Lutein is a food microconstituent that belongs to the carotenoid family, specifically the xanthophyll subclass characterised by oxygenated groups. In the human diet, lutein is mainly found in dark-green leafy vegetables and egg yolk (Chug-Ahuja et al. Reference Chug-Ahuja, Holden, Forman, Mangels, Beecher and Lanza1993; Sommerburg et al. Reference Sommerburg, Keunen, Bird and van Kuijk1998). It cannot yet be considered as a nutrient (Hendrich et al. Reference Hendrich, Lee, Xu, Wang and Murphy1994) because there is not sufficient evidence that it sustains or enhances physiological functions and/or prevents disease. Nevertheless, this xanthophyll is attracting growing interest since it selectively accumulates in the retina (Bone et al. Reference Bone, Landrum, Friedes, Gomez, Kilburn, Menendez, Vidal and Wang1997; Chan et al. Reference Chan, Leung, Lam and Tso1998), where it may protect photoreceptors against light-initiated oxidative damage (Rapp et al. Reference Rapp, Maple and Choi2000; Junghans et al. Reference Junghans, Sies and Stahl2001; Krinsky, Reference Krinsky2002), and intake of carotenoids has been associated with a lower incidence of age-related macular degeneration (Snodderly, Reference Snodderly1995; Landrum & Bone, Reference Landrum and Bone2001).
In Western diets, lutein is mostly recovered as non-esterified lutein. Lutein metabolism begins in the stomach where lutein-containing foods are subjected to acidic processing and gastric enzymes. It has been shown that lutein is partially released from spinach and transfers to the fat phase of the meal (Tyssandier et al. Reference Tyssandier, Reboul, Dumas, Bouteloup-Demange, Armand, Marcand, Sallas and Borel2003). Digestive enzymes in the duodenum continue to release lutein from its food matrix, and it is assumed that lutein is transferred to dietary lipids found in the duodenum and then into mixed micelles. Pancreatic lipase appears to facilitate the transfer of lutein from lipid droplets to mixed micelles (Borel et al. Reference Borel, Grolier, Armand, Partier, Lafont, Lairon and Azais-Braesco1996). This transfer mainly depends on pH, bile lipid concentration and carotenoid hydrophobicity (Tyssandier et al. Reference Tyssandier, Lyan and Borel2001). Mixed micelles carry lutein to the enterocyte where it is at least partly absorbed through the scavenger-receptor class B type I (Reboul et al. Reference Reboul, Abou, Mikail, Ghiringhelli, Andre, Portugal, Jourdheuil-Rahmani, Amiot, Lairon and Borel2005a). After absorption, lutein is incorporated into chylomicrons and transported to the bloodstream, via the lymphatics.
In a normal meal containing plant-derived foods, lutein is necessarily ingested with other antioxidants, including carotenoids, polyphenols and vitamins C and E. Since lutein is an antioxidant and is therefore probably subject to oxidative degradation (Siems et al. Reference Siems, Sommerburg and van Kuijk1999), it follows that other antioxidants found in the diet may protect it from degradation in the upper gastrointestinal tract, thereby enhancing its absorption efficiency. In contrast, it is also possible that dietary antioxidants may compete with lutein for absorption. Indeed, several studies have shown that carotenoids compete for their absorption (van den Berg, Reference van den Berg1999; Tyssandier et al. Reference Tyssandier, Cardinault, Caris-Veyrat, Amiot, Grolier, Bouteloup, Azais-Braesco and Borel2002).
The aims of the present study were (i) to assess whether a mixture of the main classes of dietary antioxidants (carotenoids, polyphenols, vitamin C and vitamin E) affect the bioavailability of lutein in human subjects, and (ii) to assess the individual effect of each class of antioxidant microconstituent on lutein uptake using an intestinal cell-culture model.
Material and methods
Chemicals
Lutein (96 % pure), lycopene (95·5 % pure), β-carotene (95·6 % pure) and echinenone (98 % pure) were kindly provided by DSM Ltd (formerly F. Hoffmann-La Roche, Basel, Switzerland). (R,R,R)-α-tocopherol ( ≥ 99 % pure) and (R,R,R)-γ-tocopherol ( ≥ 97 % pure) were purchased from Fluka (Vaulx-en-Velin, France). Quercetin, naringin, hesperidin, hesperetin, 2-oleoyl-1-palmitoyl-sn-glycero-3-phosphocholine (phosphatidylcholine), 1-palmitoyl-sn-glycero-3-phosphocholine (lysophosphatidylcholine), monoolein, non-esterified cholesterol, oleic acid, sodium taurocholate and pyrogallol were purchased from Sigma-Aldrich (St Quentin Fallavier, France). Naringenin, (+)-catechin, gallic acid, caffeic acid and eriodictyol were purchased from Extrasynthèse (Genay, France).
Dulbecco's modified Eagle medium containing 4·5 g/l glucose and trypsin-EDTA (at 500 mg/l and 200 mg/l, respectively) were purchased from Bio Whittaker (Fontenay-sous-Bois, France). Fetal bovine serum was purchased from Biomedia (Issy-les-Moulineaux, France), and non-essential amino acids, and penicillin and streptomycin were purchased from Gibco BRL (Cergy-Pontoise, France).
Postprandial experiment to assess the effect of an antioxidant mixture on lutein bioavailability in healthy human subjects
Since there were no available literature data on the effect of a mixture of antioxidants on lutein absorption, we were unable to perform a power analysis to calculate the number of subjects required to observe a significant effect. We therefore used a number of eight subjects because this number has allowed finding significant differences in carotenoid absorption in previous studies (Cardinault et al. Reference Cardinault, Tyssandier, Grolier, Winklhofer-Roob, Ribalta, Bouteloup-Demange, Rock and Borel2003; Riso et al. Reference Riso, Brusamolino, Ciappellano and Porrini2003; Tesoriere et al. Reference Tesoriere, Allegra, Butera and Livrea2004; Blum et al. Reference Blum, Aviram, Ben-Amotz and Levy2005; Reboul et al. Reference Reboul, Borel, Mikail, Abou, Charbonnier, Caris-Veyrat, Goupy, Portugal, Lairon and Amiot2005b). Eight male subjects were selected by the local Clinical Investigation Centre (Sainte Marguerite, Marseille, France). They should be healthy, non-smoking, aged 20–40 years and with a BMI < 24 kg/m2. They should not take medication and have no history of gastrointestinal disease, diabetes or disorders of lipid metabolism. To verify the latter points, serum glucose, TAG and cholesterol were measured by using enzymic procedures (Trinder, Reference Trinder1969; Fossati & Prencipe, Reference Fossati and Prencipe1982; Siedel et al. Reference Siedel, Hagele, Ziegenhorn and Wahlefeld1983). Subject characteristics and their daily nutrient intakes are reported in Table 1. Dietary intake was assessed by a 5 d food diary and analysed using Score-AN software (configured by Avantage Nutrition, Marseilles, France). The software's nutrient database was obtained from Ciqual and Souci's nutrition tables (Souci et al. Reference Souci, Fachmann and Kraut2000). The study was approved by the regional ethics committee in Marseilles. The objectives and requirements of the study were fully explained to the participants, and informed written consent was obtained for each subject. Each subject received each meal in a random order at a 1-month interval (cross-over design with a 1-month wash-out period). On the evening before the experiment, the subjects were asked to consume a light meal and to fast overnight. In the morning, each subject had an intravenous catheter inserted into a forearm. A first blood sample was obtained at fasting (baseline sample), and the volunteers then consumed either meal 1 (containing 18 mg lutein) or meal 2 (containing 18 mg lutein plus 500 mg vitamin C, 67 mg (100 IU) natural vitamin E, 1 g polyphenols (a mixture of grapeseed extract and citrus bioflavonoids)). Antioxidant micronutrients were from Holland & Barrett (Nuneaton, Warwickshire, UK) and were given as pills which were swallowed by the subjects during the meal. No other food was consumed during the postprandial experiment. The amount of lutein in the test meal was chosen to accurately detect lutein in the chylomicron-rich fraction. This amount of carotenoid remains nutritional as it is close to the daily total carotenoid intake, which is estimated at 14 mg/d in Europe (O'Neill et al. Reference O'Neill, Carroll, Corridan, Olmedilla, Granado, Blanco, van den Berg, Hininger, Rousell, Chopra, Southon and Thurnham2001). The amount of other antioxidant was chosen to be close to the relative proportions found in the diet. Foods were purchased from a local supermarket. Meal compositions and microconstituent characteristics are given Table 2. Additional blood samples were drawn 1, 2, 3, 4, 6 and 8 h after the beginning of the meal. Blood samples were immediately stored at 4°C and rapidly centrifuged (at 610 g for 10 min at 4°C) to isolate plasma fractions. Large chylomicrons (Sf > 400) were isolated as described by Luchoomun & Hussain (Reference Luchoomun and Hussain1999). Chylomicron-rich fraction TAG were measured using the PAP 150 Biomérieux kit (Charbonnière-les-Bains, France).
Table 1 Characteristics and nutrient intakes of the volunteers 5 d before the beginning of the study (Mean values with their standard errors)
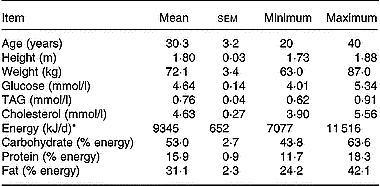
* Energy intake was assessed by a 5 d food diary analysed using Score-AN software (Avantage Nutrition, Marseille, France).
Table 2 Meal composition
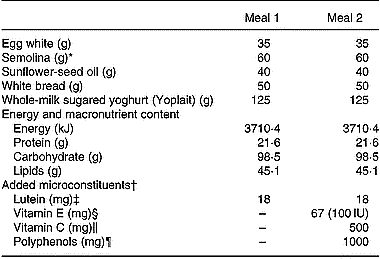
* With 180 ml hot water.
† Microconstituents were purchased from Holland & Barrett (Nuneaton, Warwickshire, UK).
‡ Lutein was free lutein (made from marigold extract).
§ Vitamin E was a natural mixture of d-α-, d-β-, d-γ- and d-δ-tocopherols.
‖ Vitamin C (ascorbic acid) was provided in a pill also containing 25 mg bioflavonoids.
¶ Polyphenols (naringin 85 mg, hesperidin 557 mg, eriodyctiol 4 mg, naringenin 3 mg, hesperetin 1 mg, plus 350 mg non-identified polyphenols) were derived from grape seeds and from citrus extracts.
Preparation of microconstituent-rich media for cell experiments
For delivery of fat-soluble microconstituents (i.e. lutein, β-carotene, lycopene, (R,R,R)-α- and (R,R,R)-γ-tocopherol) to cells, mixed micelles separately enriched with each of these microconstituents were prepared as previously described (Reboul et al. Reference Reboul, Abou, Mikail, Ghiringhelli, Andre, Portugal, Jourdheuil-Rahmani, Amiot, Lairon and Borel2005a) to obtain the following final concentrations: 0·04 mm-phosphatidylcholine, 0·16 mm-lysophosphatidylcholine, 0·3 mm-monoolein, 0·1 mm-non-esterified cholesterol, 0·5 mm-oleic acid, 5 mm-taurocholate and 0·1 to 5 μm of the relevant microconstituent. Microconstituent concentrations in the micellar solutions were checked before each experiment. The different stock solutions of micellar microconstituents were mixed to obtain the final mixtures required. Vitamin C was directly dissolved in the culture medium. Polyphenols were added to the media in dimethylsulfoxide (an identical volume of dimethylsulfoxide alone was added when there were no polyphenols). The microconstituent concentrations used are summarised in Table 3.
Table 3 Concentration of antioxidant microconstituents in the Caco-2 cell experiments of factorial design
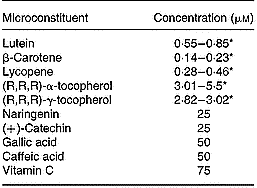
* Range of the concentrations used in the different experiments.
Measurement of lutein uptake by intestinal cells
Cell culture
Caco-2 clone TC-7 cells (Salvini et al. Reference Salvini, Charbonnier, Defoort, Alquier and Lairon2002) were kindly provided by Dr M. Rousset (U178 INSERM, Villejuif, France). Cells were cultured in the presence of Dulbecco's modified Eagle medium supplemented with 20 % heat-inactivated fetal bovine serum, 1 % non-essential amino acid and 1 % antibiotics (complete medium), as previously described (Reboul et al. Reference Reboul, Abou, Mikail, Ghiringhelli, Andre, Portugal, Jourdheuil-Rahmani, Amiot, Lairon and Borel2005a). For each experiment, cells, passage 40–60, were seeded and grown on semi-permeable filters as previously described (Reboul et al. Reference Reboul, Abou, Mikail, Ghiringhelli, Andre, Portugal, Jourdheuil-Rahmani, Amiot, Lairon and Borel2005a) to obtain differentiated confluent cell monolayers. The medium used in the apical and basolateral chambers was changed to a serum-free complete medium 12 h before each experiment. During preliminary tests, the integrity of the cell monolayers was checked by measuring transepithelial electrical resistance using a volt-ohmmeter fitted with a ‘chopstick’ electrode (Millicell ERS; Millipore, Saint-Quentin-en-Yvelines, France).
Experiment design
At the beginning of each experiment, cell monolayers were washed with PBS with 1 ml at the apical side and 2 ml at the basolateral side. The apical side of the cell monolayers received either 1 ml micellar lutein or 1 ml micellar lutein plus other micellar microconstituents, while the basolateral side received 2 ml fetal-bovine-serum-free medium. Cell monolayers were incubated at 37°C for 30 min. Media from each side of the monolayer were harvested at the end of the incubation period. Cell monolayers were washed twice with 0·5 ml PBS containing 10 mm-taurocholate to eliminate potentially adsorbed lutein, then scraped and collected in 500 μl PBS. Absorbed lutein was estimated as lutein in the scraped cells plus lutein in the basolateral chambers.
Identification of the antioxidant microconstituents that affect lutein uptake by Caco-2 cells
A full factorial design was used to identify the antioxidant classes of microconstituents that significantly affected lutein absorption. The design was constructed using Trial Run™ 1·0 (SPSS Inc., Chicago, IL, USA) software. The design comprised two levels for each factor, i.e. with or without antioxidant microconstituents. The first factor was carotenoids (we used a mixture of β-carotene and lycopene). The second factor was vitamin C. The third factor was vitamin E (we used an equimolar mixture of (R,R,R)-α-tocopherol and (R,R,R)-γ-tocopherol). The fourth factor was polyphenols (we used a mixture of gallic acid, caffeic acid, (+)-catechin and naringenin). The sixteen experiments generated by the design were performed in triplicate, and the full factorial design was performed twice. Data analysis was based on the general linear model using ANOVA.
After having identified which antioxidant classes significantly (P < 0·05) affected lutein uptake by Caco-2 cells and any potential interactions, a second series of experiments was performed to identify exactly which antioxidant microconstituents in the mixtures tested significantly affected uptake by Caco-2 cells.
All the samples were stored at − 80°C under N2 with 0·5 % pyrogallol as a preservative (reducing agent) until extraction and HPLC analysis. Aliquots of cell samples without pyrogallol were used to determine protein concentrations using a bicinchoninic acid kit (BCA kit; Pierce, Montluçon, France).
Lutein extraction and high-performance liquid chromatography analysis
Lutein was measured in chylomicron-rich fractions and in samples taken from the cell experiments according to the following method. Lutein was extracted from 500 to 800 μl samples under darkness. The carotenoid echinenone used as internal standard was added to the samples in one volume of ethanol. The mixture was extracted twice with two volumes of hexane. The hexane phases obtained after centrifugation (at 500 g for 5 min at 4°C) were completely evaporated under N2; the residue was re-dissolved in 100 μl acetonitrile–dichloromethane (50:50, v/v) and 80 μl was used for injection. Analysis was performed via a reverse-phase isocratic HPLC method as described by Lyan et al. (Reference Lyan, Azais-Braesco, Cardinault, Tyssandier, Borel, Alexandre-Gouabau and Grolier2001), using a 250 × 4·6 mm internal diameter RP C18, 5 μm Zorbax column (Interchim, Montluçon, France) and a guard column. The mobile phase was 70 % acetonitrile, 20 % dichloromethane and 10 % methanol. The flow rate was 1·5 ml/min and the column was thermostated at 25°C. The HPLC system consisted in a Dionex separation module (P680 HPLC pump and ASI-100 automated sample injector) and a Dionex UVD340U photodiode array detector (Dionex SA, Voisins le Bretonneux, France). Lutein and echinenone were quantified by their absorption at 450 nm and identified according to their retention time and absorption spectra (between 300 and 500 nm) compared with pure standards. Quantification was performed using Chromeleon software (version 6.50 SP4 Build 1000, Dionex SA, Voisins le Bretonneux, France) comparing peak areas with standard reference curves. All solvents were HPLC grade obtained from SDS (Peypin, France).
Identification of polyphenols in the polyphenol-rich pills used in the clinical study
Total polyphenol content of the pills (containing a mixture of grapeseed extract and citrus bioflavonoids) was assessed using the Folin–Ciocalteu assay as previously described (George et al. Reference George, Brat, Alter and Amiot2005). The pills contained 211 mg polyphenols/g powder. Since each pill contained 411 mg powder, eleven pills were necessary to obtain 1 g polyphenols. In order to assess which polyphenol species were found in the pills, we subsequently analysed polyphenol content by HPLC. Pill powder (6 mg) was extracted with 5 ml of a mixture of trifluoroacetic acid (TFA)-acidified water–acetonitrile (pH 4·6, 50:50, v/v) in a glass tube. Quercetin was used as an internal standard. After a 10 s vortexing step, the suspension was centrifuged at 2000 g for 10 min at 4°C. Aple of 200 μl of the clear supernatant fraction was transferred into an HPLC vial for analysis. Samples were analysed by reverse-phase HPLC using a Waters 2690 Alliance system. Separation was achieved using two monolithic type columns (Chromolith Performance RP18 E 100 × 4·6 mm; VWR, Fontenay-sous-Bois, France) in series, and maintained at 37°C. Solvents A (5 % acetonitrile, 0·006 % TFA) and B (100 % acetonitrile) were run at a flow rate of 1 ml/min. 100 % solvent A was held isocratically for 9 min, followed by an increase to 12 % of solvent B for 27 min, then increased to 55 % of solvent B for 14 min. Sample injection volume was 10 μl, and the eluent was monitored at 280 nm. Identification and quantification of polyphenols was performed using absorption spectra and calibration curves obtained with authentic standards.
Calculations and statistical analysis
Results are expressed as mean values with their standard errors. Responses in chylomicron-rich fraction TAG and lutein were assessed by calculating the area under the postprandial curves. These calculations were performed over the 0–8 h period using the trapezoidal method. Calculations were performed by subtracting the baseline concentration from the concentration value measured at each postprandial point.
For the clinical study, differences between the two groups of paired data (n 8) were tested using one-tailed paired t tests of the log-transformed data. Elaboration and data analysis of the full factorial design designed to identify which antioxidant classes affect lutein uptake by Caco-2 cells were performed using Trial Run™ 1·0 software (SPSS France SA, Paris, France ). Results obtained in the experiment designed to assess the effect of different polyphenols on lutein uptake were analysed using the Kruskal–Wallis test followed by the Mann–Whitney U test, used as a post hoc test. Statistical analyses were performed using Statview software, version 5.0 (SAS Institute, Cary, NC, USA). Differences with P < 0·05 were considered significant.
Results
Effect of a mixture of antioxidant microconstituents on postprandial chylomicron triacylglycerols and lutein responses
There was no significant difference between chylomicron-rich fraction TAG responses (expressed as area under the postprandial 0 to 8 h curves) following the ingestion of the two meals (Fig. 1). In contrast, the chylomicron-rich fraction lutein response was 23 % weaker (P = 0·07) after meal 2 (containing lutein plus the mixture of antioxidants) than after meal 1 (lutein alone) (21·9 (sem 4·6) v. 28·4 (sem 7·2) nmol × h/l, respectively; Fig. 2). Individual chylomicron-rich fraction lutein responses were very variable, ranging from 8·8 to 73·5 nmol × h/l after meal 1, but six subjects out of eight had a lower chylomicron-rich fraction lutein response after meal 2 than after meal 1, one had a similar response (28·5 and 27·2 nmol × h/l) and one had a higher response (30·0 v. 15·9 nmol × h/l).

Fig. 1 Plasma chylomicron-rich fraction TAG responses in healthy males after ingestion of meal 1 (□; 18 mg lutein) and meal 2 (■; 18 mg lutein +a mixture of antioxidant microconstituents). Data (change from fasting values) are means (n 8) with their standard errors represented by vertical bars. Inset: chylomicron-rich fraction TAG responses (area under the curve; AUC) after meal 1 and meal 2. Data are means (n 8) with their standard errors represented by vertical bars.

Fig. 2 Plasma chylomicron-rich fraction lutein responses in healthy males after ingestion of meal 1 (○; 18 mg lutein) and meal 2 (● 18 mg lutein +a mixture of antioxidant microconstituents). Data (change from fasting values) are means (n 8) with their standard errors represented by vertical bars. Inset: chylomicron-rich fraction lutein responses (area under the curve; AUC) after meal 1 and meal 2. Data are means (n 8) with their standard errors represented by vertical bars.
Effect of antioxidant microconstituents on lutein uptake by Caco-2 cells
Before describing in detail the effect of antioxidant micronutrients on lutein uptake by Caco-2 cells it should be mentioned that we found that there was no significant degradation of lutein during the duration of the experiments, whether or not there were other antioxidant micronutrients.
Identification of the antioxidant classes that affect lutein uptake
Data analysis of the results obtained in the factorial design showed that both the mixture of polyphenols (gallic acid, caffeic acid, (+)-catechin and naringenin) and the mixture of carotenoids (lycopene plus β-carotene) significantly (P < 0·05) impaired lutein uptake. In contrast, vitamins C and E had no significant effect (Table 4). No interaction was observed between the different classes of microconstituents in terms of lutein uptake.
Table 4 Effect of antioxidant microconstituents on lutein uptake in the Caco-2 cell experiments of factorial design (Mean values with their standard errors)
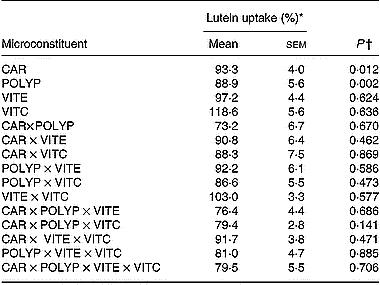
CAR, mixture of the carotenoids β-carotene and lycopene; POLYP, mixture of the polyphenols naringenin, catechin, gallic acid and caffeic acid; VITE, mixture of the main vitamin E species α- and γ-tocopherol; VITC, vitamin C.
* Lutein uptake was set at 100 % when lutein was provided without other antioxidant micronutrients.
† A P value of < 0·05 states that the compound, or the combination of compounds, significantly affected lutein uptake.
Effects of individual carotenoids
β-Carotene and lycopene, when separately co-incubated at physiological dietary concentrations (up to 0·46 μm) with lutein for 30 min, were able to decrease lutein uptake, but not significantly (data not shown).
Effects of individual polyphenols
Experiments run separately for each polyphenol showed that naringenin was the only polyphenol able to significantly impair lutein uptake (about 25 % for 25 μm- and 50 % for 150 μm-naringenin, respectively; P < 0·05, Fig. 3). We observed that the mixture of polyphenols containing 25 μm-naringenin had a similar effect to 25 μm-naringenin alone. This observation indicates that the other polyphenols tested (gallic acid, caffeic acid and (+)-catechin) had no effect on lutein uptake.

Fig. 3 Effect of a range of individual polyphenols on lutein uptake by differentiated Caco-2 TC-7 monolayers. The apical sides of the cell monolayers were rinsed with PBS and then received fetal bovine serum-free medium containing either lutein-rich mixed micelles at 0·85 μm alone (1) or 0·85 μm-lutein-rich micelles supplemented with (2) 25 μm-naringenin, (3) 150 μm-naringenin, (4) 25 μm-(+)-catechin, (5) 150 μm-(+)-catechin, (6) 50 μm-gallic acid, (7) 150 μm-gallic acid, (8) 50 μm-caffeic acid, (9) 150 μm-caffeic acid and (10) a mixture of polyphenols (25 μm-naringenin, 25 μm-(+)-catechin, 50 μm-gallic acid and 50 μm-caffeic acid). The basolateral sides received complete medium. Incubation time was 30 min. Data are means (for three assays) with their standard errors represented by vertical bars. * Mean value was significantly different from that of the control (assay performed without polyphenol) (P < 0·05).
Discussion
Given the aim of the present study was to assess the effect of dietary antioxidants on lutein absorption, we paid particular attention to the choice of both type and relative proportions of the antioxidant microconstituents. The selected antioxidants belong to the main classes of dietary microconstituents, and the relative proportions of these microconstituents were very close to those observed in the standard Western diet. In the clinical experiment, the amount of lutein contained in the test meal was set at 18 mg, i.e. about 8-fold higher than the average dietary intake, in order to accurately detect newly absorbed lutein in the chylomicron fraction (Tyssandier et al. Reference Tyssandier, Cardinault, Caris-Veyrat, Amiot, Grolier, Bouteloup, Azais-Braesco and Borel2002). Nevertheless, this amount remains close to the median daily total carotenoid intake (14 mg/d; O'Neill et al. Reference O'Neill, Carroll, Corridan, Olmedilla, Granado, Blanco, van den Berg, Hininger, Rousell, Chopra, Southon and Thurnham2001). The amounts of other antioxidant microconstituents added in the postprandial experiment were calculated in order to be close to the relative proportions found in the Western diet. We opted not to add carotenoids to the antioxidant mixture used in the clinical study since it has been established that carotenoids compete with each other for absorption (van den Berg, Reference van den Berg1999; Tyssandier et al. Reference Tyssandier, Cardinault, Caris-Veyrat, Amiot, Grolier, Bouteloup, Azais-Braesco and Borel2002), and we did not want this effect to mask an effect of other antioxidants. In the Caco-2 cell experiments, the concentration of lutein in mixed micelles was set to about 0·7 μm in order to accurately detect absorbed lutein. This represents the same concentration as found in the human duodenum after ingestion of a standard Western meal (Tyssandier et al. Reference Tyssandier, Reboul, Dumas, Bouteloup-Demange, Armand, Marcand, Sallas and Borel2003). In the Caco-2 cell experiments, the competitive carotenoids chosen were β-carotene and lycopene, which are the main dietary carotenoids. The mixture of vitamin E contained equal amounts of α-tocopherol and γ-tocopherol, which are the main dietary species of vitamin E. Finally, the selected polyphenols, i.e. naringenin, (+)-catechin, gallic acid and caffeic acid, represent the two main dietary classes of these microconstituents, i.e. flavonoids and non-flavonoids (mainly phenolic acids).
The postprandial experiment suggested that the mixture of antioxidant microconstituents diminished lutein bioavailability ( − 23 %; P = 0·07). This difference was barely not significant probably due to the high inter-individual variability in chylomicron lutein response. In fact, based on the data obtained, i.e. standard deviations and between-group differences, we calculated that twenty-five subjects would be required to establish a significant decrease in chylomicron lutein response with a power efficiency of 80 %.
Since we provided a mixture of antioxidants, we could not reach definitive conclusions on the individual effect of each class of antioxidants or on possible synergies or antagonism between antioxidants. Furthermore, since it was unfeasible to run a clinical study to assess the effect of all the combinations of antioxidants on lutein absorption, we opted for an approach using the intestinal Caco-2 cell model in order to provide insight into the possible interactions between dietary antioxidants on lutein absorption. The Caco-2 cell line was selected because it is the most frequently used model for evaluating the intestinal absorption and uptake of lipids and carotenoids (Garrett et al. Reference Garrett, Failla and Sarama1999; Ferruzzi et al. Reference Ferruzzi, Failla and Schwartz2001; Sugawara et al. Reference Sugawara, Kushiro, Zhang, Nara, Ono and Nagao2001; During et al. Reference During, Hussain, Morel and Harrison2002) and because it expresses the scavenger receptor class B type I, which facilitates lutein uptake (Reboul et al. Reference Reboul, Abou, Mikail, Ghiringhelli, Andre, Gleize, Kaloustian, Portugal, Amiot and Borel2003). We used the TC-7 clone because it is more homogeneous than the parental Caco-2 cell line (Gres et al. Reference Gres, Julian, Bourrie, Meunier, Roques, Berger, Boulenc, Berger and Fabre1998) and possesses β-carotene 15,15′-monoxygenase (During et al. Reference During, Albaugh and Smith1998), which is a key enzyme in carotenoid metabolism. The factorial design showed that carotenoids and polyphenols were the only two classes of antioxidants that had a significant effect on lutein uptake. The fact that vitamin C had no significant effect on lutein uptake apparently disagrees with a recent report stating that vitamin C facilitates lutein absorption (Tanumihardjo et al. Reference Tanumihardjo, Li and Dosti2005). However, it should be stressed that although vitamin C apparently enhanced lutein absorption rate in that study, it did not significantly affect blood lutein response, i.e. lutein bioavailability. The fact that both the mixture of carotenoids and the mixture of polyphenols significantly impaired lutein uptake raised the question as to which carotenoid(s) or polyphenol(s) were responsible for this effect. Therefore, we conducted a second series of experiments to measure the individual effect of each carotenoid and polyphenol on lutein uptake. The present results, as well as those obtained in another study (Reboul et al. Reference Reboul, Abou, Mikail, Ghiringhelli, Andre, Portugal, Jourdheuil-Rahmani, Amiot, Lairon and Borel2005a), showed that the carotenoid effect was not specific for β-carotene or lycopene but, rather, was dependent on the total concentration of carotenoids. This inhibitory effect of carotenoids on lutein uptake, which is in agreement with previous studies (van den Berg, Reference van den Berg1999; Tyssandier et al. Reference Tyssandier, Cardinault, Caris-Veyrat, Amiot, Grolier, Bouteloup, Azais-Braesco and Borel2002), is probably the result of competition between lutein and other carotenoids for uptake through the scavenger receptor class B type I located at the enterocyte brush-border membrane (Reboul et al. Reference Reboul, Abou, Mikail, Ghiringhelli, Andre, Portugal, Jourdheuil-Rahmani, Amiot, Lairon and Borel2005a). The second set of experiments also showed that naringenin was the only polyphenol that impaired lutein uptake. The specific effect exerted by naringenin needs to be further elucidated by additional experiments, but it is nevertheless significant that narignenin is the most lipophilic of all the polyphenols tested (log P = 2·52 v. 0·86, 0·82 and 0·38 for gallic acid, caffeic acid and (+)-catechin, respectively (Cooper et al. Reference Cooper, Webb and Peters1997)). It is possible that naringenin affects lutein uptake through an interaction with scavenger receptor class B type I, which is known to transport lipophilic molecules with low substrate specificity. A second hypothesis might be related to an interaction between naringenin and membrane lipids (Tachibana et al. Reference Tachibana, Fujimura and Yamada2004) which may affect the invagination of lipid raft domains containing lutein receptors.
The moderate inhibitory effect of the antioxidant mixture observed in the clinical study, as compared with the effect observed in the Caco-2 experiments, can be explained by two factors: (i) the polyphenol supplement used in the clinical study contained only 4 mg aglycone flavanones (3 mg naringenin +1 mg hesperitin), giving a lutein:aglycone flavanone ratio of 4·5 compared with 0·025 in the Caco-2 cell experiments; (ii) the flavanone glycosides (naringin and hesperidin) may not affect lutein absorption and are not very efficiently hydrolysed to their corresponding aglycones in the human digestive tract (Nemeth et al. Reference Nemeth, Plumb, Berrin, Juge, Jacob, Naim, Williamson, Swallow and Kroon2003; Walle et al. Reference Walle, Browning, Steed, Reed and Walle2005).
In conclusion, the results obtained show that some dietary antioxidants (vitamin C, vitamin E, flavones and phenolic acids) have no significant effect on lutein bioavailability, whereas it is apparently impaired by other antioxidants (carotenoids, naringenin and probably other aglycone flavanones). These findings should be taken into account when antioxidant supplements are consumed over long periods, as well as in the design of optimal human diets.
Acknowledgement
The present study was supported by INSERM and INRA (ATC Nutrition 2002, project no. A02256AS).









