Trans-fat is a class of unsaturated fatty acids that possess at least one double bond in the trans configuration. The most common trans-fatty acids in the diet are trans-octadecenoic acids (18 : 1; Steinhart et al. 2003), consisting of a large number of positional isomers (trans-4 to trans-16). Ruminant-derived products (milk and meat) contain trans-fatty acids in smaller quantities (1–8 % fatty acids, with vaccenic acid (trans-11-18 : 1) as the major trans isomer) than partially hydrogenated fats and industrially prepared food (up to 60 % fatty acids with trans-9- and trans-10-18 : 1 as the major trans isomers; Aro et al. Reference Aro, Kosmeijeir-Schuil, van den Bovenkamp, Hulshof, Zock and Katan1998; Craig-Schmidt, Reference Craig-Schmidt, Sebedio and Christie1998).
Conjugated linoleic acids (CLA) refer to a group of geometrical and positional isomers of linoleic acid (Delmonte et al. Reference Delmonte, Roach, Mossoba, Losi and Yurawecz2004). The most abundant naturally occurring CLA isomer is the cis-9, trans-11 (c9, t11)-CLA which is widely found in ruminant-related products (Kraft et al. Reference Kraft, Collomb, Moeckel, Sieber and Jahreis2003). It is formed both by anaerobic biohydrogenation of linoleic acid in the rumen (Bauman & Griinari, Reference Bauman and Griinari2003), but mainly by endogenous Δ9-desaturation (via stearoyl-CoA desaturase (SCD); EC 1·14·99·5) in the mammary gland and other tissues with trans-11-18 : 1 as the precursor (Mosley et al. Reference Mosley, Shafii, Moate and McGuire2006). This endogenous CLA synthesis has also been observed in non-ruminant animals and human subjects (Turpeinen et al. Reference Turpeinen, Mutanen, Salminen, Basu, Palmquist and Griinari2002; Kraft et al. Reference Kraft, Hanske, Moeckel, Zimmermann, Haertl, Kramer and Jahreis2006; Kuhnt et al. Reference Kuhnt, Kraft, Moeckel and Jahreis2006a).
The average daily intake of trans-fatty acids is higher in US and Canadian populations (about 5·8 g/d; 2·6 % energy intake; Food & Drug Administration, 2003, 2006) than in European populations (about 2·2 g/d; 0·9 % energy intake; van de Vijver et al. Reference van de Vijver, Kardinaal and Couet2000). Interestingly, in the USA and Canada approximately 80 % of total trans-fatty acids are currently derived from industrially processed food products containing hydrogenated vegetable oils. In contrast, in the European Union about 40 % are derived from hydrogenated vegetable oils. In the European Union, the intake of total trans-18 : 1 from ruminant fats was estimated to be from 1·3 to 1·8 g/d (Wolff, Reference Wolff1995). Thus, trans-11-18 : 1 intake was estimated at 1·0 g/d whereas CLA intake was lower and ranged between 0·1 and 0·5 g/d (Fremann et al. Reference Fremann, Linseisen and Wolfram2002; Jahreis & Kraft, Reference Jahreis and Kraft2002).
The impact of dietary trans-fatty acids and CLA on inflammatory processes and on the immune system in human subjects requires further evaluation. Trans-fatty acid intake has been related to endothelial dysfunction (Lopez-Garcia et al. Reference Lopez-Garcia, Schulze, Meigs, Manson, Rifai, Stampfer, Willett and Hu2005), inflammation (Mozaffarian et al. Reference Mozaffarian, Pischon, Hankinson, Rifai, Joshipura, Willett and Rimm2004a, Reference Mozaffarian, Rimm, King, Lawler, McDonald and Levyb), type 2 diabetes (Bray et al. Reference Bray, Lovejoy, Smith, DeLany, Lefevre, Hwang, Ryan and York2002; Lefevre et al. Reference Lefevre, Lovejoy, Smith, DeLany, Champagne, Most, Denkins, de Jonge, Rood and Bray2005) and to an increased risk of CVD (Lemaitre et al. Reference Lemaitre, King, Raghunathan, Pearce, Weinmann, Knopp, Copass, Cobb and Siscovick2006; Mensink et al. Reference Mensink, Zock, Kester and Katan2003; Mozaffarian et al. Reference Mozaffarian, Katan, Ascherio, Stampfer and Willett2006). Several studies have shown that trans-fatty acids affect plasma markers of inflammation, such as pro-inflammatory cytokines (for example, IL-6, TNFα), acute-phase proteins (for example, C-reactive protein (CRP)), and adhesion molecules (for example, intercellular adhesion molecule (ICAM)-1) (Baer et al. Reference Baer, Judd, Clevidence and Tracy2004; Lopez-Garcia et al. Reference Lopez-Garcia, Schulze, Meigs, Manson, Rifai, Stampfer, Willett and Hu2005).
In contrast to trans-fatty acids, CLA (for example, isomer dependent; c9, t11 and trans-10, cis-12) were found in cell and animal studies to have anti-inflammatory activity (suppressing eicosanoid synthesis; for example, prostaglandin E2 and prostaglandin I2 (prostacyclin; PGI2) (Bulgarella et al. Reference Bulgarella, Patton and Bull2001) and pro-inflammatory cytokines, for example, TNFα and IL-8) (Jaudszus et al. Reference Jaudszus, Foerster, Kroegel, Wolf and Jahreis2005; Ringseis et al. Reference Ringseis, Mueller, Herter, Gahler, Steinhart and Eder2006) and to reduce fatty streak formation (Kritchevsky et al. Reference Kritchevsky, Tepper, Wright, Czarnecki, Wilson and Nicolosi2004). Furthermore, CLA can alter immune function (for example, cell proliferation (Bassaganya-Riera et al. Reference Bassaganya-Riera, Pogranichniy, Jobgen, Halbur, Yoon, O'Shea, Mohede and Hontecillas2003; Hontecillas et al. Reference Hontecillas, Wannemeulher, Zimmerman, Hutto, Wilson, Ahn and Bassaganya-Riera2003); immunglobulins (Bontempo et al. Reference Bontempo, Sciannimanico, Pastorelli, Rossi, Rosi and Corino2004)). However, the majority of human CLA studies reported less consistent responses than those of the animal studies (Kelley et al. Reference Kelley, Simon, Taylor, Rudolph, Benito, Nelson and Mackey2001; Albers et al. Reference Albers, van der Wielen, Brink, Hendriks, Dorovska-Taran and Mohede2003; Tricon et al. Reference Tricon, Burdge, Kew, Banerjee, Russell, Grimble, Williams, Calder and Yaqoob2004).
The present study was designed to investigate the effects of a 6-week dietary supplementation of 3·0 g trans-11-18 : 1 and 3·0 g trans-12-18 : 1 and endogenous CLA synthesis on several biomarkers (for example, IL-6, 8, TNFα, CRP, ICAM-1, leptin, adiponectin, N metabolites, PGI2, activity of phospholipase A2, and transaminases). In addition, we determined the fatty acid composition and the incorporation of trans-11-18 : 1 and trans-12-18 : 1 and their Δ9-desaturation products (c9, t11-CLA and cis-9, trans-12-18 : 2) into lipids of peripheral blood mononuclear cells (PBMC) and the phagocytic activity of granulocytes.
Subjects and methods
Subjects and diets
The study was approved by the ethics committee of the Friedrich Schiller University of Jena (Germany). The study design and diets have been described in detail previously (Kuhnt et al. Reference Kuhnt, Kraft, Moeckel and Jahreis2006a). Twenty-four healthy subjects participated in the present study (Table 1). Throughout the entire study (8 weeks) the consumed basal diet of each subject had to contain only marginal amounts of trans-fatty acids and CLA. The subjects received written instructions to keep the conditions of the trans-fatty acid-free and CLA-free basal diet.
Table 1 Baseline data of female and male subjects at the beginning of the intervention period(Mean values and standard deviations)
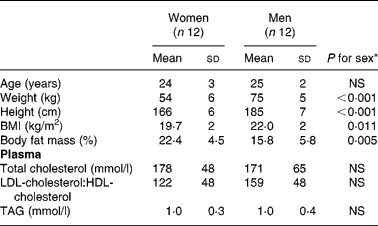
* t test; P ≤ 0·05.
The subjects were randomly assigned and divided into the control group and the test group (each group, n 12). Each study group consisted of six men and six women. The study started with a 2-week adaptation period (baseline) without supplementation. During this period all volunteers consumed daily 20 g pure commercial chocolate spread (% fatty acid methyl esters (FAME): 18 : 1, 60 %; 16 : 0, 18 %; 18 : 2, 13 %) to make the adaptation diet isoenergetic compared with the intervention diet. During the intervention period the diet of the test group was supplemented with 3·0 g trans-11-18 : 1/d and 3·0 g trans-12-18 : 1/d (% FAME in trans-isomer mixture: trans-11- and trans-12-18 : 1, 60 %; cis-11- and cis-12-18 : 1, 20 %; 18 : 0, 11 %; Natural ASA, Hovdebygda, Norway). The diet of the control group was supplemented with control oil free of CLA and trans-fatty acids to make the intervention diets isoenergetic. The control oil was a mixture of palm kernel oil and rapeseed oil (1 : 1) with a fatty acid distribution almost similar to the chocolate spread (% FAME: 18 : 1, 50 %; 16 : 0, 14 %; 18 : 2, 12 %). In order to standardise the dietary food before blood collection all subjects received fresh food every day from our department during the last week of both study periods (Table 2). Both preparations (control oil and trans-isomer mixture) were added to chocolate spread to achieve a good acceptability during the intervention period. Each subject consumed daily 20 g chocolate spread enriched with the trans-isomer mixture or the control oil.
Table 2 Daily dietary intake during the standardised diet of the study(Mean values and standard deviations)

TE, tocopherol equivalents.
* No significant treatment × sex interactions.
† Significantly different from the control group with baseline value as covariate.
‡ 1 mg TE = 1 mg α-tocopherol = 10 mg γ-tocopherol.
Blood sampling
Blood samples were collected on the last day of the standardised diet of the adaptation period (baseline; day 0) and the intervention period (day 42). Blood samples were taken between 07·30 and 08·30 hours after overnight fasting by venepuncture into EDTA-vacutainer™ tubes (BD Vacutainer Systems, Heidelberg, Germany). In addition, for eicosanoid determination the EDTA blood was mixed im- mediately with indomethacin (0·5 mmol/ml distilled water; Sigma-Aldrich, St Louis, MO, USA), an inhibitor for cyclo- oxygenase.
Preparation of peripheral blood mononuclear cells
Fresh EDTA blood was diluted 1 : 1 with PBS. The diluted blood was layered carefully onto Histopaque® (density 1·077 g/l, diluted blood : Histopaque ratio was 4 : 3; Sigma-Aldrich, Munich, Germany) and centrifuged for 30 min at 400 g at 20°C. The uppermost layer of fluid (plasma) was removed and then the opaque PBMC layer (mixture of monocytes and lymphocytes) was collected from the interphase. The PBMC were washed with PBS twice (centrifugation at 250 g, 10 min) to lower the degree of erythrocyte contamination. Cell count was determined using a Neubauer haematocytometer counting chamber (Roth, Karlsruhe, Germany).
Analysis of lipids of peripheral blood mononuclear cells
The detailed procedures and results of lipid analysis have been previously described (Kuhnt et al. Reference Kuhnt, Kraft, Moeckel and Jahreis2006a). Briefly, total lipids of PBMC (at least 20 × 106) were extracted with chloroform–methanol–water (2:1:1, by vol.). Tricosanoate (TAG, C23 : 0) was added to each lipid extract as an internal standard. FAME were prepared with 1,1,3,3-tetramethylguanidine in methanol (1 : 4, v/v, 5 min, 100°C; Sigma-Aldrich) and purified by TLC on silica gel plates (Merck, Darmstadt, Germany). FAME were separated by two different GC procedures (GC-17 V3; Shimadzu, Kyoto, Japan) and detected with a flame ionisation detector. The first GC procedure determined the fatty acid distribution from C4 to C25 carbon length including CLA using a fused silica capillary column (DB-225 ms, 60 m × 0·25 mm internal diameter with 0·25 μm film thickness; J&W Scientific, Folsom, CA, USA). The second GC method separated the cis and trans isomers of 18 : 1 using a fused silica capillary column (CP-select, 200 m × 0·25 mm internal diameter with 0·25 μm film thickness; Varian, Middelburg, The Netherlands). For both procedures the injector and detector temperatures were maintained at 260 and 270°C, respectively. H2 was used as the carrier gas at 2·22 ml/min. The first GC method was as follows: the initial oven temperature was maintained at 70°C for 2 min, then increased at 10°C/min to 180°C, then increased at 2°C/min to 220°C and held for 5 min and finally, it was increased at 2°C/min to 230°C and held for 15 min. The second GC method required isothermal conditions at 181°C. The distribution of the CLA isomers was determined using Ag+-HPLC (LC10A; Shimadzu) according to Kraft et al. (Reference Kraft, Collomb, Moeckel, Sieber and Jahreis2003). Fatty acids were identified by comparison with standard FAME (Sigma-Aldrich and Larodan, Malmö, Sweden) run previously.
Immunophenotyping
The two-colour immunophenotyping was conducted by flow cytometry in a flow cytometer FACScan™ employing simulSET™ software, simultest™ IMK-Lymphocyte test kit, and several different fluorochrome-labelled monoclonal antibodies (BD Biosciences, Heidelberg, Germany). The percentage of lymphocytes, monocytes, and granulocytes of total leucocytes (CD45 carrying cells) was determined by using CD14/CD45 gating. The fluorochrome-labelled monoclonal antibodies utilised in the subpopulations of leucocytes determinations included: total T (CD3+) lymphocytes, B (CD19+) lymphocytes, helper/inducer T (CD3+CD4+) lymphocytes, suppressor/cytotoxic T (CD3+CD8+) lymphocytes, natural killer lymphocytes (identified as CD3− CD16+ and/or CD56+) and several subsets of lymphocytes such as the activated T (CD3+/HLA-DR+) lymphocytes, CD25 (α-chain of the IL-2- receptor), CD4+CD25+ (helper cell carrying IL-2 receptor), CD54 (ICAM-1) and CD130 (IL-6 receptor-associated signal transducer).
Phagocytic process
The quantitative analysis of leucocyte phagocytosis in human blood was conducted as an ex vivo multifactorial process according to the manufacturers' instructions for the various required testing assays: Migratest® to measure chemotaxis, Phagotest® to measure ingestion of microbes, and Phagoburst® to measure oxidative burst (ORPEGEN Pharma, Heidelberg, Germany). The cell preparations were analysed by flow cytometry (FACScan™; BD Biosciences, San Jose, CA, USA) and fluorescence data were analysed with the use of CELLQUEST™ software (BD Biosciences). The Migratest® allows the quantitative determination of the chemotactic activity of neutrophilic granulocytes which have migrated through a membrane (pore size 3·0 μm) towards a gradient of the chemoattractant N-formyl-Met-Leu-Phe (fMLP). In addition, the expression of leucocyte-endothelial cell adhesion molecule-1 and the cell shape change with the forward scatter signals were determined. These measurements were conducted under fMLP (stimulated positive test samples, +fMLP) conditions and compared with incubation buffer (negative control, − fMLP) conditions. The Phagotest® and Phagoburst® measured the percentage of neutrophilic granulocytes which demonstrated phagocytosis (ingestion of bacteria) and oxidative burst rates (intracellular killing by O2-dependent mechanisms). The median fluorescence intensity enabled the measurement of the number of ingested bacteria per cell and burst activity per cell.
Cytokines
Increases in plasma concentrations of various soluble cytokines (IL-8, 1β, 6, 10, 12p70) and TNFα are indicators of inflammation. These plasma factors were analysed via a human inflammation Cytometric Bead Array kit using flow cytometry (FACScan™ instruments and CELLQUEST™ software; BD Biosciences). Samples were analysed as triplicates. Intra-assay and inter-assay CV of IL-8, 1β, 6, 10 and 12p70 were lower than 13 % (69–78 pg/ml).
Adipokines
Adipose tissue secretes a variety of biologically active molecules, adipokines, such as leptin and adiponectin. Plasma concentration of leptin was measured using an in-house RIA as described previously (Kratzsch et al. Reference Kratzsch, Berthold, Lammert, Reuter, Keller and Kiess2002). Adiponectin concentration was also measured by RIA (Linco Research, St Charles, MO, USA). Samples were analysed as duplicates. Intra-assay and inter-assay CV of leptin and adiponectin were 12·5 % (5 ng/ml) and 9·6 % (6 ng/ml), respectively.
Prostacyclin and secretory phospholipase A2 activity
The effects of the trans-11- and trans-12-18 : 1 supplementation on secretory phospholipase A2 (sPLA2) activity in plasma were assessed using an sPLA2 assay kit (Cayman Chemical, Ann Arbor, MI, USA). PGI2, an endothelial prostglandin, is quickly hydrated to its more stable metabolite 6-keto-prostaglandin F1α (6-keto-PGF1α). The plasma 6-keto-PGF1α metabolite concentrations were utilised to estimate PGI2 concentrations and were analysed by an EIA kit (Cayman Chemical). Samples were analysed as triplicates and intra-assay and inter-assay CV of 6-keto-PGF1α were lower than 15 % (50 pg/ml).
Activity of transferases and the concentrations of creatinine, bilirubin, uric acid, urea, and C-reactive protein in plasma
The activity of several transferases, specific for liver injury (γ-glutamyltransferase (EC 2·3·2·2), aspartate aminotransferase (EC 2·6·1·1), alanine aminotransferase (EC 2·6·1·2)), and plasma concentrations of total bilirubin, creatinine, uric acid and urea were determined by enzymic assays using the Synchron LX®20-system (Beckman Coulter, Fullerton, CA, USA) according to the methods of the International Federation of Clinical Chemistry and Laboratory Medicine. As an indicator of acute inflammation, CRP concentration was quantified by using a turbidimetric immunoassay assay on the Synchron LX®20-system (Beckman Coulter).
Statistical analysis
All statistical analyses were performed using SPSS software package, version 11·5 (SPSS Inc., Chicago, IL, USA). The P value ≤ 0·05 was regarded as significant. Values are reported as mean values and standard deviations. Sex-related baseline data were compared using the t test. The Kolmogorov–Smirnov test was used to test the distribution of the data. All measures were normally distributed. Data analyses were conducted as two-factor (sex and diets) ANOVA with interaction. Analysis of covariance (baseline as covariate) was used to compare data of the two treatments. Correlations were calculated by using Pearson correlation analysis.
Results
Fatty acid distribution of peripheral blood mononuclear cells
Trans-11- and trans-12-18 : 1 were incorporated into the membrane lipids of PBMC. Trans-12-18 : 1 was more readily incorporated than trans-11-18 : 1 (Table 3). c9, t11-CLA was also significantly increased. Despite the elevated trans-12-18 : 1 content in membrane lipids of PBMC, the cis-9, trans-12-18 : 2 remained unchanged. After the intervention period, the 22 : 6n-3 proportion of the test groups' PBMC membrane lipids was significantly lower than that of the control group. Other fatty acids were not affected (Table 3).
Table 3 The effects of dietary supplementation of trans-11- and trans-12-18 : 1 isomers (6 g/d; 1 : 1) on the fatty acid profile of human peripheral blood mononuclear cells lipids (% total fatty acid methyl esters)(Mean values and standard deviations)

* No significant treatment × sex interactions.
† Significantly different from the control group with baseline value as covariate.
‡ Conjugated linoleic acid.
Clinical, immunological, and inflammatory parameters
In general, the trans-isomer treatment produced no significant differences in the clinical, immunological and inflammatory parameters analysed for the two treatment groups. No treatment effects were shown on sex subgroups (Tables 4 and 5).
Table 4 The effects of the dietary supplementation of trans-11- and trans-12-18 : 1 isomers (6 g/d; 1 : 1) on the circulating immune cells and subtypes of lymphocytes(Mean values and standard deviations)

CD, cluster of differentiation.
* No significant treatment × sex interactions; no significant differences between the control and test groups with baseline value as covariate.
Table 5 The effects of the dietary supplementation of trans-11- and trans-12-18 : 1 isomers (6 g/d; 1 : 1) on the concentrations of plasma biomarkers(Mean values and standard deviations)

γ-GT, γ-glutamyltransferase; ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase; sPLA2, secretory phospholipase A2.
* No significant treatment × sex interactions; no significant differences between the control and test groups with baseline value as covariate.
Phagocytic process
The examination of the phagocytic process of granulocytes included their migration, ingestion and oxidative burst rates. No significant differences in the number of chemotactic cells after stimulation were observed between the study groups after supplementation of the intervention treatments (control group 6812 (sd 4156); test group 6459 (sd 4588)). The percentage of phagocytic granulocytes in both treatment groups was unaffected by the intervention treatments (control group 97·4 (sd 1·7) %; test group 97·8 (sd 1·9) %). In addition, no significant differences for both treatment groups were observed between the percentage of granulocytes with oxidative burst (control group 94·1 (sd 4·3) %; test group 95·8 (sd 4·2) %) and their individual cell activity.
Plasma concentrations of nitrogen metabolites, C-reactive protein and the activity of transferases
The plasma concentrations of N metabolites (total bilirubin, urea, uric acid and creatinine) did not significantly differ when the treatment groups were compared (Table 5). The concentrations of urea, uric acid and creatinine were positively correlated with total bilirubin in both groups after baseline and intervention periods (data not shown; P ≤ 0·017). Furthermore, the concentration of urea correlated with uric acid (control group r 0·614, P = 0·034; test group r 0·399, P = 0·199) and creatinine (control group r 0·586, P = 0·045; test group r 0·709, P = 0·010). The plasma concentration of CRP did not exceed 3 mg/l.
A decrease in the activity of plasma γ-glutamyltransferase, alanine aminotransferase, and aspartate aminotransferase was observed after the intervention period. However, no significant differences between the treatment groups were demonstrated. The activity of these enzymes correlated positively with each other during both study periods (data not shown; P ≤ 0·05).
Plasma concentrations of cytokines, adipokines, and 6-keto-prostaglandin F1α
The plasma concentrations of cytokines in both treatment groups were unaffected by the intervention treatment (Table 5). There were significant correlations between IL-6 and TNFα (adaptation period: control group r 0·813, P = 0·001; test group r 0·634, P = 0·027) and IL-8 and TNFα (intervention period: control group r 0·562, P = 0·057; test group r 0·763, P = 0·004).
No significant differences in the plasma concentrations of leptin and adiponectin were observed (Table 5). Sex differences were observed in plasma leptin concentrations that were independent of the treatment group; female subjects possessed higher plasma leptin concentrations than their male counterparts (female 10·5 (sd 9·4) ng/ml v. male 2·3 (sd 2·1) ng/ml; P = 0·007). In contrast, females' adiponectin concentrations were lower than that of their male counterparts (female 11·3 (sd 4·6) ng/ml v. male 14·8 (sd 4·5) ng/ml; P = 0·073). No significant correlation between plasma adiponectin and leptin was observed. The correlation between plasma adiponectin and leptin was negative but not significant for both treatment groups during both study periods (data not shown).
Another sex difference was observed in the percentage of body fat (BIA 2000-C; Data Input GmbH, Darmstadt, Germany); female subjects demonstrated a significantly higher percentage of body fat than their male counterparts independent of the treatment group (adaptation period 22·3 (sd 4·7) v. 15·8 (sd 5·8) %; intervention period 21·6 (sd 5·0) v. 14·8 (sd 5·8) %). The plasma leptin concentration was positively correlated with body fat in both sexes (adaptation period: male r 0·848, P < 0·001; female r 0·774, P = 0·005; intervention period: male r 0·786, P = 0·002; female r 0·779, P = 0·005). The plasma adiponectin concentration correlated negatively with body fat in both sexes of both study periods, but without significance (data not shown).
The activity of the sPLA2 and the plasma concentration of 6-keto-PGF1α were not different between the treatment groups.
Discussion
The incorporation of fatty acids into cellular lipids can influence their physiological functions (Kew et al. Reference Kew, Banerjee, Minihane, Finnegan, Williams and Calder2003, Reference Kew, Mesa, Tricon, Buckley, Minihane and Yaqoob2004). Trans-fatty acid intake is positively associated with inflammation and increased insulin resistance in human subjects (Baer et al. Reference Baer, Judd, Clevidence and Tracy2004; Mozaffarian et al. Reference Mozaffarian, Pischon, Hankinson, Rifai, Joshipura, Willett and Rimm2004a). Systemic inflammation has been reported as an independent risk factor for heart disease (Libby, Reference Libby2002). Therefore, changes in the long-term trans-fatty acid concentrations in human tissues result in changes to the risk of developing and/or to the rate of progression of coronary artery disease (Ascherio et al. Reference Ascherio, Katan, Zock, Stampfer and Willett1999; Mensink et al. Reference Mensink, Zock, Kester and Katan2003).
Generally, the differentiation of the physiological effects between individual trans isomers (trans-9-, trans-11-18 : 1, etc) or between different trans classes (for example, trans-16 : 1, trans-18 : 1 and trans-18 : 2 acids) has rarely been reported in the scientific literature (Mensink et al. Reference Mensink, Zock, Kester and Katan2003). In fact, recent studies demonstrated that higher levels of trans-18 : 2 and lower levels of trans-18 : 1 in erythrocyte membranes and plasma lipids are associated with higher risks of fatal IHD and sudden heart death (Lemaitre et al. Reference Lemaitre, King, Raghunathan, Pearce, Weinmann, Knopp, Copass, Cobb and Siscovick2006; Kew et al. Reference Kew, Banerjee, Minihane, Finnegan, Williams and Calder2003). In contrast, CLA, especially the c9, t11 isomer, appear to possess anti-inflammatory and anti-atherogenic properties (Kritchevsky et al. Reference Kritchevsky, Tepper, Wright, Czarnecki, Wilson and Nicolosi2004; Jaudszus et al. Reference Jaudszus, Foerster, Kroegel, Wolf and Jahreis2005; Ringseis et al. Reference Ringseis, Mueller, Herter, Gahler, Steinhart and Eder2006).
During the present study the supplemented trans isomers incorporated into the lipids of PBMC were increased. In addition, the c9, t11-CLA proportion of PBMC lipids was significantly increased as expected. The source of the increase of c9, t11-CLA concentration was most probably from Δ9-desaturation by SCD with trans-11-18 : 1 as the precursor. On the contrary, trans-12-18 : 1 was not converted to cis-9, trans-12-18 : 2. The quantity of trans-12-18 : 1 incorporated into the PBMC lipids was approximately 2-fold higher than that of trans-11-18 : 1.
In general, changes to the types and quantities of dietary fats consumed could influence the production of various cytokines and immune cell function in man (Kew et al. Reference Kew, Banerjee, Minihane, Finnegan, Williams and Calder2003). The proportion of trans-fatty acids of erythrocyte membranes has been associated with the increase of primary cardiac arrest (Lemaitre et al. Reference Lemaitre, King, Raghunathan, Pearce, Weinmann, Knopp, Copass, Cobb and Siscovick2002) and the increased concentration of biomarkers of systemic inflammation (TNFα, CRP) in patients with heart diseases (Mozaffarian et al. Reference Mozaffarian, Rimm, King, Lawler, McDonald and Levy2004b). However, there was no evidence of a relationship between trans-fatty acid concentrations in adipose tissue and sudden cardiac death (Roberts et al. 1995). In the present study, the trans-11- and trans-12-18 : 1 supplementation had no observable effect on the immune cell function and inflammation biomarkers. The determined concentrations of several cytokines, adipokines and N metabolites did not correlate with the changes of the fatty acid profiles of PBMC (data not shown).
It is possible that the increased c9, t11-CLA could compensate for the effects of the incorporated trans isomers – if they have any effects – whereby the presently synthesised concentrations of c9, t11-CLA (about 0·7 g/d, trans-11 conversion rate 25 %) are lower than in previous supplementation studies (2·4–3·0 g/d; Tricon et al. Reference Tricon, Burdge, Kew, Banerjee, Russell, Grimble, Williams, Calder and Yaqoob2004; Risérus et al. Reference Risérus, Vessby, Arnlov and Basu2004). However, despite a c9, t11-CLA-rich diet (2·4 g/d) and a diet naturally enriched with c9, t11-CLA (1·4 g) and trans-11-18 : 1 (4·7 g) in the studies of Burdge et al. (Reference Burdge, Lupoli and Russell2004), (Reference Burdge, Tricon and Morgan2005), the c9, t11-CLA content of PBMC lipids did not exceed 0·22 and 0·27 % FAME, respectively. The intake of 0·6 g c9, t11-CLA/d (Burdge et al. Reference Burdge, Lupoli and Russell2004) compared with endogenously synthesised amounts of c9, t11-CLA (0·7 g/d) during the present study (about 0·7 g/d) showed with similar baseline values (0·08 % FAME) slightly lower c9, t11-CLA incorporation into lipids of PBMC than of the endogenously synthesised c9, t11-CLA (0·12 v. 0·16 % FAME). In addition, the content of CLA in cellular lipids is dependent on their dietary intake but in general it is not proportional to the CLA intake. The CLA incorporation into cellular lipids is relatively low which can cause the inconclusive and variable effects of CLA supplementation in human subjects (Calder, Reference Calder2002).
It has been well documented that the composition of cell membranes influences the form and function of these membranes and, thus, potentially affects human health (Han et al. Reference Han, Leka, Lichtenstein, Ausman, Schaefer and Meydani2002). The supplemented trans-18 : 1 isomers were readily incorporated into lipids of PBMC and can potentially affect cell membrane functions, and transport and signalling pathways (Katz, Reference Katz2002).
Trans-fatty acids could also modulate fatty acid metabolism and, possibly, inflammatory responses of adipocytes (Mozaffarian et al. Reference Mozaffarian, Katan, Ascherio, Stampfer and Willett2006). Adipose tissue acts as an endocrine organ and synthesises adipokines which are suspected of playing a role in inflammation (Nakanishi et al. Reference Nakanishi, Yamane, Kamei, Nojima, Okubo and Kohno2005). Generally, leptin is secreted at concentrations which are proportional to the amount of stored lipids in the human body, and this tendency was observed in the present study. Adiponectin is related to CVD and the metabolic syndrome (Kumada et al. Reference Kumada, Kihara and Sumitsuji2003). No changes of leptin and adiponectin concentrations after the trans-11- and trans-12-18 : 1 supplementation were shown during the present study (Table 5). It is known that leptin is involved in the regulation of SCD which is responsible for the conversion of trans-11-18 : 1 to c9, t11-CLA. Leptin suppressed the expression and activity of SCD in mice (Cohen & Friedman, Reference Cohen and Friedman2004). However, in the present study, the concentration of leptin was not associated to the activity of SCD. The SCD activity was estimated by desaturation indices of serum fatty acids (cis-9-18 : 1/18 : 0 and cis-9-16 : 1/16 : 0; Santora et al. Reference Santora, Palmquist and Roehrig2000).
Trans-fatty acids (for example, trans-11- and trans-12-18 : 1) as well as CLA are suspected of inducing oxidative stress (8-iso-PGF2α, an isoprostane biomarker of oxidative stress; Turpeinen et al. Reference Turpeinen, Mutanen, Salminen, Basu, Palmquist and Griinari2002; Risérus et al. Reference Risérus, Basu, Jovinge, Fredrikson, Arnlov and Vessby2002). In contrast, the biomarker of oxidative stress was not affected in a recent study with the supplementation of 3·6 g trans-11-18 : 1/d over 5 weeks (Tholstrup et al. Reference Tholstrup, Raff, Basu, Nonboe, Sejrsen and Straarup2006). Nakanishi et al. (Reference Nakanishi, Yamane, Kamei, Nojima, Okubo and Kohno2005) stated that the plasma adiponectin and leptin concentrations were associated with oxidative stress levels. After the application of intervention treatments in the present study, the urinary 8-iso-PGF2α concentrations were observed at higher levels in the test group than those levels observed in the control group (Kuhnt et al. Reference Kuhnt, Wagner, Kraft, Basu and Jahreis2006b). However, no correlation between urinary 8-iso-PGF2α concentrations to leptin and adiponectin concentrations as well as to trans-11-18 : 1, trans-12-18 : 1 and CLA of cellular lipids was found (data not shown).
Trans-fatty acids can also modulate monocyte and macrophage activity as manifested by increased production of cytokines (Han et al. Reference Han, Leka, Lichtenstein, Ausman, Schaefer and Meydani2002). The concentrations of TNFα, IL-1β, IL-6 as well as CRP were considerably increased during the development and progression of inflammation and were reported to be involved in the development of atherosclerotic lesions in man. CRP is increasingly acknowledged as an independent risk factor for CVD and metabolic syndrome (Ridker, Reference Ridker2003).
Recent studies showed that changes in quantity of intake of trans-fatty acids were positively related to changes in plasma IL-6, TNFα (Han et al. Reference Han, Leka, Lichtenstein, Ausman, Schaefer and Meydani2002) and CRP concentrations (Baer et al. Reference Baer, Judd, Clevidence and Tracy2004; Mozaffarian et al. Reference Mozaffarian, Pischon, Hankinson, Rifai, Joshipura, Willett and Rimm2004a; Lopez-Garcia et al. Reference Lopez-Garcia, Schulze, Meigs, Manson, Rifai, Stampfer, Willett and Hu2005). In addition, the serum IL-6 concentration was strongly associated with PBMC phospholipid concentrations (Kew et al. 2003). Tholstrup et al. (Reference Tholstrup, Raff, Basu, Nonboe, Sejrsen and Straarup2006) reported in a butter supplementation study with healthy men (normal BMI) that both trans-11-18 : 1 and c9, t11-CLA concentrations increased in plasma, but the plasma CRP concentrations were unchanged. In contrast, in a study of CLA supplementation in human subjects, especially with trans-10, cis-12-CLA, plasma CRP concentrations were increased (Risérus et al. Reference Risérus, Basu, Jovinge, Fredrikson, Arnlov and Vessby2002). In the present study, no significant differences in the plasma concentrations of any IL, TNFα and CRP were observed during the trans-11- and trans-12-18 : 1 intervention. Nevertheless, it is important to note that the plasma cytokine concentration represents the general overall level of the complete body (dilution effects) and not the concentration at the endothelium. Furthermore, the method of CRP concentration determination was fairly insensitive, possessing a detection limit of 3 mg/l. However, at present little is known about the relevance of low concentrations of CRP (0·3 to 1·5 mg/l) in apparently healthy subjects. In a recent study the correlation of plasma CRP and CHD was assessed as relatively moderate (Danesh et al. Reference Danesh, Wheeler, Hirschfield, Eda, Eiriksdottir, Rumley, Lowe, Pepys and Gudnason2004). Thus, it is arguable whether the correlation of trans-fatty acid intake and plasma CRP is evident. Moreover, the pro-inflammatory effects of dietary trans-fatty acids were observed in women with increased BMI (Mozaffarian et al. Reference Mozaffarian, Pischon, Hankinson, Rifai, Joshipura, Willett and Rimm2004a). This observation suggests that the trans-fatty acid intake could be related to effects on and responses of adipose tissue or stored fat. In subjects with normal BMI no significant relationship was observed in the present study and in the studies of Mozaffarian et al. (Reference Mozaffarian, Pischon, Hankinson, Rifai, Joshipura, Willett and Rimm2004a) and Tholstrup et al. (Reference Tholstrup, Raff, Basu, Nonboe, Sejrsen and Straarup2006).
Trans-fatty acids could change lipoprotein metabolism (Mensink et al. Reference Mensink, Zock, Kester and Katan2003). However, in the present study with normocholesterolaemic subjects the total cholesterol:HDL-cholesterol ratio and the LDL-cholesterol:HDL-cholesterol ratio were not affected by the study treatments. In the study with 3·6 g trans-11-18 : 1/d over 5 weeks no changes of serum lipids were observed as well (Tholstrup et al. Reference Tholstrup, Raff, Basu, Nonboe, Sejrsen and Straarup2006).
One cell study confirmed that CLA can directly reduce the production of 6-keto-PGF1α in human vein endothelial cells (Torres-Duarte et al. Reference Torres-Duarte and Vander Hoek2003). Trans-fatty acids could also affect thrombogenesis due to their influence on the eicosanoid synthesis. However, in a human intervention study the platelet activation and endothelial PGI2 production was unchanged during a 5-week diet supplemented with trans-fatty acids from hydrogenated vegetable oils compared with a diet containing SFA (both about 9 % energy intake; Turpeinen et al. Reference Turpeinen, Wuebert, Aro, Lorenz and Mutanen1998). In addition, in a rat study, a diet rich in trans-18 : 1 fatty acids decreased the arachidonic acid of aorta and platelet phospholipids, yet no observable effects were detected in the plasma PGI2 and TXB2 concentrations which might result from an adequate supply of linoleic acid (Mahfouz & Kummerow, Reference Mahfouz and Kummerow1999). The sPLA2 could be additionally a relevant biomarker for atherogenesis. However, in the present study the sPLA2 activity and the plasma concentration of 6-keto-PGF1α were not influenced by the trans-11- and trans-12-18 : 1 supplement treatment. No correlation with trans-11- and trans-12-18 : 1, or c9, t11-CLA proportions of PBMC lipids of both treatment groups were observed (data not shown).
Trans-fatty acid intake has been reported to increase the concentrations of biomarkers related to endothelial dysfunction (Lopez-Garcia et al. Reference Lopez-Garcia, Schulze, Meigs, Manson, Rifai, Stampfer, Willett and Hu2005). Diets rich in CLA (c9, t11 and trans-10, cis-12) and trans-11-18 : 1 did not affect blood pressure and arterial elasticity in healthy men (Raff et al. Reference Raff, Tholstrup, Sejrsen, Straarup and Wiinberg2006). The present study showed no effects on ICAM-1 on total leucocytes. Unfortunately, we did not investigate other variables that represent endothelial function (for example, E-selectin, blood pressure).
Dietary fatty acids could affect immune-relevant cells, for example, decrease of lymphocyte proliferation and/or activation, (Thies et al. Reference Thies, Nebe-von-Garon, Powell, Yaqoob, Newsholme and Calder2001) and oxidative burst rate by neutrophils (Varming et al. Reference Varming, Schmidt, Svaneborg, Moller, Lervang, Grunnet, Jersild and Dyerberg1995). A diet high in hydrogenated fats, however, did not affect lymphocyte proliferation (Han et al. Reference Han, Leka, Lichtenstein, Ausman, Schaefer and Meydani2002). The trans-fatty acid composition of the membranes could influence the activity of monocytes and macrophages and this might be relevant for atherosclerotic processes. At present, little if any research has been published concerning the influence of trans-fatty acid isomers on the phagocytic process in human subjects. In the present study, regardless of the extent of trans-11- and trans-12-18 : 1 incorporation, no significant effects were observed in the cell migration, ingestion and oxidative burst of active cells.
Some studies have reported an association between the intake of trans-fatty acids and the increased risk of CHD in general. Unfortunately, most of these data are from epidemiological studies (for example, Nurses' Health Study) which are often inconclusive. At present, it is still unknown whether there are any distinctly different effects from the sources of trans-fatty acids (ruminant or industrial; Weggemans et al. Reference Weggemans, Rudrum and Trautwein2004), their isomeric distribution, and their general proportion of individual isomers (trans-9- v. trans-11-18 : 1).
In our opinion, conducting long-term trials to test the effects of trans-fatty acid intake would be unethical considering the suggested adverse effects on serum lipids and inflammation. Therefore, in the present study the supplementation period with the high amount of 6 g trans-fatty acid isomers/d over 6 weeks can be classified as a period of high impact on the immune system.
Both supplemented trans isomers (trans-11- and trans-12-18 : 1) and the synthesised c9, t11-CLA were incorporated into PBMC lipids at least without influencing biomarker concentrations of inflammation and immune function. The Δ9-desaturation of trans-11-18 : 1 appears to be the key in differentiating the naturally derived trans-11-18 : 1 isomer from trans-9-18 : 1, trans-10-18 : 1, and as presently shown from the trans-12-18 : 1.
Nevertheless, due to the observed increase of the biomarker 8-iso-PGF2α and the inconsistent and limited published research concerning the effects of trans-fatty acids in human subjects, it is still highly advisable that a general reduction of daily trans-fatty acid intake is recommended, especially in the US and Canadian populations. Further research is required to investigate the effects of the consumption of individual trans-fatty acid isomers on human health.
Acknowledgements
The present study was supported by The German Research Foundation (DFG), JA 893.







