Intra-uterine growth retardation (IUGR) is usually defined as impaired growth and development of the embryo and/or its organs during gestation(Reference Wu, Bazer and Cudd1). In humans, IUGR has been observed in about 23·8 % of newborns and approximately thirty million babies worldwide suffer from IUGR every year(Reference de Onis, Blossner and Villar2). Previous studies have shown that IUGR neonates are associated with higher postnatal morbidity and mortality(Reference Aucott, Donohue and Northington3, Reference Garite, Clark and Thorp4). Due to developmental and growth restriction, IUGR neonates often appear immature with regard to the digestive and immune systems compared with their normal counterparts(Reference D'Inca, Gras-Le Guen and Che5, Reference D'Inca, Kloareg and Gras-Le Guen6). For example, changes in histopathology and thymus size have been observed(Reference Cromi, Ghezzi and Raffaelli7, Reference Lang, Baker and Khoury8), and lower numbers of T cells in the thymus(Reference Contreras, Yu and Hale9) as well as an abnormal cytokine profile in serum(Reference Zhong, Li and Huang10) and the intestine(Reference D'Inca, Gras-Le Guen and Che5, Reference Zhong, Li and Huang10, Reference Chatelais, Jamin and Gras-Le Guen11) have been reported in IUGR neonates.
In order to achieve catch-up growth, human IUGR neonates are generally fed a high-protein formula(Reference Premji, Fenton and Sauve12) or a special formula containing a high density of nutrients(Reference Young, Morgan and McCormick13). However, catch-up growth in the first few weeks of postnatal life renders IUGR neonates to an increased risk of the metabolic syndrome such as obesity or other obesity-related diseases in later life(Reference Stettler, Stallings and Troxel14). In addition, evidence in poultry has shown that high nutrient density could decrease the immune function(Reference Guo, Li and Chen15), whereas decreased feed intake can optimise the immune system(Reference Jang, Kang and Ko16). Although it has been widely reported that IUGR impairs intestinal development and function, the intestinal innate immune response and the role of early high nutrient intake (HNI) in regulating innate immunity are still vague. Because of the physiological and genomic similarities between pigs and humans(Reference Humphray, Scott and Clark17), the pig has been recognised as an ideal model for the study of clinical nutrition. Moreover, as a multifetal domestic animal, pigs display severe naturally occurring IUGR due to uteroplacental insufficiency(Reference Wu, Bazer and Wallace18).
Therefore, studying the immunological response of IUGR piglets to HNI may provide useful information on IUGR human infants fed a nutrient-enriched formula. The aim of the present study was to assess the difference in growth and immune function between IUGR and normal-birth weight (NBW) piglets in response to HNI during the suckling period.
Materials and methods
Animal care and formula milk
The animal use and care protocol was approved by the Animal Care and Use Committee of Sichuan Agricultural University. The basic formula milk powder (Table 1) was formulated according to previous studies(Reference Chatelais, Jamin and Gras-Le Guen11, Reference Dourmad, Noblet and Etienne19). The basic nutrient-level formula milk was prepared by mixing 1 kg of formula powder (DM 87·5 %) with 4 litres of water to a milk solution, which was similar to sow milk composition. The high nutrient-level formula milk was prepared by mixing 1·73 kg of formula powder with 4 litres of water to the milk solution, whose nutrient contents were about 1·5-fold those of the former.
Table 1 Composition and nutrient level of the basal formula milk powder (87·5 % DM basis, %)

CP, crude protein.
* Vitamin premix provided per kg powder diet: vitamin A, 0·94 mg; vitamin D3, 0·01 mg; vitamin E, 20 mg; vitamin K3, 1 mg; vitamin B12, 0·04 mg; riboflavin, 5 mg; niacin, 20 mg; pantothenic acid, 15 mg; folic acid, 1·5 mg; thiamin, 1·5 mg; pyridoxine, 2 mg; biotin, 0·1 mg.
† Mineral premix provided per kg powder diet: Zn, 90 mg; Mn, 4·0 mg; Fe, 90 mg; Cu, 6·0 mg; I, 0·2 mg; Se, 0·3 mg.
Animal housing and experimental design
Piglets with a birth weight near the mean litter birth weight (sd 0·5) were identified as NBW, whereas those with at least 1·5 sd lower birth weight were defined as IUGR according to our previous study(Reference Che, Thymann and Bering20). The average birth weights of NBW and IUGR piglets (Duroc × (Landrace × Yorkshire)) used in the present study were 1·52 (sd 0·06) and 0·87 (sd 0·04) kg, respectively. Piglets were fed with liquid diets at 50 ml/kg body weight (BW) per meal with a feeding bottle seven times per d at 3 h intervals between 06.00 and 24.00 hours. Therefore, piglets receiving the basic nutrient-level formula milk had adequate nutrient intake (ANI), whereas those receiving the high nutrient-level formula milk had HNI. A total of twelve pairs of IUGR and NBW piglets, regardless of sex, at 7 d of age from twelve sows were selected and allotted to one of the two dietary groups. This produced four experimental groups (birth weight/nutrient intake (NI)): IUGR/ANI, NBW/ANI, IUGR/HNI and NBW/HNI (n 6 per group). All pigs were housed individually in metabolism cages (0·8 m × 0·7 m × 0·4 m) at an ambient temperature of 30°C in an environmentally controlled room. Room humidity was controlled between 50 and 60 % during the experimental period of 21 d. Piglets had free access to water. The BW and the formula milk intake of pigs were recorded daily. The average daily DM intake (ADMI) was calculated by multiplying the average daily intake of formula milk by its corresponding DM content. Formula milk intake was calculated as the difference between the offered amounts and the refusals.
Blood sampling and analyses
Blood samples were collected by venepuncture on the morning (08.00 hours) of days 14 and 21 after an overnight fast. A part of the sample was injected into Eppendorf tubes containing sodium heparin for the examination of leucocytes. The rest were allowed to coagulate for 40 min before centrifugation (3500 g, 10 min). Eppendorf tubes were immediately placed on ice until they arrived at the veterinary hospital for leucocyte determination (within 2 h). The isolated serum samples were then stored at − 80°C until analysis. Leucocyte examination (neutrophil, lymphocyte and monocyte counts) was done through an automatic blood analyser. Serum TNF-α, IL-1β and IL-10 were assayed using corresponding commercially available porcine ELISA kits (R&D Systems). The minimum detectable concentrations of TNF-α, IL-1β and IL-10 were 7, 30 and 8 pg/ml, respectively.
Tissue sample collection
At the end of the experiment, all piglets were anaesthetised with an intravenous injection of pentobarbital sodium (15 mg/kg BW) and slaughtered. The liver, spleen, kidney and pancreas of each piglet were weighed immediately. The length and weight of the small intestine were measured after the removal of luminal contents. Duodenal, jejunal and ileal samples of approximately 2 cm in length were stored in 4 % methanal solution for histological analyses. The rest of the ileum was frozen in liquid N2, and then stored at − 80°C.
Small-intestinal morphology
Duodenal, jejunal and ileal samples stored in 4 % methanal solution were prepared after staining with haematoxylin and eosin using standard paraffin embedding procedures. A total of five intact, well-oriented crypt–villus units were selected in triplicate for each intestine of piglets. Villous heights and crypt depths were measured using an image processing and analysis system (Optimus software version 6.5; Media Cybergenetics).
Total RNA extraction and real-time RT-PCR
Total RNA was isolated from ileal samples using TRIzol (catalogue no. 15 596-026; Invitrogen). RNA quality was verified by both agarose gel (1 %) electrophoresis and spectrometry (A260/A280, Beckman DU-800; Beckman Coulter, Inc.). Real-time RT-PCR was performed in duplicate to amplify the target gene and the reference gene of the ileum using the one-step SYBR® PrimeScript™ RT-PCR kit II (catalogue no. DRR086A; Takara). Briefly, the reaction mixture (10·0 μl) contained 5·6 μl of a freshly premixed one-step SYBR Green RT-PCR Master mix and a PrimeScript™ Enzyme Mix, 0·8 μl of the primer pair and 3·6 μl RNA template that contained about 150 ng RNA. PCR consisted of one cycle at 42°C for 5 min, one cycle at 95°C for 10 s and forty cycles at 95°C for 5 s and 60°C for 34 s, followed by a dissociation step at 95°C for 15 s, 60°C for 60 s and 95°C for 15 s. To confirm specific amplification, melt curve analysis was performed (ABI 7900HT; Applied Biosystems).
Relative mRNA abundance was determined using the Δ cycle threshold (ΔC t) method, as outlined in the protocol of Applied Biosystems. In brief, a ΔC t value is the C t difference between the target gene and the reference gene (![]() $$\Delta C _{t} = C _{t}^{target} - C _{t}^{reference} $$). For each of the target genes, the ΔΔC t values of all the samples were calculated by subtracting the average ΔC t of the corresponding IUGR/ANI group. The ΔΔC t values were then converted to fold differences by raising 2 to the power − ΔΔC t (
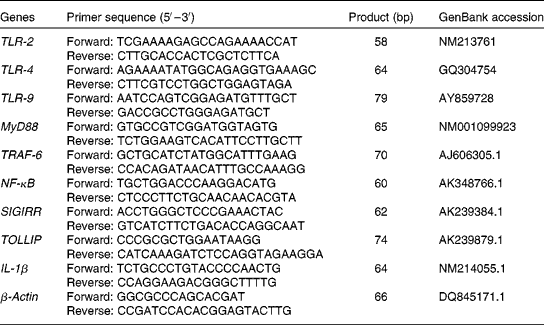
$$\Delta C _{t} = C _{t}^{target} - C _{t}^{reference} $$). For each of the target genes, the ΔΔC t values of all the samples were calculated by subtracting the average ΔC t of the corresponding IUGR/ANI group. The ΔΔC t values were then converted to fold differences by raising 2 to the power − ΔΔC t (![]() $$2^{ - \Delta \Delta C _{t}} $$). Further details on relative gene expression analysis have been described previously(Reference Livak and Schmittgen21). Primers (Table 2) for the assayed genes and the reference gene were designed using Primer Express 3.0 (Applied Biosystems).
$$2^{ - \Delta \Delta C _{t}} $$). Further details on relative gene expression analysis have been described previously(Reference Livak and Schmittgen21). Primers (Table 2) for the assayed genes and the reference gene were designed using Primer Express 3.0 (Applied Biosystems).
Table 2 Primer sequences of the target and reference genes

TLR, Toll-like receptor; MyD88, myeloid differentiation factor 88; TRAF-6, TNF receptor-associated factor 6; SIGIRR, single Ig IL-1-related receptor; TOLLIP, Toll-interacting protein.
Statistical analysis
Data of blood leucocytes and serum cytokines were analysed as repeated measures using the MIXED procedure of Statistical Product and Service Solutions 17.0 (SPSS, Inc.) according to the following model:
where μ is the mean; αi is the effect of BW (i= IUGR, NBW); βj is the effect of NI (j= ANI, HNI); αβ ij is the interaction between BW and NI; U k is the litter (k= 1, 2,…, 12); ωl is the time (days 14 and 21), αω il is the interaction between BW and time; βω jl is the interaction between NI and time; αβω ijl is the interaction between BW, NI and time; ɛijkl ~ N(0, σ2) represents the random error. Data of intestinal morphology were also analysed as repeated measures according to the model; however, ωl here refers to the segment (duodenum, jejunum and ileum), αω il refers to the interaction between BW and segment, βω jl refers to the interaction between NI and segment and αβω ijl refers to the interaction between BW, NI and segment. Data on growth performance, organ indices and gene expressions were analysed according to the model, but omitting the effect of time and the interaction between time, BW and NI. Results are presented as means with their standard errors. Differences between groups were analysed using the general linear model procedure followed by Duncan's test. P< 0·05 was considered as statistically significant.
Results
Growth performance
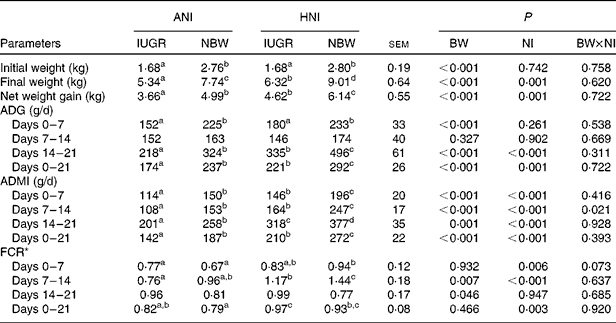
In the present study, regardless of the NI, the initial BW, final BW and BW gain of IUGR piglets were lower (P< 0·001) than those of NBW neonates (Table 3). However, relative to NBW piglets with ANI, IUGR piglets receiving HNI had a comparable BW gain, but the final BW was still lower ( − 18·3 %, P< 0·05). Regardless of the NI, IUGR piglets had a lower (P< 0·001) average daily gain during the 1st and 3rd weeks compared with NBW piglets; as a result, the overall average daily gain was lower (P< 0·001) in IUGR piglets relative to NBW piglets. HNI increased (P= 0·001) the final BW and BW gain of piglets and increased the average daily gain (P= 0·001), the ADMI (P< 0·001) and the feed conversion ratio (P= 0·003) throughout the experimental period. BW and NI had no interaction effect on growth performance, except for the ADMI during the 2nd week (P= 0·021). Furthermore, IUGR piglets with HNI had a similar average daily gain to NBW piglets receiving ANI due to the similar ADMI throughout the experimental period.
Table 3 Effects of the level of nutrient intake on the growth performance of intra-uterine growth-retarded (IUGR) and normal-birth weight (NBW) neonates (Mean values with their standard errors)

ANI, adequate nutrient intake; HNI, high nutrient intake; BW, body weight; NI, nutrient intake; ADG, average daily gain; ADMI, average daily DM intake; FCR, feed conversion ratio.
a,b,c,dMean values within a row with unlike superscript letters were significantly different (P< 0·05).
* FCR was calculated by dividing the ADMI by its corresponding ADG.
Organ indices
As shown in Table 4, BW (P< 0·001) and NI (P= 0·005) had a significant effect on the intestinal length:BW ratio in piglets. HNI decreased (P= 0·005) the relative intestinal length but increased (P= 0·096) the relative liver weight. The relative intestinal weight (P= 0·002), intestinal length (P< 0·001), liver weight (P< 0·001) and pancreas weight (P= 0·023) of IUGR piglets were significantly higher than those of NBW piglets. The relative intestinal weight, intestinal length and liver weight of IUGR piglets with HNI were increased (P< 0·05) by 27·8, 15·3 and 29·3 % than those of NBW piglets with ANI, respectively. However, the relative weights of the spleen and kidney were not affected by IUGR or NI. No interaction was found between BW and NI for any of the relative weights of the organs.
Table 4 Effects of the level of nutrient intake on the organ indices of intra-uterine growth-retarded (IUGR) and normal-birth weight (NBW) neonates (Mean values with their standard errors)

ANI, adequate nutrient intake; HNI, high nutrient intake; BW, body weight; NI, nutrient intake.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Composition of peripheral leucocytes
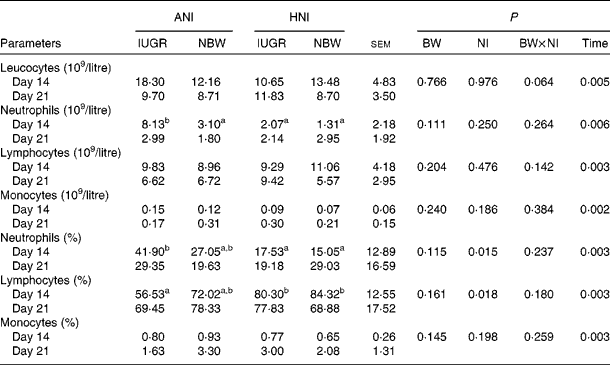
No effect of BW or the interaction between BW and NI was observed on the count or percentage of neutrophils, lymphocytes and monocytes (Table 5). HNI significantly decreased (P= 0·015) neutrophil percentage, but increased (P= 0·018) lymphocyte percentage. The counts of leucocytes (P= 0·005), neutrophils (P= 0·006) and lymphocytes (P= 0·003) were decreased on day 21. However, the count (P= 0·002) and percentage (P= 0·003) of monocytes were increased on day 21. In addition, the neutrophil count of IUGR piglets with ANI was increased (P< 0·05) by 163 % compared with that of NBW piglets receiving HNI on day 14.
Table 5 Effects of the level of nutrient intake on the count and percentage of blood leucocytes, neutrophils, lymphocytes and monocytes in intra-uterine growth-retarded (IUGR) and normal-birth weight (NBW) neonates (Mean values with their standard errors)

ANI, adequate nutrient intake; HNI, high nutrient intake; BW, body weight; NI, nutrient intake.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Serum concentrations of TNF-α, IL-1β and IL-10
As shown in Table 6, IUGR decreased serum concentrations of TNF-α (P= 0·002) and IL-1β (P< 0·001), as well as the ratios of TNF-α:IL-10 (P< 0·001) and IL-1β:IL-10 (P< 0·001) in piglets. HNI increased the concentration of IL-10 (P< 0·001) but decreased IL-1β concentration (P= 0·045), as well as the ratios of TNF-α:IL-10 (P< 0·001) and IL-1β:IL-10 (P< 0·001). BW and NI had significant interaction effects on IL-10 concentration (P= 0·002), as well as on the ratios of TNF-α:IL-10 (P< 0·001) and IL-1β:IL-10 (P< 0·001). The concentrations of TNF-α (P= 0·011), IL-1β (P= 0·010) and IL-10 (P= 0·004) as well as the IL-1β:IL-10 ratio (P= 0·002) were higher on day 21 compared with those on day 14. However, the TNF-α:IL-10 ratio (P= 0·002) decreased on day 21.
Table 6 Effects of the level of nutrient intake on the concentrations of TNF-α, IL-1β and IL-10 in intra-uterine growth-retarded (IUGR) and normal-birth weight (NBW) neonates (Mean values with their standard errors)
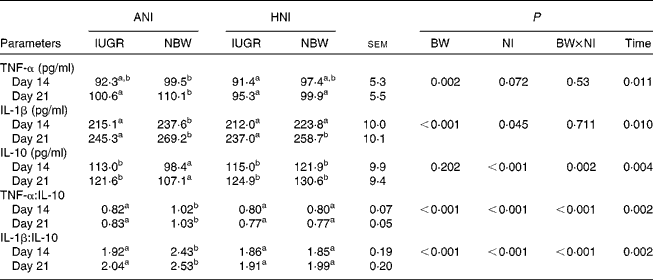
ANI, adequate nutrient intake; HNI, high nutrient intake; BW, body weight; NI, nutrient intake.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Intestinal morphology
Irrespective of the NI, IUGR decreased (P= 0·038) the intestinal villous height:crypt depth ratio (VCR) in piglets (Table 7). No effect of NI was observed on intestinal morphology. BW and NI had a significant interaction effect on villous height (P= 0·001). The villous height (P= 0·002), the crypt depth (P= 0·013) and the VCR (P= 0·002) were significantly affected by the segment in the small intestine, with the duodenum having the highest villous height and the deepest crypt depth and the jejunum having the highest VCR. Furthermore, compared with NBW piglets receiving ANI, the duodenal and ileal crypt depths were deeper (15–18 %, P< 0·05), but the ileal VCR was higher ( − 20 %, P< 0·05) in IUGR piglets receiving HNI, respectively.
Table 7 Effects of the level of nutrient intake on the intestinal morphology of intra-uterine growth-retarded (IUGR) and normal-birth weight (NBW) neonates (Mean values with their standard errors)

ANI, adequate nutrient intake; HNI, high nutrient intake; BW, body weight; NI, nutrient intake; VCR, villous height:crypt depth ratio.
a,b,cMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Gene expression in the ileum
The mRNA abundance of Toll-like receptor (TLR)-4 (P= 0·039), TLR-9 (P= 0·003), TNF receptor-associated factor 6 (TRAF-6, P= 0·034), IL-1β (P= 0·021) and Toll-interacting protein (TOLLIP, P= 0·053) was decreased in the ileum of IUGR piglets relative to NBW piglets (Table 8). HNI decreased the mRNA abundance of NF-κB (P= 0·034) and IL-1β (P= 0·015), but increased TLR-9 (P= 0·005) mRNA expression in the ileum. BW and NI had no interaction effect on the mRNA abundance of these genes in the ileum, except for TLR-9 (P= 0·003). Moreover, IUGR piglets receiving HNI had a lower (P< 0·05) mRNA abundance of TLR-4, NF-κB and TOLLIP in the ileum than NBW piglets receiving ANI.
Table 8 Effects of the level of nutrient intake on the mRNA abundance of innate immune-related genes in the ileum of intra-uterine growth-retarded (IUGR) and normal-birth weight (NBW) neonates (Mean values with their standard errors)

ANI, adequate nutrient intake; HNI, high nutrient intake; BW, body weight; NI, nutrient intake; TLR, Toll-like receptor; MyD88, myeloid differentiation factor 88; TRAF-6, TNF receptor-associated factor 6; SIGIRR, single Ig IL-1-related receptor; TOLLIP, Toll-interacting protein.
a,bMean values within a row with unlike superscript letters were significantly different (P< 0·05).
Discussion
The present study was one of the rare studies documenting the effect of HNI during the suckling period on the growth and immune function of IUGR piglets reared in well-controlled conditions. The intriguing findings were that IUGR piglets exhibited a differential immune response to HNI compared with NBW piglets. Particularly, the increased NI during the 21 d of the suckling period impaired the systematic immune response of IUGR piglets by decreasing the number of leucocytes, altering the serum cytokine profile and the intestinal expression of innate immune-related genes.
The lower BW gain in IUGR pigs could be resulting from the inadequate intake of nutrients, as indicated by the markedly decreased average DM intake in IUGR pigs. However, IUGR pigs are able to exert a similar growth rate to normal pigs when receiving a similar DM intake. The present results showed that there was a comparable BW gain between the IUGR pigs with HNI and NBW pigs with ANI. These findings indicate that IUGR piglets receiving HNI achieved catch-up growth. The difference in the BW of piglets was due to the ADMI and the corresponding different nutrient contents of the formula milk.
IUGR could lead to a relatively longer intestine in neonates, as previously described in pigs(Reference Xu, Mellor and Birtles22), rabbits(Reference Cellini, Xu and Arriaga23) and sheep(Reference Avila, Harding and Rees24). In the present study, consistently, IUGR piglets exhibited a relatively longer intestine and a heavier liver and pancreas, indicating the potential metabolic priority over key organs relative to whole-body growth(Reference Bauer, Walter and Hoppe25, Reference Mostyn, Litten and Perkins26). The liver plays a major role in the metabolism of dietary nutrients and other substances(Reference Jobgen, Fried and Fu27). The higher relative liver weight in IUGR piglets with HNI may presumably be due to the compensatory hypertrophy of the liver, which is in accordance with the report of Rompala et al. (Reference Rompala, Johnson and Rumpler28) showing that rams with a high level of feed intake resulted in a greater liver weight:empty BW ratio.
Growth rate was increased in IUGR piglets receiving HNI. However, it seems that catch-up growth would impair the immune system according to the results of serum cytokines. The neonatal period was the intense period of changes in the expression of molecules involved in the recognition of bacteria by epithelial and immune cells, such as TLR and cytokines(Reference Chatelais, Jamin and Gras-Le Guen11). Cytokine concentrations and their ratios were sharply changed in IUGR piglets with HNI relative to NBW piglets, which suggested the compromised immune function of piglets.
In addition, during the neonatal period, cells of the innate immune system, predominantly neutrophils, macrophages and natural killer cells, are mainly responsible for the clearance of foreign antigens. In neonates, cells involved in triggering innate immunity are functional, but they are present in lower numbers and have lower enzyme activity than their adult counterparts(Reference Kovarik and Siegrist29). In the present study, we did not test macrophages and natural killer cells, but the number and percentage of neutrophils were decreased along with the increase of lymphocyte percentage in IUGR piglets receiving HNI on day 14. As the events in the neonatal period allow the maturation of the immune system, peripheral lymphocyte subsets also showed certain changes. Comans-Bitter et al. (Reference Comans-Bitter, de Groot and van den Beemd30) and de Vries et al. (Reference de Vries, de Bruin-Versteeg and Comans-Bitter31) found that an increase in T- and B-lymphocytes occurred during the 1st weeks of life, while natural killer cells declined after birth(Reference Comans-Bitter, de Groot and van den Beemd30, Reference de Vries, de Bruin-Versteeg and Comans-Bitter31).
Small-intestinal morphology containing the villous height, the crypt depth and the VCR of the duodenum, jejunum and ileum is one of the major indicators reflecting gut health in piglets. The increasing villous height implied the increased surface area for nutrient absorption(Reference Caspary32), whereas the deeper crypt suggested a fast new villous tissue turnover in response to normal sloughing or inflammation from a pathogen(Reference Yason, Summers and Schat33). In the present study, IUGR piglets with HNI had higher jejunal and ileal villous heights, compared with piglets with ANI, which could be an important reason for the catch-up growth. However, the jejunal and ileal crypt depths in IUGR piglets receiving HNI were deeper. The present results are consistent with a previous study which suggested that piglets with a high level of feed intake had a higher villous height and a deeper crypt depth(Reference van Beers-Schreurs, Nabuurs and Vellenga34).
Moreover, the gastrointestinal tract is the largest immune organ in the body, and, as such, is the location for the majority of lymphocytes and immune effector cells with pattern recognition receptors(Reference Kelly and Coutts35), which sense luminal antigens and mediate the inflammatory response(Reference Newburg and Walker36). TLR are typical pattern recognition receptors in mediating mucosal innate host defence and in maintaining mucosal and commensal homeostasis(Reference Newburg and Walker36). MyD88, TRAF-6 and NF-κB are downstream signalling molecules and transcription factors shared by TLR-2, -4 and -9(Reference Takeda and Akira37), while single Ig IL-1-related receptor and TOLLIP are crucial negative regulators(Reference Shibolet and Podolsky38). It has been demonstrated that the TLR-4–Myd88–NF-κB signal pathway is involved in inflammation(Reference Kawai and Akira39). Nenci et al. (Reference Nenci, Becker and Wullaert40) suggested that the down-regulation of NF-κB at the mRNA level might be a regulatory mechanism to augment long-term inflammatory responses. The decreased expressions of TLR-4, TLR-9, NF-κB and IL-1β in IUGR piglets receiving HNI suggested that HNI during the suckling period would reduce the intestinal innate immunity of IUGR piglets.
In summary, the present results suggest that HNI during the suckling period would lead to an abnormal immune function of neonatal piglets with IUGR. Further investigations are warranted to determine whether IUGR pigs with HNI would have a persistent impact on the immune system.
Acknowledgements
The present study was supported by the International Cooperation in Science and Technology Project of Sichuan Province (no. 2010HH0014), the National 973 Project (2012CB124701) and the National Natural Science Foundation (no. 31101727). F. H., Y. X., X. D., Y. L. and S. B. participated in the experimental design and data interpretation, and helped in the drafting of the manuscript. L. C. and K. Z. conceived the study, directly supervised the project and participated in the experimental design and data interpretation. F. H., S. H. and L. H. carried out the animal feeding trial and molecular experiment. F. H. was responsible for the writing of the manuscript. There are no conflicts of interest to declare.