The gastrointestinal tract is a complex system that actively participates in the protection of the host against aggressions from the external environment. The defence system of the gut comprises three components, namely the microflora, the mucosal barrier and the local immune system, which need to be in permanent contact and continuously communicating with each otherReference Bourlioux, Koletzko, Guarner and Braesco1. The intestinal epithelial cells act as an essential link in communicating with the immune cells in the underlying mucosa and the microflora in the lumen via the expression of regulatory cytokines.
The gastrointestinal epithelium is covered by a protective mucus gel composed primarily of mucin that is synthesised and secreted by goblet cellsReference Gaskins and Deplancke2. One of the major components of human mucin glycoproteins and glycolipids is l-fucose, a six-carbon deoxyhexose having a galacto-configurationReference Albermann, Distler and Piepersberg3. l-Fucose can also be found at the terminal position of many cell-surface oligosaccharide ligands that mediate cell-recognition and adhesion-signalling pathwaysReference Mäki and Renkonen4. Fucose is also present in certain foods, such as in Undaria pinnatifida, a brown seaweed which is one of the richest known sources of fucoseReference Mori, Kamei, Nishide, Nisizawa, Hoppe and Levring5.
Research has shown that fucose can be released from mucin through the hydrolytic activity of pathogens such as Vibrio cholerae Reference Macfarlane, Hay and Gibson6 and Candida albicans Reference de Repentigny, Aumont, Bernard and Belhumeur7 to facilitate the dispersion of the pathogens along the intestinal tract, and to aid in their penetration of the mucin barrier. Some indigenous bacteria such as those from the genera Bacteriodes Reference Bourlioux, Koletzko, Guarner and Braesco1, Ruminococcus and Bifidobacterium Reference Hoskins8 were found to degrade mucin, leading to the release of free fucose into the intestinal lumen, which was then utilised as an energy source. This would be advantageous for the intestinal colonisation of fucose-utilising bacteria.
However, it is still unclear if free fucose released from intestinal mucin and consumed food through the digestive activity of the intestinal microflora and/or the host plays any role in the microbe–host interaction in the gastrointestinal tract.
The aim of the present study was to determine the immunomodulating effect of fucose in an in vitro differentiated Caco-2 cell model.
Materials and methods
Epithelial cells and culture conditions
Caco-2 cells were obtained from the American Type Culture Collection (Rockville, MD, USA). This human colon adenocarcinoma cell line was cultured in minimal essential medium (MEM; Gibco-BRL, Grand Island, NY, USA) that contained 25 mm-glucose, 20 % (v/v) heated inactivated fetal calf serum (Gibco-BRL) and 1 % non-essential amino acids (Gibco-BRL). Cells were grown at 37°C in an atmosphere of 5 % (v/v) CO2 in air. The cells were allowed to differentiate into enterocytes by seeding at a density of 1 × 105 cells/well in twenty-four-well tissue culture dishes (Nunc, Roskilde, Denmark) and culturing them for 14 d, changing the medium every alternate day.
Experimental protocols
Differentiated Caco-2 cells were grown in medium supplemented with or without 0·5 % (w/v) l-fucose and incubated for 24 h at 37°C. The culture medium was collected thereafter and stored at − 20°C. The cultured Caco-2 cells were also harvested for subsequent RNA extraction.
Ribonucleic acid extraction and cDNA array analysis
RNA was isolated from cells in three independent experiments for each treatment using Trizol® reagent (Gibco-RBL). The integrity of the RNA was analysed in Nanodrop®ND-1000 (Nanodrop Technologies, Wilmington, DE, USA). The RNA was processed, labelled and hybridised to the non-radioactive human common cytokines GEArray Q-series Kit (Superarray Inc., Bethesda, MD, USA) according to the cDNA GEArray® Q and S Series Kits user manual (version 8.5; Superarray Inc.). The probed arrays were scanned by flatbed scanner (Amersham Biosciences, Little Chalfont, Bucks, UK) and analysed by MagixScanner software (Amersham Biosciences). The GEArray Expression Analysis Suite was used for calculation of absolute and comparison data.
Reverse transcription-polymerase chain reaction
Semi-quantitative reverse transcription was carried out using an oligo(dT) primer, Superscript™ III RT (Invitrogen, Carlsbad, CA, USA) and 1 μg total RNA from at least three independent experiments. The resulting cDNA was amplified using the Platinum®Taq DNA Polymerase kit (Invitrogen) and primers designed. PCR were optimised to the linear amplification range and run in thirty amplification cycles of denaturation (94°C for 30 s), annealing (55°C for 30 s) and extension (72°C for 1 min), followed by a final extension (72°C for 7 min). The PCR products were visualised by agarose gel electrophoresis, and β-actin was used as an endogenous control. Table 1 showed the primer sequences of the various genes assayed for in the RT-PCR analysis.
Table 1 Primer sequences used in reverse transcription-polymerase chain reaction analysis

TGF, transforming growth factor; TLR, toll-like receptor; TRAF, TNF receptor-associated factor; Mekk, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase; RelA, ppGpp synthetase.
The bands obtained after gel electrophoresis were analysed using densitometry software (Gene Tools; Syngene, Cambridge, Cambs, UK), a semi-quantitative method to compare gel band intensities. The PCR reactions were normalised to the expression of the gene encoding β-actin. Thereafter, the samples were compared against the control well (without fucose) to obtain the differences in their gene expression.
Cytokine protein assay
The quantification of cytokines TNF-α, IL5, IL8, IL12 and IL17 was performed by a Bio-Plex Human 5-Plex Assay (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer's protocol. The amounts of various cytokines in the cell-culture supernatant fractions were quantified in samples obtained from at least three different experiments.
High-performance liquid chromatography analysis of fucose
The fucose concentrations in the samples collected were determined by HPLC (Perkin Elmer series 200; Boston, MA, USA). The isocratic HPLC separation was performed using the 300 × 7·8 mm aminex HPX 87C column (Bio-Rad Laboratories). The mobile phase was reverse osmosis water obtained from the Sartorius arium 611VF water purification system (Sartorius, Goettingen, Germany). The system was operated at a flow rate of 0·8 ml/min at 85°C with operating pressure of 390 pounds per square inch (psi). After each sample analysis, the mobile phase was allowed to run for 10 min. Triplicates were done for each condition.
Statistical analysis
All statistical analyses in the present study were carried out using SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). The independent-samples t test was used to compare the means for two groups of data. If the significance value for the Levene test was high (typically greater than 0·05), equal variances for both groups were assumed. A low significance value for the t test (typically less than 0·05) indicated significant difference between the two group means. In addition, if the CI for the mean difference did not contain zero, this indicated that the difference was significant.
Results
Human common cytokine arrays
The topmost single layer of intestinal epithelial cells is an integral and essential component of the mucosal immune system. In the present study, we analysed the gene expression in human intestinal epithelial cells (differentiated Caco-2 cells) incubated with or without fucose. After 24 h of incubation, total RNA harvested were hybridised to microarrays containing cDNA of ninety-six selected human cytokine genes.
Almost 70 % of the genes represented on the microarray were detected in Caco-2 cell culture grown in normal MEM. The expressions of the genes were considered to be significantly increased or decreased (P < 0·05; Student's t test) when they showed an average change of at least two-fold in the experimental group (with fucose).
The up regulated genes in the cells treated with MEM supplemented with 0·5 % fucose (Fig. 1) were associated with the development and proliferation of immune cells as well as the modulation of both innate and adaptive immune immunity, involved in both cellular and humoral immunity. These genes included TNFSF5, TNFSF7, TNF-α, IL5, IL12, IL17, IL18, bone morphogenetic protein (BMP)-4, thromobopoietin and erythropoietin.

Fig. 1 Up regulation of human common cytokine genes in differentiated Caco-2 cells grown in 0·5 % fucose compared with cells grown in the absence of fucose. Positive values greater than 2 indicate up regulation by fucose. Values are mean-folds of increase in gene expression from three individual experiments as compared with the control group, with standard deviations represented by vertical bars. BMP, bone morphogenetic protein; EPO, erythropoietin; FGF, fibroblast growth factor; THPO, thrombopoietin.
Besides the immune genes found up regulated in the cells incubated with fucose, another set of genes responsible for epithelial cell restitution was found to be up regulated as compared with the control group. These included fibroblast growth factor (FGF)-2 and transforming growth factor (TGF)-β.
The four selected cytokine genes, namely IL5, IL12, IL17 and TNF-α, detected by the microarrays were confirmed using the Bio-Plex Human 5-Plex Assay (data shown in the Secretion of cytokines by Caco-2 cells section). Cytokine IL8 was selected for it is an inflammation marker, although there was no significant variation in the gene expression assay. Protein detection assays for BMP, erythropoietin, FGF, IL18 and thrombopoietin are not available. The gene expression could be further confirmed by other techniques such as quantitative real-time PCR, and this will be performed in a future study.
Reverse transcription-polymerase chain reaction
We first characterised the array of genes associated with the toll-like receptor (TLR)-2 signalling pathway in the control cultures of Caco-2 cells grown in normal MEM. Low levels of mRNA of TLR2, TNF, TGF-β, TRAF6, RelA and Mekk3 were found in the control cells (Fig. 2).

Fig. 2 RT-PCR results. Lane 1 is the TrackIt™ 1 kb Plus DNA ladder (Invitrogen, Carlsbad, CA, USA). Lane 2 denotes samples extracted from Caco-2 cells grown in normal minimal essential medium (MEM) (control group). Lane 3 denotes Caco-2 cells grown in MEM supplemented with 0·5 % fucose. Lane 4 denotes a negative PCR control done using sterile water instead of the extracted RNA as the template to check for contamination of reagents. RelA, v-rel reticuloendotheliosis viral oncogene homolog A; TRAF, TNF receptor-associated factor; Mekk, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase; TLR, toll-like receptor.
When the Caco-2 cells were incubated with fucose, the TLR2 gene expression was up regulated by 10-fold as compared with the control group (Figs. 2 and 3). The expressions of genes downstream of TLR2 in the TLR2 signalling pathway such as TRAF6, Mekk3, RelA, TNF and TGF-β were also elevated significantly (Figs. 2 and 3). Incubating Caco-2 cells with medium supplemented with fucose resulted in an approximate 6-fold increase in the mRNA levels for TNF, a 5-fold increase in the Mekk3 mRNA levels, a 50-fold increase in the message levels of RelA, a 6-fold increase in the TNF mRNA levels and a 10-fold increase in the TGF-β mRNA levels in comparison with the control cells (Figs. 2 and 3).
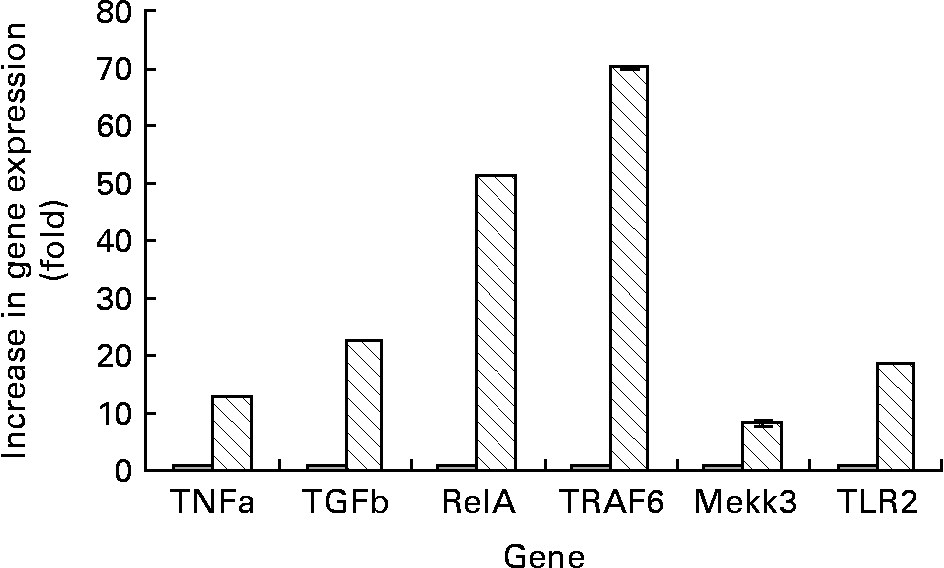
Fig. 3 Increase of expression of various genes when differentiated Caco-2 cells were incubated with 0·5 % fucose (![]() ), using normal untreated Caco-2 cells as the control (
), using normal untreated Caco-2 cells as the control (![]() ). Values are mean-folds of increase in gene expression from three independent experiments as compared with the control group. The standard deviations were too minute in value to express. TGF, transforming growth factor; RelA, ppGpp synthetase; TRAF, TNF receptor-associated factor; Mekk, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase; TLR, toll-like receptor.
). Values are mean-folds of increase in gene expression from three independent experiments as compared with the control group. The standard deviations were too minute in value to express. TGF, transforming growth factor; RelA, ppGpp synthetase; TRAF, TNF receptor-associated factor; Mekk, mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase; TLR, toll-like receptor.
Secretion of cytokines by Caco-2 cells
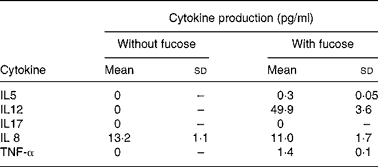
The protein endproducts of the detected genes were assayed using the available kitReference Greenbaum, Colangelo, Williams and Gerstein9. Concentrations of five selected cytokines, IL5, IL8, IL12, IL17 and TNF-α, in the culture medium were assayed. It is known that some cytokines such as IL8 and TNF-α are expressed and secreted from intestinal epithelial cellsReference Satsu, Matsuda, Toshimitsu, Mori, Mae, Tsukagawa, Kitahara and Shimizu10. In cell cultures incubated with normal MEM, none of the tested cytokines was detected except for IL8 where a concentration of 13·2 (sd 1·1) pg/ml was detected.
It was found that in the presence of fucose, 0·3 (sd 0·05) pg IL5 protein/ml and 49·9 (sd 3·6) pg IL12 protein/ml were detected (Table 2). This is in agreement with the level of gene expression of IL5 and IL12 in the human cytokine gene microarray assay (Fig. 1). This shows that these cytokines were not produced and secreted under normal conditions and that their production and secretion are regulated by fucose.
Table 2 Protein levels of selected cytokines secreted*
(Mean values and standard deviations)
* Obtained from at least three different experiments.
The gene expressions of IL17 were found to be up regulated in the conditions where the cells were treated with fucose (Fig. 1); however, the protein of IL17 was not detected (Table 2). This might be due to the poor correlation of mRNA expression and protein synthesis. It is also possible that the expression levels of the IL17 cytokine were below the detection limit of the method.
In line with up regulated TNF-α gene expression in the presence of fucose (Fig. 1), 1·37 pg TNF-α/ml was detected in the culture supernatant fraction (Table 2).
Since Caco-2 cells originate from colon adenocarcinoma, it is not surprising to detect the pro-inflammatory cytokine IL8 protein in differentiated Caco-2 cells grown in normal media (Table 2). The secreted protein level of IL8 detected for the differentiated Caco-2 cells incubated in media supplemented with fucose was significantly lower as compared with the control group (Table 2).
Fucose uptake by Caco-2 cells
After 24 h of incubation with media supplemented with 5 mg fucose/ml, the average concentration of free fucose in the supernatant fractions collected was 4·7 mg/ml. This suggested that the differentiated Caco-2 cells did not use exogenous fucose significantly. Hence, the immune responses of differentiated Caco-2 cells observed in the presence of fucose were a result of the interaction of free fucose with the Caco-2 cells rather than the cells' metabolism of the sugar.
Discussion
When differentiated enterocyte-like Caco-2 cells were treated with fucose, the expression of fourteen cytokine genes was found increased and none of the genes measured decreased their expression (Fig. 1). From the results obtained from the gene expression studies, fucose seems to play a role in activating genes involving in inflammatory responses. The pro-inflammatory genes up regulated include those for TNF-α, IL12, TNFSF5, TNFSF7, IL17 and IL18.
TNFSF5 (CD40L) regulates B cell function by engaging CD40 on the B cell surfaceReference Hollenbaugh, Grosmaire, Kullas, Chalupny, Braesch-Andersen, Noelle, Stamenkovic, Ledbetter and Aruffo11 and this interaction helps to drive B cells into the cell cycleReference Roitt, Brostoff and Male12. This signal is essential for the germinal centre development and antibody responses to T-cell dependent antigensReference Roitt, Brostoff and Male12.
The ligand TNFSF7 induces the proliferation of co-stimulated T cells, enhances the generation of cytolytic T cells, and contributes to T cell activationReference Goodwin, Alderson and Smith13. IL17B codes for the T-cell-derived cytokine that shares sequence similarity to IL17. It was reported that IL17B is capable of inducing the release of TNF-α and IL1B from a monocytic cell lineReference Shi, Ullrich and Zhang14.
IL18 codes for the pro-inflammatory cytokine that has interferon-γ- and TNF-α-inducing activity that contributes to the development of various inflammatory diseasesReference Matsui, Tsutsui and Nakanishi15. It has been reported that the combinatorial effect of IL12 and IL18 inhibits the production of IgE by the induction of interferon-γ production by activated B cells, which may present a unique approach to the treatment of allergyReference Yoshimoto, Okamura, Tagawa, Iwakura and Nakanishi16.
Another interesting observation noted was that the gene expression of TLR2 was elevated 10-fold in comparison with the control group. The expressions of genes downstream of TLR2 in the TLR2-signalling pathway such as TRAF6, Mekk3 and RelA were also elevated. This suggests that in a condition where fucose exceeds that of the physiological concentration, the intestinal epithelial cells may be primed for inflammatory reactions via the TLR2-signalling pathway (Figs. 2 and 3).
NF-κB regulates a wide variety of genes encoding for cytokines such as IL1, IL2, IL6, IL8, IL12, TNF-α, lymphotoxin-α, lymphotoxin-β and granulocyte-macrophage colony-stimulating factorReference Ghosh and Karin17. As mentioned earlier, both the IL12 and TNF-α gene expressions were found to be up regulated, suggesting that fucose may have evoked the transcription of target cytokine genes of NF-κB via the TLR2 pathway. Furthermore, the up regulated gene expression of TNF-α detected in both the microarrays and RT-PCR analysis was in agreement with the detected level of TNF-α protein (Table 1). IL12 may serve as an essential inducer of T helper-1 cell developmentReference Roitt, Brostoff and Male12. Similarly, TNF-α activates immune cells such as macrophages and granulocytesReference Roitt, Brostoff and Male12.
It appears that fucose modulates both innate and adaptive immune immunity, and is involved in both the cellular and humoral immunity of the latter.
Besides modulating the immune reactions in differentiated Caco-2 cells, fucose induced a set of cytokine genes that are involved in the development and proliferation of immune cells. This set of genes includes BMP2, BMP4, IL5, thrombopoietin and erythropoietin (Fig. 1).
The BMP group consists of BMP2 and BMP4, whose gene expressions were elevated approximately 3- and 2-fold respectively as compared with the control group (Fig. 1). Both BMP2 and BMP4 are members of the TGF-β superfamily. Of the two BMP genes found up regulated, BMP4 can regulate the development and proliferation of human haematopoietic stem cells by acting as a survival factor, which aids in preserving stem cell function under conditions that normally lead to stem cell lossReference Bhatia, Bonnet, Wu, Murdoch, Wrana, Gallacher and Dick18. Undifferentiated human haematopoietic stem cells are crucial in the immune system since they differentiate under the influence of the microenvironment, such as cell-to-cell interactions and presence of soluble or membrane-bound cytokines, to give rise to many cells that are involved in the immune responseReference Roitt, Brostoff and Male12.
IL5 acts as a growth and differentiation factor for both B cells and eosinophils. This cytokine is a main regulator of eosinopoiesis, eosinophil maturation and activationReference Azuma, Tanabe, Konishi, Kinashi, Noma, Matsuda, Yaoita, Takatsu, Hammarstrom and Smith19.
Thrombopoietin is a humoral growth factor that is necessary for megakaryocyte proliferation and maturation, as well as for thrombopoiesisReference de Sauvage, Hass and Spencer20.
The colony-stimulating factor erythropoietin plays a role in the process of erythropoiesisReference Baig, Patel, Coussons and Grant21. Erythropoietin gene expression has been found to induce the expression and secretion of TNF-α, which plays a key role in the inflammatory responseReference Jacobs-Helber, Roh, Bailey, Dessypris, Ryan, Chen, Wickrema, Barber, Dent and Sawyer22.
In addition to the cytokine genes that are involved in the development and proliferation of immune cells, members of the FGF family were found up regulated by fucose in the present study. FGF family peptides possess broad mitogenic and cell-survival activities, and are involved in a variety of biological processes, including embryonic development, cell growth, morphogenesis, tissue repair, tumour growth and invasionReference Smallwood, Munoz-Sanjuan, Tong, Macke, Hendry, Gilbert, Copeland, Jenkins and Nathans23. Besides having an effect on the fibroblast cells, FGF especially the acidic FGF (FGF2) can also exert their effects on gastrointestinal cells. The up regulated gene expression of FGF2 may help to promote epithelial cell restitution in conjunction with the enhanced expression of TGF-β mRNA (Figs. 2 and 3) since the process of intestinal epithelial cell turnover is highly dynamic, occurring every 24–96 h among different mammalian speciesReference Dignass, Tsunekawa and Podolsky24.
It was noted that the exogenous source of fucose was not metabolised by the differentiated Caco-2 cells. Hence it is the direct interaction of fucose with differentiated Caco-2 cells that brought about the modulation of inflammatory reactions, the regulation of the development and proliferation of the haematopoietic stem cells as well as the possible restitution of the intestinal epithelial cells in the in vitro model.
Fucose is reported to modulate the attachment of enteric pathogens such as V. cholerae on the intestinal surfaceReference Hultgren, Abraham, Caparon, Falk, St Geme and Normark25. Earlier studies suggested that fucose-containing structures on eukaryotic cells may function as receptors for vibrio adhesion and therefore may be important determinant of host susceptibilityReference Jones and Freter26. V. cholerae produces the enzyme neuraminidase that attacks the intestinal glycoproteins and gangliosides, thereby unmasking receptor sites for cholera toxinReference Kabir, Ahmad and Ali27. Fucose is a major component of mucus of the gut; a sudden increase in the concentration of fucose in the gut lumen may be indicative of a breach of the intestinal barrier of mucus by enteroinvasive pathogens. We would like to propose that free fucose serves as a danger signal for the first line of defence in the intestine leading to up regulation of the expression of cytokine genes involved in the development of immune cells as well as induction of the adaptive and innate immunity. It is known that some intestinal indigenous bacteria are able to degrade mucin, releasing fucoseReference Bourlioux, Koletzko, Guarner and Braesco1, Reference Hoskins8, and commensal bacteria have been reported to stimulate host immune reactionsReference Saavedra and Tschernia28. It is likely that the fucose danger-signalling mechanism is not specific for pathogens, but a response to the breach of the mucosal barrier.
In conclusion, free fucose appears to play the role of a mediator in the modulation of immune responses in human intestinal epithelium from cDNA studies. The presence of free fucose in the human intestinal tract may signal the invasion of mucin-hydrolysing microbes and breakage of the mucosal barrier. The intestinal epithelial cells respond by up regulation and secretion of cytokines, pre-empting the actual invasion of pathogens. This signalling mechanism may also be used by commensal (probiotic) bacteria in the enhancement of host immune responses and in the maintenance of mucosal barrier integrity.







