basolateral membranes. The presence of SLC23A1 on the apical membrane may account for transepithelial AA absorption from intestinal lumen(Reference MacDonald, Thumser and Sharp9), whereas that of SLC23A2 at the basolateral pole may play a role in AA uptake by enterocytes from blood for their own requirements during fasting periods(Reference Boyer, Campbell and Sigurdson10).
The liver plays a central role in vitamin C homoeostasis because, even in the species capable of synthesising vitamin C, synthesis is restricted to the liver (and pancreas) from where it is distributed to the organism(Reference Rivas, Zuniga and Salas-Burgos5). Rat orthologues Slc23a1 and Slc23a2 also behave as sodium-dependent AA transporters with K m in the micromolar range, as has been also calculated for human isoforms in different expression systems(Reference Savini, Rossi and Pierro11). In rat liver, the expression of these transporters has been reported to be sensitive to changes in the status of oxidative challenge and the administration of antioxidant agents(Reference Perez, Castano and Gonzalez-Buitrago12, Reference Perez, Castano and Jimenez13). This, together with changes in the expression of enzymes accounting for AA regeneration from DHAA, is probably involved in the existence of alterations of vitamin C that have been found in pathological conditions in the liver content, such as in patients with alcoholic liver disease or primary biliary cirrhosis(Reference Beattie and Sherlock14). However, in spite of the importance of the liver in maintaining vitamin C homoeostasis, and the role of this vitamin in the detoxification processes carried out by this organ, available information about the cell-specific distribution of these transporters and their expression under pathological conditions is scarce.
The aim of the present study was therefore to investigate whether chronic liver diseases are accompanied by changes in the expression of SLC23A1 and SLC23A2, whether these transporters are differentially expressed in different cells of the hepatic tissue and whether this is affected by long-term exposure to bile acid-induced oxidative stress. Long-term obstructive cholestasis in rats was used as a model of secondary biliary cirrhosis because it was considered a good example of chronic liver disease accompanied by oxidative stress due to the accumulation of bile acids. This was preferred as an alternative to other available models of liver cirrhosis in rats, which primarily affect hepatocyte metabolism, such as those induced by the administration of toxic compounds.
Experimental methods
Human liver samples
Liver samples were obtained from patients undergoing needle biopsies at the University Hospital, Salamanca, Spain. The research protocol, which conformed to the ethical guidelines of the 1975 Declaration of Helsinki, was reviewed and approved by the human subjects committee of the Hospital, and written consent was obtained in all the cases. Only tissue remaining from samples collected for diagnostic purposes was used in this study. Fresh tissue specimens collected in the operating room were immediately immersed in RNAlater solution (Ambion, BioNova Cientifica, Madrid, Spain). A total of twenty-three liver specimens were selected from patients diagnosed at the Gastroenterology Division of the University Hospital with one of the following liver diseases: hepatocellular cholestasis (n 6), primary biliary cirrhosis I–II (n 5), haemochromatosis (n 4) and non-alcoholic steatohepatitis (n 4). Biopsies with a confirmed diagnosis of absence of any liver disease (n 4) were considered as controls. Age of patients (42–67 years) and sex distribution (approximately same proportion of males and females) were similar in all groups.
Animals
Aged Wistar rats were obtained from Harlan Iberica (Barcelona, Spain), the rest of the animals used in this study, also Wistar rats, were obtained from the Animal House, University of Salamanca. Animals received humane care according to the criteria outlined by the National Academy of Sciences(15). They were used in experimental protocols approved by the ethics committee of the University of Salamanca. Rats were anaesthetised with isoflurane and a sham operation (control) or bile duct ligation was performed(Reference Monte, Morales and Arevalo16). Animals were used 8 weeks after the surgery. To collect tissue samples, we anaesthetised the animals with sodium pentobarbital and immediately immersed the samples in RNAlater solution or in liquid N2 and stored them at − 80°C. Blood was collected from the inferior vena cava and the serum obtained was stored at − 20°C.
Measurement of mRNA and protein levels
Total RNA extraction from human liver samples and rat kidney, duodenum, jejunum, ileum, lung and brain was performed as previously described(Reference Perez, Castano and Gonzalez-Buitrago12, Reference Perez, Castano and Jimenez13). Real-time quantitative PCR was performed using AmpliTaq Gold polymerase in an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Madrid, Spain) with the following thermal conditions: a single cycle of 95°C for 10 min, followed by forty cycles of 95°C for 15 s and 60°C for 60 s. Primer oligonucleotides (Table S1, supplementary material for this article can be found at http://www.journals.cambridge.org/bjn) obtained from Genosys (Cambridge, UK) were designed with the assistance of primer Express software (Applied Biosystems) for cDNA fragments in described sequences, and their specificity was checked using BLAST. The results obtained for each rat sample were normalised using polyubiquitin-C, β-actin and 18S rRNA. The latter was measured with the Taqman Ribosomal Control Reagents kit (Applied Biosystems). Human samples were normalised with the Taqman β-actin Control Reagents kit (Applied Biosystems). Expression levels were calculated as 2− ΔΔCt, where ΔC t was the difference of C t in each sample between the target gene and the normaliser. This was used to calculate ΔΔC t as the difference of this value between control and experimental groups.
For immunohistochemistry analysis goat anti-Slc23a1 (D-19) and anti-Slc23a2 (G-19) polyclonal antibodies, and mouse anti-cytokeratin-7 (CK-7) and anti-glyceraldehyde-3-phosphate dehydrogenase (6C5) monoclonal antibodies from Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-CD163 (ED2) and anti-Reca1 (HIS52) from AbD Serotec (Madrid, Spain); and rabbit polyclonal antibody anti-Oatp1b2 (K22), which was a generous gift from Dr B. Stieger (Zürich, Switzerland), were used. Immunoblotting analyses of liver homogenates were carried out in 7·5 SDS-PAGE. The following dilutions of primary antibodies were used: anti-rat Slc23a1 and Slc23a2 (1:300) and anti-rat glyceraldehyde-3-phosphate dehydrogenase (1:1000). After incubation with appropriate IgG horse-radish peroxidase-linked secondary antibody, immunodetection was performed with ECL (Amersham Pharmacia Biotech, Freiburg, Germany). Analysis of confocal laser-scanning immunofluorescence microscopy (Zeiss LSM 510; Barcelona, Spain) was performed after immunostaining 5 μm rat liver cryosections that had been air-dried for 2 h. Cryosections were fixed in cold methanol. Primary antibodies were diluted in 2 % fetal calf serum in PBS: Slc23a1 (1:100), Slc23a2 (1:100), CK-7 (1:100), CD163 (1:50), Oatp1b2 (1:200) and Reca1 (1:25). Anti-mouse, -rabbit or -goat Alexa 488 or Alexa 594 (Molecular Probes) antibodies were diluted 1:1000. Regarding the specificity of anti-Slc23a1 and anti-Slc23a2 antibodies, in both cases the signal was blocked in displacement assays carried out in western blotting and immunohistochemistry studies using specific peptides (sc-9921P and sc-9927P for Slc23a1 and Slc23a2, respectively) also supplied by Santa Cruz Biotechnology.
Histological examination
Livers from animals of the control group and from animals with bile duct ligation were processed to investigate their histological structure. Cryosections (5 μm) were fixed with neutral buffered formalin for 20 min and stained with haematoxylin and eosin following standard procedures, and were examined by light microscopy.
Biochemical and statistical analyses
Rat serum samples were used for routine biochemical tests that were performed in a Vitros 5,1 FS Chemistry System (Ortho-Clinical Diagnostics, Madrid, Spain), and a Modular DPP analyzer (Roche Diagnostics, Barcelona, Spain). Total bile acid concentrations in serum were assayed by an enzymatic/fluorimetric method(Reference Mashige, Imai and Osuga17). Rat bile samples were collected from the common bile duct, as previously described(Reference Garcia-Marin, Villanueva and Esteller18), and used to determine vitamin C concentrations. After maximal oxidation by incubation with 100 μm CuCl2 for 120 min, vitamin C concentration was measured by HPLC-MS/MS (6410 Triple Quad LC/MS; Agilent Technologies, Santa Clara, CA, USA) using electrospray ionisation in negative mode. When pure commercial AA standard was put in aqueous solution and treated with CuCl2, three major precursor ions were detected. Based on previous reports(Reference Pastore, Rizzetto and Curcuruto19), these were assumed to be DHAA (m/z 173), open-chain hydrated hemiketal (m/z 209) and the methanolic derivative of this compound (m/z 241). Fragmentation of these ions results in all three cases in (5R)-hydroxymethyl-2,3,4(5H)-furantrione (m/z 143). Thus, for quantitative analyses, data acquisition in multiple reaction-monitoring modes using m/z 143 as product ion was carried out. After performing an ANOVA test, we calculated the statistical significance of differences among groups using the Student's t test or the Bonferroni method of multiple-range testing, as appropriate.
Results
Changes in SLC23A1 and SLC23A2 expression in human liver disease
The amount of SLC23A1 mRNA was enhanced in several liver diseases (haemochromatosis>hepatocellular cholestasis ≥ primary biliary cirrhosis>non-alcoholic steatohepatitis). The expression of SLC23A2 was also enhanced, although the order of mRNA abundance was different (primary biliary cirrhosis>haemochromatosis>hepatocellular cholestasis ≥ non-alcoholic steatohepatitis). In contrast, no significant change was observed in the mRNA levels of OATP1B1, OATP1B3 and OATP1A2 (Fig. 1).

Fig. 1 Expression of sodium-dependent transporters of vitamin C (a) SLC23A1 and (b) SLC23A2 in comparison with the transporters of organic anions (c) OATP1A2, (d) OATP1B1 and (e) OATP1B3 in human liver. Steady-state levels of mRNA were measured by real-time quantitative RT-PCR from biopsies of human liver tissue collected from control subjects with a confirmed diagnosis of the absence of liver disease (n 4) and patients with hepatocellular cholestasis (n 6), primary biliary cirrhosis I–II (n 5), haemochromatosis (n 4) and non-alcoholic steatohepatitis (n 4). Values are means, with their standard errors represented by vertical bars. Samples were analysed in triplicate, and expressed as the relative abundance of each mRNA in control liver. α-Actin mRNA was used as the normaliser. * Mean values were significantly different from the control using the Bonferroni method of multiple-range testing (P < 0·05). 1, control; 2, cholestasis; 3, primary biliary cirrhosis; 4, heamochromatosis; 5, non-alcoholic steatohepatitis.
Ontogeny–associated changes in the mRNA of Slc23a1 and Slc23a2 in rat liver
In spite of limitations in extrapolating to human subjects of results obtained in laboratory animals, their use often offers the possibility to carry out more detailed investigations. In the present study we have taken advantage of the existence, in rats, of two orthologues for human SLC23A isoforms. Obstructive cholestasis maintained for 2 months in rats was chosen as the experimental model. Before carrying out the experiments, the time course of the expression of Slc23a1 and Slc23a2 during the rat life was investigated.
To look for a gene to normalise mRNA measurements, we determined the expression levels of polyubiquitin-C, β-actin and 18S rRNA in the liver of rats with different ages. During ontogenic development levels of polyubiquitin and β-actin do not change substantially, although levels of 18S RNA were more stable (data not shown). This was the reason why we used 18S RNA to compare values over the lifetime in rats. However, since values of C t for β-actin were closer to those of target genes, this was preferred as the normaliser when different experimental groups of individuals of the same age were compared. In these cases, similar expression levels of β-actin were found.
The ontogeny-associated patterns of three important organic anions transporters in rat liver, Oatp1a1, Oatp1a4 and Oatp1b2(Reference Kullak-Ublick, Stieger and Meier20), studied here for comparison, differed in some way or another (Fig. 2). Their expression levels were low at birth, but then increased progressively over the first (Oatp1a4) or the first and second (Oatp1a1 and Oatp1b2) months of life before adult levels were attained. During senescence, a significant decrease in expression levels of Oatp1b2 was found, whereas those of Oatp1a1 and Oatp1a4 were maintained (Fig. 2). The expression of Slc23a1 was low at birth and along the first 2 weeks of life. Then, it increased up to adult levels by the third postnatal week, was maintained over adulthood, and decreased during senescence (Fig. 2). In contrast, Slc23a2 mRNA levels at birth were similar to those found in adults and remained unchanged during senescence (Fig. 2).
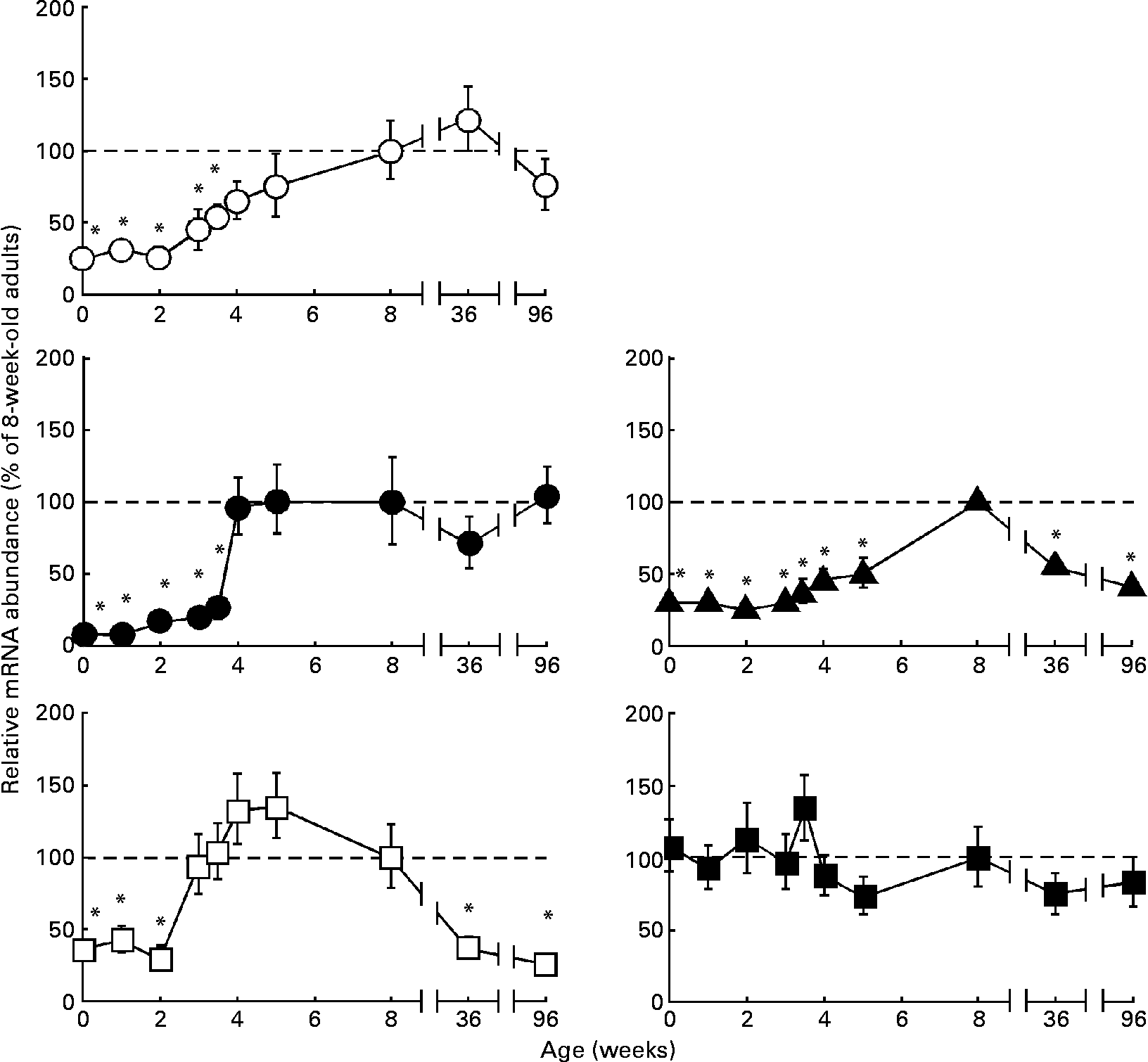
Fig. 2 Ontogeny-associated changes in the expression of transporters of vitamin C in rat liver. Determination by real-time quantitative RT-PCR of the relative abundance of the mRNA of Oatp1a1 (○), Oatp1a4 (●), Oatp1b2 (▲), Slc23a1 (□) and Slc23a2 (■) during postnatal rat development, adulthood and senescence. Values are means, with their standard errors represented by vertical bars (n 6 animals, collected from three litters). * Mean values were significantly different from those of the group of adult rats (8-week-old) by the Bonferroni method of multiple-range testing (P < 0·05).
Comparative expression of Slc23a1 and Slc23a2 in rat organs
To evaluate the relevance of changes in hepatic expression of vitamin C transporters in the overall picture of the different organs involved in the handling of the vitamin, we compared the expression levels of Slc23a1 and Slc23a2 in kidney, intestine, lung and brain with those of liver. As previously described(Reference Kuo, MacLean and McCormick21) both Slc23a1 and Slc23a2 are expressed in rat liver. Only kidneys presented Slc23a1 levels similar to the liver (Table 1), while the rest of tissues studied here had lower levels than hepatic tissue. Regarding Slc23a2 (Table 1), the expression levels were similar in liver and kidney. The expression levels in brain, duodenum and lung were higher, whereas those in jejunum and ileum were lower.
Table 1 Expression levels of Slc23a1 and Slc23a2 in rat organs
(Mean values with their standard errors, n 5)
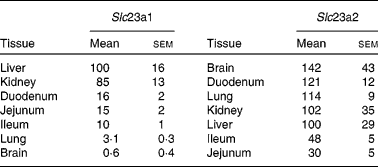
The abundances of mRNA were determined by real-time RT-PCR and are expressed as compared to that in the liver of the same animal (100 %).
Effect of long-term cholestasis on Slc23a1 and Slc23a2 expression
We have previously(Reference Perez, Macias and Duran22) shown that there is a marked oxidative stress after 1 week of complete obstructive cholestasis in rats. To simulate chronic liver diseases in human subjects with long-term exposure to high levels of bile acids, and hence presumably oxidative stress, we have, in the present study, prolonged obstructive cholestasis for 8 weeks in young adult rats (8-week-old). This situation induced high mortality (20 %), lower gained body weight ( − 14 %) and hepatomegaly ( × 3). Changes in serum biochemical parameters typical of cholestasis were found (Table 2). Signs of renal damage, such as increased levels of serum urea and creatinine (Table 2), were also observed.
Table 2 Serum parameters of control and bile duct-ligated rats for 8 weeks
(Mean values with their standard errors, n 5)
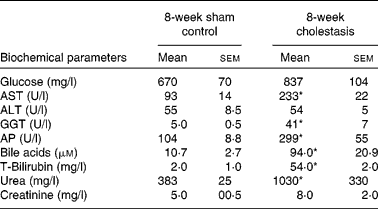
AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, γ-glutamyl transpeptidase; AP, alkaline phosphatase.
* Mean values were significantly different between cholestatic rats and sham-operated animals (P < 0·05).
A marked decrease in mRNA levels of all Oatps studied here was found 8 weeks after bile duct ligation (Fig. 3). Regarding mRNA levels of Slc23a1 and Slc23a2, these were significantly decreased and increased, respectively (Fig. 3). These changes were consistent with those observed when protein levels were measured (Fig. 3).

Fig. 3 Effect of long-term (8 weeks) obstructive cholestasis due to bile duct ligation on liver mRNA levels of Oatp1a1, Oatp1a4, Oatp1b2, Slc23a1 and Slc23a2, measured by real-time quantitative RT-PCR. Values are means, with their standard errors represented by vertical bars for four samples analysed in triplicate for each experimental group, expressed as the relative abundance of mRNA compared with control rat liver used as the calibrator. α-Actin mRNA was used as the normaliser. Representative samples of liver homogenate were analysed by western blotting to determine the effect of 8-week bile duct ligation on liver protein expression of Slc23a1 and Slc23a2. * Mean values were significantly different with control using the Student's t test (*P < 0·05). □, control; ■, cholestasis.
Detection of subtissular distribution of Slc23a isoforms in rat liver
In control livers, positive staining of Slc23a1 in hepatocytes was co-localised with Oatp1b2 immunolabeling (Fig. 4(a)–(c)). Slc23a1 was also detected in bile duct cells (Fig. 5(a)–(c)), endothelial cells (Fig. 6(a)–(c)), Kupffer cells (Fig. 7(a)–(c)) and stellate cells (Fig. 8(a)–(c)), as suggested by comparative localisation with CK-7 as a marker for cholangiocytes, Reca1 for endothelial cells, CD163 for Kupffer cells and α-smooth muscle actin for stellate cells, respectively. Interestingly, in cholangiocytes, Slc23a1 was apparently located at the apical membrane (Fig. 5(a)–(c)).

Fig. 4 Cell-specific distribution and cholestasis-associated changes in the expression of transporters of vitamin C in rat hepatocytes. Immunofluorescence of Slc23a1 (a–f) or Slc23a2 (g–l) and Oatp1b2 in control (a–c, g–i) and 8-week obstructive cholestasis (d–f, j–l) rat liver cryosections. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Bars = 20 μm.

Fig. 5 Cell-specific distribution and cholestasis-associated changes in the expression of transporters of vitamin C in rat biliary epithelial cells. Immunofluorescence of Slc23a1 (a–f) or Slc23a2 (g–l) and CK-7 in control (a–c, g–i) and 8-week obstructive cholestasis (d–f, j–l) rat liver cryosections. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Bars = 20 μm, except panels f and l, where bar = 10 μm. CK-7, cytokeratin-7.

Fig. 6 Cell-specific distribution and cholestasis-associated changes in the expression of transporters of vitamin C in rat liver endothelial cells. Immunofluorescence of Slc23a1 (a–f) or Slc23a2 (g–l) and Reca1 as marker for endothelial cells in control (a–c, g–i) and 8-week obstructive cholestasis (d–f, j–l) rat liver cryosections. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Bars = 20 μm.

Fig. 7 Cell-specific distribution and cholestasis-associated changes in the expression of transporters of vitamin C in rat liver Kupffer cells. Immunofluorescence of Slc23a1 (a–f) or Slc23a2 (g–l) and CD163 in control (a–c, g–i) and 8-week obstructive cholestasis (d–f, j–l) rat liver cryosections. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Bars = 20 μm.

Fig. 8 Cell-specific distribution and cholestasis-associated changes in the expression of transporters of vitamin C in rat liver stellate cells. Immunofluorescence of Slc23a1 (a–f) or Slc23a2 (g–l) with α-smooth muscle actin (α-SMA), in control (a–c, g–i) and 8-week obstructive cholestasis (d–f, j–l) rat liver cryosections. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole. Bars = 20 μm.
Immunoreactivity for Slc23a2 was strong in endothelial cells (Fig. 6(g)–(i)) and Kupffer cells (Fig. 7(g)–(i)), weak in hepatocytes (Fig. 4(g)–(i)) and stellate cells (Fig. 8(g)–(i)) and could not be detected in cholangiocytes (Fig. 5(g)–(i)). The above cell distribution was observed in all liver specimens studied (n 4). No signal was observed when the primary antibody was omitted (data not shown).
To understand the potential role of a SVCT at the luminal membrane of cholangiocytes (Fig. 5(a)–(c)), we investigated the presence of vitamin C in bile. Using HPLC-MS/MS, we carried out measurement of vitamin C in basal rat bile. The present study revealed that under physiological circumstances rat bile contains approximately 10–20 (15 (sem 4) μM; n 5) vitamin C.
Effect of long-term cholestasis on subtissular Slc23a1 and Slc23a2 expression
Long-term cholestasis produced a severe disorganisation of the liver parenchyma, typical of secondary biliary cirrhosis, with areas of ductular proliferation, leucocytes infiltration and fibrosis (Fig. S1, supplementary material for this article can be found at http://www.journals.cambridge.org/bjn). This was accompanied by a weaker Slc23a1 immunoreactivity in the plasma membrane of hepatocytes (Fig. 4(d)–(f)), cholangiocytes – even in areas of ductular proliferation (Fig. 5(d)–(f)) – and endothelial cells (Fig. 6(d)–(f)). Only Kupffer cells showed a clear staining for Slc23a1 in cholestasis (Fig. 7(d)–(f)).
Regarding Slc23a2 expression, a strong immunoreactivity in the liver parenchyma was found. This was not presumably due to hepatocytes because it did not co-localise with Oatp1b2 (Fig. 4(j)–(l)). The signal due to Slc23a2 expression was weak in proliferating bile ducts (Fig. 5(j)–(l)) and endothelial cells (Fig. 6(j)–(l)). The strongest immunopositive signal was confined to the areas with an enhanced abundance of Kupffer cells (Fig. 7(j)–(l)).
In stellate cells, a decreased staining for Slc23a1, together with a marked increase in that for Slc23a2, was observed in rats with cholestasis (Fig. 8(j)–(l)).
Discussion
An interesting and initial finding of the present study was that levels of SLC23A1 and SLC23A2 were up-regulated in the liver of patients with several chronic liver diseases, which share the characteristic of being under a maintained and prolonged oxidative stress. The enhancement in the expression of both isoforms was less marked in non-alcoholic steatohepatitis, whereas expression of SLC23A1 was more strongly up-regulated in haemochromatosis and that of SLC23A2 in primary biliary cirrhosis. Although the limited sample size recommends cautious interpretation of these results, the results were consistent with a differential response of these genes to stimuli and the existence of heterogeneous expression of SLC23A1 and SLC23A2 in cells of liver parenchyma, as was later confirmed in rat liver tissue. The dissimilar ontogeny-related expression pattern observed for Slc23a1 and Slc23a2 in rat liver supports the hypothesis of different transcriptional regulation for both genes.
Regarding the relative abundance of these transporters in different organs, the results suggest that, at least in rats, both Slc23a1 and Slc23a2 in liver and kidney may play a key role in vitamin C homoeostasis. In the intestine, Slc23a2, mainly in duodenum, seems to be the major isoform involved in vitamin C absorption. In organs with a role as consumers of this vitamin, such as brain and lung, the expression of Slc23a2 is higher than in liver.
Long-term obstructive cholestasis in rats was used as a model of chronic bile acid accumulation, which is known to be accompanied by oxidative stress(Reference Perez and Briz23). Obstructive cholestasis for 3 or 7 d has previously been found to induce decreased expression of rat liver Oatps(Reference Donner, Schumacher and Warskulat24). The authors of this study found a decreased expression of Oatp1a1, Oatp1a4 and Oatp1b2 at 3 d of bile duct ligation, whereas at 7 d the expression of Oatp1b2 was restored. In other models of cholestasis in rats, a decreased expression of the three Oatp isoforms in liver was also observed(Reference Geier, Wagner and Dietrich25). In long-term cholestasis studied here, these changes were more marked.
Regarding Slc23a(1/2), the results obtained here confirm those of recent studies by our group, showing that cholestasis imposed for the last 7 d of rat pregnancy induced an enhanced expression of Slc23a2 in the maternal and fetal livers and in the placenta as part of a complex defensive response to the oxidative stress brought about by the accumulation of biliary compounds(Reference Perez, Castano and Gonzalez-Buitrago12). As found in the previous studies(Reference Perez, Castano and Gonzalez-Buitrago12, Reference Perez, Castano and Jimenez13), overall liver Slc23a1 expression was less sensitive than Slc23a2 to 1-week cholestasis, whereas after long-term cholestasis the overall expression of Slc23a1 in the whole liver tissue was markedly decreased.
The response of the transcriptional regulation of Slc23as to cholestasis in rats was not similar to that found for SLC23A(1/2) in human liver with hepatocellular cholestasis, in which the expression of both isoforms was enhanced (SLC23A1>SLC23A2). This was consistent with data obtained using HepG2 hepatoblastoma cells, in which bile acids induced only SLC23A2 expression(Reference Perez, Castano and Jimenez13). Interestingly, in HepG2 cells, bilirubin was able to induce the expression of both SLC23A1 and SLC23A2(Reference Perez, Castano and Jimenez13). Other diseases characterised by a certain degree of retention of bile components and/or oxidative stress, such as haemochromatosis, primary biliary cirrhosis and non-alcoholic steatohepatitis, were also accompanied by an increased expression of SLC23A1 and SLC23A2 in human liver.
To understand the role of both isoforms in liver physiology and pathophysiology, we carried out studies on subtissular distribution. Since Slc23a1 was detected in all liver cell types, it was surmised that upon ontogenic maturation this would guarantee the ability of all liver cells to obtain vitamin C from external sources.
Although human SLC23A1 and SLC23A2 share 66 % sequence identity, these isoforms usually localise differently in the apical and basolateral membranes of epithelial cells(Reference Daruwala, Song and Koh6, Reference Marin, Serrano, Perez and Columbus8). This is because SLC23A2 contains a basolateral targeting sequence in the N terminus, which is conserved among mammalian SLC23A2 forms. In contrast, the less conserved N terminus of SLC23A1 is not required for apical localisation(Reference Varma, Sobey and Campbell26). Indeed, co-localisation of Oatp1b2 indicates the presence of Slc23a1 at the basolateral plasma membrane of rat hepatocytes, which suggests that this isoform could be mainly responsible for the uptake of the AA that reaches sinusoidal blood after being taken up by the intestine. The findings that Slc23a1 is expressed at the apical membrane of cholangiocytes, together with the presence of vitamin C in bile at micromolar concentrations, suggest that these cells could be able to carry out the re-uptake of vitamin C from bile.
It is believed that SLC23A1 is involved in whole-body homoeostasis of vitamin C, whereas SLC23A2 protects metabolically active cells against oxidative stress. Whether these isoforms are functional exchangeable in non-polarised cells is not known. Although there is a marked diversity in values of K m calculated for these isoforms, most studies agree that SLC23A2 is a carrier with higher affinity for AA. However, differences are often slight and values of K m for both transporters are in the 10–100 μm range(Reference Savini, Rossi and Pierro11). It should be considered that normal values for serum concentrations are approximately 60 μm. These are much higher than normal concentrations of DHAA (approximately 2 μM), which is taken up by GLUT1, GLUT3 and GLUT4(Reference Rumsey, Kwon and Xu3–Reference Rivas, Zuniga and Salas-Burgos5). This implies that although a role of GLUTs in vitamin C handling by the cells does probably exist, under physiological circumstances, this is expected to be minor as compared with that of SLC23As. Differential regulation of SLC23A1 and SLC23A2 expression regarding both cell and isoform specificities may serve for preferential accumulation of AA where it is more needed(Reference Savini, Rossi and Pierro11). The results of the present study support this hypothesis. Thus, in addition to Slc23a1, endothelial cells and Kupffer cells also expressed Slc23a2. AA is essential for preventing endothelial dysfunction, because vitamin C is needed for type IV collagen synthesis and enhancement of cell proliferation(Reference Aguirre and May27). Moreover, Kupffer cells need AA to buffer the oxidative stress related to their activation and to enhance several aspects of their function(Reference Aguirre and May27).
In long-term cholestasis, the appearance of signs of liver parenchyma disorganisation was consistent with a parallel loss of Oatp1b2 and Slc23a1. The change in the overall expression of Slc23a1 in rat liver (decreased) and SLC23A1 in human liver (increased) may result from a dissimilar combination of changes in the predominance of different cell types in whole tissue on the one hand and the expression level of this isoform in different cell types on the other.
The proliferation of biliary ducts in the group with cholestasis was accompanied by increased expression of CK-7+cells, i.e., presumably cholangiocytes, which also expressed Slc23a1. In endothelial cells (Reca+), the intensity of staining for Slc23a1 and Slc23a2 did not seem to be affected by cholestasis. A marked increase in the abundance of Kupffer cells was observed in rats with cholestasis, as revealed by the enhanced abundance of CD163+cells. These cells were also positive for Slc23a1 and Slc23a2. Thus, increased expression of Slc23a2 in whole liver may be partly because of an enhanced abundance of cells expressing this isoform, mainly Kupffer cells. This may also be the cause of the enhanced abundance of SLC23A2 in human liver diseases studied here.
In conclusion, although we cannot rule out that other factors such as inflammatory mediators may also affect SLC23A expression, these results suggest that the increased expression of both SLC23A(1/2) isoforms may contribute to a protective mechanism of the liver under pathological conditions, such as accumulation of bile acids. Moreover, liver cell heterogeneity may play an important role in the expression and transcriptional regulation of SLC23A1 and SLC23A2, which may determine the overall ability of this organ to take up vitamin C in health and diseases. In both cases, an additional level of complexity that may affect vitamin C homoeostasis and hence nutrient recommendations is based on the existence of differences in population-specific variants for both SLC23A1 and SLC23A2(Reference Eck, Erichsen and Taylor28).
Acknowledgements
The authors thank Dr L. Muñoz, J. F. Martin and J. Villoria for care of the animals. Technical help by E. Keck and revision of the English spelling, grammar and style of the manuscript by N. Skinner are also gratefully acknowledged. This study was supported in part by the Instituto de Salud Carlos III, FIS (grant no. PI051547); the Junta de Castilla y León (grant nos. SA021B06, SA033A08, SAN673SA07/08, BIO39/SA27/10 and GR75-2008), Spain; the Ministerio de Ciencia y Tecnología, Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica (grant nos. SAF2009-08493 and SAF2010-15517), Spain and the Fundación Investigación Médica, Mutua Madrileña (Convocatoria VI, 2009), Spain. The group is member of the Network for Cooperative Research on Membrane Transport Proteins (REIT), co-funded by the Ministerio de Educación y Ciencia, Spain and the European Regional Development Fund (ERDF) (grant no. BFU2007-30688-E/BFI) and belongs to the CIBERehd (Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas) Instituto de Salud Carlos III, Spain. R. I. R. M. and J. J. G. M. designed the study; R. I. R. M., J. J. G. M., C. H. and S. C. D. J. carried out experimental work; R. I. R. M., F. J. and F. G. S. M. performed study on human samples. All the authors were involved in discussion of the results. R. I. R. M. and J. J. G. M. prepared the manuscript. All the authors revised the manuscript. The authors confirm that there are no conflicts of interest.












