Obesity is associated with chronic, low-grade inflammation, which has been suggested to underlie the pathogenesis of various obesity-related morbidities including type 2 diabetes, CVD and the metabolic syndrome( Reference Esser, Legrand-Poels and Piette 1 – Reference Chawla, Nguyen and Goh 3 ). The inflammatory state of adipose tissue resulting from either genetically or diet-induced obesity has been well described (reviewed in Wensveen et al.( Reference Wensveen, Valentic and Sestan 4 ) and Huh et al.( Reference Huh, Park and Ham 5 )), and it has been suggested that obesity-induced inflammation may also be systemic( Reference Fontana, Eagon and Trujillo 6 ). Emerging evidence suggests that, as a consequence of increased adiposity, cell-mediated immune functions are dysregulated and impaired, which may contribute to compromised host defence( Reference Costanzo, Taylor and Dutt 7 , Reference Foulds, Boysen and Crane 8 ). Impaired host defence is the main factor contributing to increased susceptibility to infection in obese individuals( Reference Bochicchio, Joshi and Bochicchio 9 , Reference Fuhrman, Bonmarin and Bitar 10 ). The spleen is the largest lymphoid organ in the body involved in the regulation of peripheral immune responses (reviewed in Bronte & Pittet( Reference Bronte and Pittet 11 )). In rodents, diet-induced obesity reduces splenic mRNA expression of pro-inflammatory cytokines( Reference Lamas, Martinez and Marti 12 ), in contrast to the inflammatory state in adipose tissue. Further, spleen-derived IL-10, a potent anti-inflammatory cytokine, has been shown to mediate inflammatory processes in other metabolic tissues, namely adipose and liver( Reference Gotoh, Inoue and Masaki 13 ). This suggests that the systemic inflammatory processes, in the context of obesity, may be involved in mediating inflammation in other metabolically active tissues. However, information regarding the impact of high-fat diet consumption and the resulting obesity on inflammatory and adaptive immune responses in peripheral lymphoid tissues is limited.
Dietary intervention is an appealing approach to ameliorating metabolic consequences and co-morbidities of obesity. Many plant-based foods and plant-derived bioactive phytochemicals have been shown to have varied efficacy in this regard. Among them, blueberry has been associated with several cardiovascular and metabolic health benefits( Reference Kay and Holub 14 – Reference Stull, Cash and Johnson 16 ). The beneficial effects of blueberry may be related to the fact that they are rich in anthocyanins, phenolics, ascorbic acid and other bioactive compounds that have antioxidant and anti-inflammatory properties( Reference Prior, Cao and Prior 17 , Reference Kang, Thakali and Jensen 18 ). As individual bioactive compounds, anthocyanins have been shown to reduce the concentration of pro-inflammatory cytokines and mediators, both in vivo and in vitro ( Reference Zhu, Ling and Guo 19 ). As a whole food, supplementation with blueberry to genetically( Reference Vendrame, Daugherty and Kristo 20 ) and diet-induced( Reference DeFuria, Bennett and Strissel 21 ) obese rodents reduced expression of pro-inflammatory cytokines in adipose tissue. These observations suggest that blueberry or blueberry-derived bioactive components may attenuate obesity-associated dysfunctional inflammatory processes within adipose tissue. However, little is known about the effect of blueberry supplementation on immune and inflammatory responses in peripheral lymphoid tissues and thus on host defence and regulation of inflammation. To address this issue, in this study we investigated the effects of dietary blueberry supplementation on cell-mediated immune and inflammatory responses in mice made obese by high-fat-diet (HFD) feeding.
Methods
Animals and diets
Male C57BL/6 mice were obtained from Jackson Laboratories at 5 weeks of age and housed individually at the Jean Mayer USDA Human Nutrition Research Center on Aging, as previously described( Reference DeFuria, Bennett and Strissel 21 , Reference Strissel, Stancheva and Miyoshi 22 ). After acclimation for several days, mice were randomly assigned to receive one of three pelleted experimental diets (n 9–11/group). Diets were fed ab libitum for either 8 or 12 weeks. Experimental diets (from Research Diets) were as follows: low-fat diet (LFD) containing 10 % energy from fat (no. D12450B), HFD containing 60 % energy from fat (no. D12492) or HFD supplemented with 4 % (wt:wt) freeze-dried whole blueberry powder (HFD+B). Experimental diet composition and anthocyanin composition of the blueberry powder has been previously described and reported by DeFuria et al.( Reference DeFuria, Bennett and Strissel 21 ). The total anthocyanin content of the blueberry powder was 31·44 g/kg dry weight and the major anthocyanins included peonidin-3-glucoside (44·3 %), malvidin-3-arabinoside (29·0 %), peonidin-3-arabinoside (13·9 %) and delphinidin-3-galactoside (4·4 %)( Reference DeFuria, Bennett and Strissel 21 ). Briefly, the blueberry powder provided by US Highbush Blueberry Council was a 1:1 blend of Vaccinium ashei (Tifblue) and Vaccinium corumbosym (Rubel). Energy from total carbohydrates and sucrose was balanced in the HFD and HFD+B diets. The blueberry power provided 14·5 MJ/kg, and contributed 2·7 % of total energy in the HFB+B diet. The relevance of the experimental diet supplemented with 4 % blueberry powder to human consumption was calculated based on previous calculations( Reference Pae, Ren and Meydani 23 ). On the basis of the average consumption of 3 g diet/d per mouse, a diet containing 4 % blueberry powder would provide mice with a daily intake of 0·12 g of blueberry powder or 4·8 g/kg body weight for a mouse weighing 25 g. The intake per body weight in mice was converted to intake per body weight in humans by using an isoenergetic calculation (based on mice consuming 50 kJ/d (12 kcal/d) and humans consuming 8368 kJ/d (2000 kcal/d) or using a factor of 14). As such, 4·8 g/kg body weight for mice is equivalent to 0·34 g of blueberry powder/kg body weight for humans or 24 g/d for a 70-kg person. As fresh blueberries contain approximately 85 % water, consuming 24 g of dried blueberries/d is equivalent to 160 g of fresh blueberries, or roughly one cup (150 g) per day.
The institutional and national guidelines for the care and use of animals were followed, and all experimental procedures involving animals were approved by the Tufts University Institutional Animal Care and Use Committee.
Ex vivo T-cell proliferation
After 8 or 12 weeks, mice were euthanised and spleens were collected aseptically, weighed and single-cell suspensions were prepared as previously described( Reference Wu, Guo and Ren 24 ). Splenocytes were seeded into ninety-six-well round-bottom cell culture plates (2×105/well) and incubated in the presence of T-cell mitogens: concanavalin A (Con A) (Sigma-Aldrich) at 0·5, 1·5 or 3 µg/ml; phytohaemagglutinin (PHA) (Difco Laboratories) at 2·5, 5 or 20 µg/ml; or plate-coated anti-T-cell receptor antibodies (anti-CD3) at 1 or 5 µg/ml without or with soluble anti-CD28 (anti-CD3/CD28; BD Biosciences) at 2 µg/ml for 72 h. Cultures were pulsed with 0·5 µCi [3H]-thymidine during the final 4 h of incubation. Cells were harvested onto glass fibre filter mats, and cell proliferation was quantified as the amount of [3H]-thymidine incorporation into DNA by liquid scintillation counting in a 1205 Betaplate counter (Wallac). Data are expressed as counts per min.
Ex vivo cytokine production
Splenocytes in twenty-four-well culture plates (3×106/well) were cultured in the presence of Con A (1·5 µg/ml) or lipopolysaccharide (LPS, 1 µg/ml) for 24 h for IL-1β, IL-6, TNF-α or in the presence of Con A (1·5 µg/ml) or anti-CD3 (5 µg/ml)/anti-CD28 (2 µg/ml) for 48 h for interferon-γ (IFN-γ), IL-2, IL-4 and IL-10 production. The supernatants were collected at the end of incubation and measured using ELISA kits according to the manufacturer’s instructions for IL-1β (R&D Systems), IL-6, TNF-α, IFN-γ, IL-2, IL-4 and IL-10 (all from BD Biosciences). All measurements were recorded in duplicate with CV<10 %.
Statistical analyses
Data are reported as means with their standard errors unless indicated otherwise. Data were analysed using one-way ANOVA in IBM SPSS Statistics (version 22) with diet as the main effect. In cases in which a significant main effect of diet was found, post hoc analysis was performed using the Duncan adjustment to determine differences between diet groups. Sample size was determined by power calculation using the SYSTAT Software (Systat Software, Inc.). A clinically relevant and meaningful change at 30 % in inflammatory markers was used to assess statistical power of the study. The conditions were as follows: one-way ANOVA, three groups, expected mean difference at 30 % with standard deviation at 20 %, with power >80 % and α=0·05. Expected mean difference of 30 % is a meaningful and clinically relevant level of change, and 20 % sd is a common level of variation for C57BL/6 mice in terms of individual differences and response to intervention. Variables that were not normally distributed were log-10-transformed before statistical analysis. The following variables were log-10-transformed before statistical analysis: liver weight, subcutaneous fat weight, gonadal fat weight, 12-week proliferation with Con A 3, PHA 5, PHA 20 and anti-CD3, 8-week IL-2 with Con A, 8-week IL-10 with CD3/28 and PHA. Differences at P≤0·05 (two-sided) were considered significant.
Results
Effects of blueberry on high-fat diet-induced body weight gain and fat accretion
After 12 weeks, mice in both HFD groups had gained significantly more weight (Fig. 1), and had greater final body weights (Fig. 1, Table 1) and fat mass (both subcutaneous and visceral depots) compared with LFD-fed mice (Table 1). Among the two HFD groups, HFD+B-fed mice gained insignificantly more weight (10 %) than HFD-fed mice, reflecting significantly greater fat accretion in both subcutaneous and visceral depots (P<0·001; Table 1). Greater weight gain and adipose mass among HFD+B mice can be attributed to approximately 17 % greater daily energy intake as compared with mice fed HFD alone (Table 1).
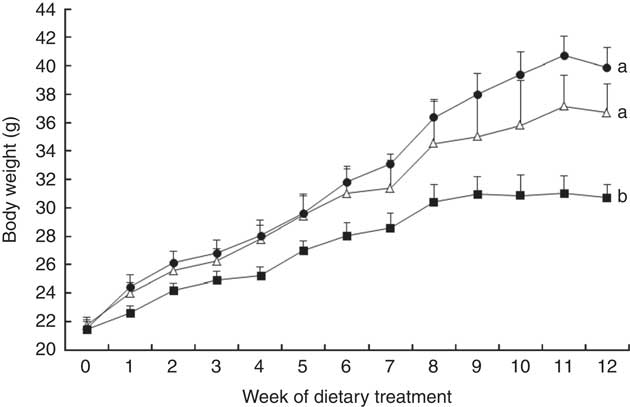
Fig. 1 Body weight of C57BL/6 mice fed a low-fat diet (LFD, ![]() ), high-fat diet (HFD,
), high-fat diet (HFD, ![]() ) or a high-fat diet with blueberry (HFD+B,
) or a high-fat diet with blueberry (HFD+B, ![]() ) for 12 weeks. Values are means with their standard errors. Multiple comparisons between diet groups have been performed with Duncan adjustment. a,b Mean body weights from each diet group with unlike letters were significantly different (P<0·05).
) for 12 weeks. Values are means with their standard errors. Multiple comparisons between diet groups have been performed with Duncan adjustment. a,b Mean body weights from each diet group with unlike letters were significantly different (P<0·05).
Table 1 Anthropometric measurements and energy intake of C57BL/6 mice fed a low-fat diet (LFD), high-fat diet (HFD) or a high-fat diet with blueberry (HFD+B) for 12 weeksFootnote * (Mean values with their standard errors)
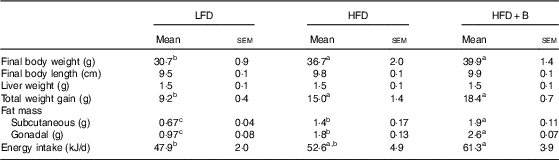
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* Multiple comparisons between diet groups have been performed with Duncan adjustment.
Ex vivo T-cell proliferation
In the ex vivo cell proliferation experiments, different concentrations of Con A, PHA, anti-CD3 or anti-CD3/CD28 were used. The optimal concentrations of each stimulation used to induce T-cell proliferation are shown in Fig. 2 (12 weeks) and online Supplementary Fig. S1 (8 weeks). Similar results were obtained with the non-optimal concentrations of Con A (0·5 µg/ml) and PHA (2·5 µg/ml) (results not shown).
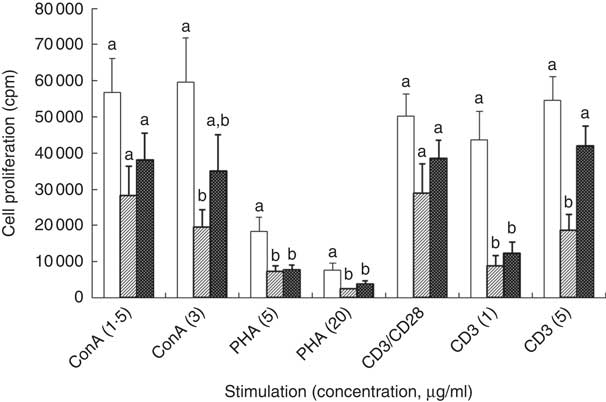
Fig. 2 Effect of feeding a low-fat diet (LFD, ![]() ), high-fat diet (HFD,
), high-fat diet (HFD, ![]() ) or a high-fat diet with blueberry (HFD+B,
) or a high-fat diet with blueberry (HFD+B, ![]() ) for 12 weeks on T-cell proliferation following stimulation of C57BL/6 mice. ConA, concanavalin A; PHA, phytohaemagglutinin; CD, cluster of differentiation. Splenocytes were cultured in the presence of T-cell mitogens Con A (1·5 or 3 µg/ml), PHA (5 or 20 µg/ml) or anti-T cell receptor (anti-CD3; 1 or 5 µg/ml) without or with soluble anti-CD28 (2 µg/ml) (CD3/CD28) for 72 h. Cultures were pulsed with [3H]-thymidine during the final 4 h of incubation. T-cell proliferation was quantified as the amount of [3H]-thymidine incorporation into DNA by liquid scintillation counting, and data are expressed as counts per min (cpm). Multiple comparisons between diet groups have been performed with Duncan adjustment. a,b T-cell proliferation from each diet group with unlike letters were significantly different (P<0·05).
) for 12 weeks on T-cell proliferation following stimulation of C57BL/6 mice. ConA, concanavalin A; PHA, phytohaemagglutinin; CD, cluster of differentiation. Splenocytes were cultured in the presence of T-cell mitogens Con A (1·5 or 3 µg/ml), PHA (5 or 20 µg/ml) or anti-T cell receptor (anti-CD3; 1 or 5 µg/ml) without or with soluble anti-CD28 (2 µg/ml) (CD3/CD28) for 72 h. Cultures were pulsed with [3H]-thymidine during the final 4 h of incubation. T-cell proliferation was quantified as the amount of [3H]-thymidine incorporation into DNA by liquid scintillation counting, and data are expressed as counts per min (cpm). Multiple comparisons between diet groups have been performed with Duncan adjustment. a,b T-cell proliferation from each diet group with unlike letters were significantly different (P<0·05).
Impacts of HFD and HFD+B feeding on T-cell proliferation were observed after 12 weeks of feeding (Fig. 2), but not after 8 weeks (online Supplementary Fig. S1). Relative to mice fed LFD, T-cell proliferation in response to Con A was reduced in mice fed HFD (Fig. 2). Notably, the inhibitory effect of HFD on T-cell proliferation in response to Con A or anti-CD3 was partially attenuated by HFD+B feeding (Fig. 2). In response to 5 ug/ml anti-CD3, T cells from HFD+B-fed mice evinced 1·2-fold greater cell proliferation as compared with HFD-fed mice (P=0·028), making it not significantly different from that observed in LFD-fed mice (Fig. 2).
Ex vivo production of cytokines by splenocytes
CD3/CD28-stimulated splenocytes from mice fed HFD or HFD+B for 8 weeks produced significantly (54 %) less IL-4 as compared with splenocytes from mice fed LFD (Table 2). Feeding HFD or HFD+B diet for 8 weeks had no significant effect on the ex vivo production of any other cytokines measured (Table 2). When feeding extended to 12 weeks, splenocytes from HFD-fed mice continued to produce less IL-4 in response to anti-CD3/CD28; additionally, they produced significantly less IL-6 and TNF-α in response to LPS and less IFN-γ in response to Con A as compared with splenocytes from LFD-fed mice (P<0·05 for all; Table 3). These results indicate that 12 weeks of HFD feeding compromised the acute inflammatory response (see Discussion). Notably, the protective effects of blueberry supplementation on cytokine production emerged after 12 weeks of dietary treatment. At this time, splenocytes from mice fed HFD+B produced IL-4 comparable with that seen in LFD-fed mice but significantly higher compared with HFD-fed mice (Table 3). No difference was found among diet groups in production of IL-1β, IL-2 or IL-10 (Table 3).
Table 2 Effect of feeding a low-fat diet (LFD), high-fat diet (HFD) or a high-fat diet with blueberry (HFD+B) for 8 weeks on ex vivo cytokine production by stimulated splenocytesFootnote * of C57BL/6 mice (Mean values with their standard errors)
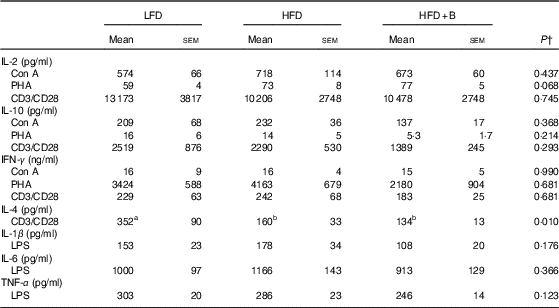
Con A, concanavalin A; PHA, phytohaemagglutinin; CD, cluster of differentiation; IFN-γ, interferon-γ; LPS, lipopolysaccharide.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* Cytokine concentrations in spleen supernatant after culture for 24 h (IL-1β, IL-6, TNF-α) stimulated with LPS (1 µg/ml), or 48 h (IL-2, IL-4, IL-10 and IFN-γ) with mitogens (Con A (1·5 µg/ml), CD3 (5 µg/ml) or CD28 (2 µg/ml). Each of the measures were conducted in duplicate (CV<10 %).
† P value of the main effect of diet analysed by one-way ANOVA. Multiple comparisons between diet groups have been performed with Duncan adjustment.
Table 3 Effect of feeding a low-fat diet (LFD), a high-fat diet (HFD) or a high-fat diet with blueberry (HFD+B) for 12 weeks on ex vivo cytokine production by stimulated splenocytesFootnote * of C57BL/6 mice (Mean values with their standard errors)
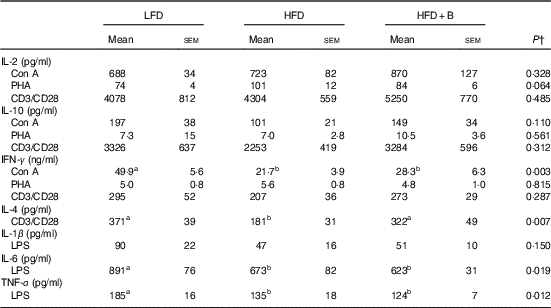
Con A, concanavalin A; PHA, phytohaemagglutinin; CD, cluster of differentiation; IFN-y, interferon-γ; LPS, lipopolysaccharide.
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* Cytokine concentrations in spleen supernatant after culture for 24 h (IL-1β, IL-6, TNF-α) with LPS (1 µg/ml) or 48 h (IL-2, IL-4, IL-10 and IFN-γ) with mitogens (Con A (1·5 µg/ml), CD3 (5 µg/ml) and CD28 (2 µg/ml). Each of the measures were conducted in duplicate (CV<10 %).
† P value of the main effect of diet analysed by one-way ANOVA. Multiple comparisons between diet groups have been performed with Duncan adjustment.
Discussion
The bioactive components anthocyanins, as well as phenolics and ascorbic acid, present in blueberry have been demonstrated to have high antioxidant capacity and anti-inflammatory potential( Reference Kang, Thakali and Jensen 18 , Reference Johnson, de Mejia and Fan 25 , Reference Nile and Park 26 ). It has recently been suggested that dietary supplementation with blueberry may also ameliorate the immune dysfunction that occurs in the context of obesity (reviewed in Shi et al.( Reference Shi, Loftus and McAinch 27 )). In the present study, we demonstrate that diet-induced obesity impairs peripheral T-cell-mediated and inflammatory responses and show for the first time that supplementing a HFD with blueberry powder may partially attenuate these impaired functions.
As expected, feeding a HFD for 12 weeks resulted in obesity with elevated final body weight and fat mass compared with LFD-fed mice. Mice fed the HFD supplemented with 4 % (w/w) blueberry powder (HFD+B) were not protected against diet-induced obesity, which is consistent with the findings in human studies that dietary blueberry supplementation does not reduce weight gain in obese subjects( Reference Basu, Du and Leyva 15 , Reference Stull, Cash and Johnson 16 , Reference Qin, Xia and Ma 28 ). Rather, HFD+B-fed mice were approximately 3 g heavier at the end of the 12-week dietary treatment and had greater gonadal and subcutaneous fat accretion compared with mice fed HFD (Table 1). These findings of enhanced weight and adiposity in mice fed HFD+B are consistent with the previous studies using mouse models but with varied obesogenic diets, blueberry formulations and/or feeding time courses( Reference DeFuria, Bennett and Strissel 21 , Reference Prior, Wu and Gu 29 , Reference Prior, Wilkes and Rogers 30 ). The enhanced weight gain and fat accretion reported for mice fed blueberry-supplemented obesogenic diets reflects, in whole or part, greater food (thus energy) intake (Table 1;( Reference Prior, Wu and Gu 29 , Reference Prior, Wilkes and Rogers 30 )). As the HFD and HFD+B diets used in our earlier( Reference DeFuria, Bennett and Strissel 21 ) and present study are balanced for total carbohydrate and sucrose content, greater intake of the HFD+B diet is likely to reflect greater palatability. Clearly, pair-feeding studies are required to rigorously determine the effects of blueberry or blueberry constituents on body mass and composition, independent of energy intake.
Inflammation is the body’s protective response to injury from infection and trauma, and it is vital for eliminating both the causes of injury (e.g. microbes) and the consequences of injury (e.g. dead tissues). Inflammation is resolved after recovery from injury. In contrast, obesity-associated inflammation is a chronic (non-resolving), low-grade inflammatory state, which is suggested not only to be involved in the pathogenesis of several major chronic, non-infectious diseases (reviewed in Lumeng & Saltiel( Reference Lumeng and Saltiel 31 )), but also to impair normal immune responses against pathogens (reviewed in Milner & Beck( Reference Milner and Beck 32 )). In the present study, we used bacterial endotoxin LPS and T-cell mitogens/T-cell receptor (TCR) antibodies as ex vivo stimulatory agents to mimic antigen/pathogen-induced inflammatory and T-cell-mediated responses within the spleen, a major organ of the peripheral immune system. We observed that feeding a HFD for 12 weeks inhibited T-cell proliferation, indeed suggesting that diet-induced obesity results in dysfunction of the systemic immune system. Supplementation of HFD with blueberry attenuated the HFD-associated reduction in ex vivo T-cell proliferation when splenocytes were stimulated with anti-CD3 and also, to a less degree, when stimulated with Con A depending on the concentrations used. Protective actions of blueberry in the context of HFD were also observed when other effector functions of T cells were examined, specifically the anti-CD3/CD28-stimulated production of T helper (Th) 1 cytokine IFN-γ and Th2 cytokines IL-4 and IL-10 (Table 3). IL-4 is an anti-inflammatory cytokine and hallmark of Th2 differentiation( Reference Nelms, Keegan and Zamorano 33 , Reference Swain, Weinberg and English 34 ), in which Th2-polarised cells are involved in a positive feedback loop to produce more IL-4, among other Th2 cytokines (IL-13). In response to IL-4, in vitro adipocyte macrophage differentiation is polarised to an M2, or ‘alternatively activated’ macrophage phenotype( Reference Odegaard and Chawla 35 ). M2 macrophages are associated with the resolution of inflammation by down-regulating production of pro-inflammatory cytokines and by reciprocally up-regulating anti-inflammatory cytokine production (reviewed in Gordon( Reference Gordon 36 )). Therefore, we hypothesise that restoration of IL-4 production may be an alternative mechanism whereby the systemic immune system mediates the previously demonstrated anti-inflammatory actions of blueberry on adipose tissue( Reference DeFuria, Bennett and Strissel 21 ). Impaired lymphocyte proliferation and cytokine production observed in our study are consistent with previously reported impaired T-cell proliferative responses in animal studies using HFD-induced obesity mouse models( Reference Lamas, Martinez and Marti 12 , Reference Sato Mito, Suzui and Yoshino 37 ), as well as human studies in obese human subjects compared with non-obese controls( Reference Tanaka, Inoue and Isoda 38 ). The fact that a diet supplemented with 4 % blueberry powder was able to restore some of the obesity-associated systemic immune dysfunction in mice is very promising. This is equivalent to a human (based on a body weight of 70 kg) consuming approximately one cup (150 g) of fresh blueberries per day, which is a physiologically relevant and attainable amount of blueberry that could be easily incorporated into the human diet.
Relative to the spontaneous, low-grade inflammatory state at the systemic level, stimulation-induced inflammation is a key step in initial host defence responses. In this study, we examined the production of pro-inflammatory cytokines IL-1β, IL-6 and TNF-α after LPS stimulation. The primary sources of these cytokines in spleen are innate immune cells including monocytes/macrophages, natural killer cells and dendritic cells, in particular macrophages. LPS as a bacterial endotoxin can bind to the pathogen recognition receptor, toll-like receptor-4, to activate macrophages eventually leading to gene activation and synthesis of cytokines. In addition, one of the most important effector functions of CD4+T cells (helper T cells) is to activate macrophages by releasing IFN-γ. Therefore, the decrease in IFN-γ production following a HFD is also hypothesised to result in impaired macrophage activities. However, one limitation of this study is that we did not examine cell phenotypes (proportion of cell type) present in the spleen. Thus, we cannot determine whether the production of different cytokines following stimulation is due to alterations in cell activity, numbers or both. The lower cytokine production observed in spleen of HFD-fed mice in our study is consistent with a previous study demonstrating a lower IL-6 and TNF-α mRNA expression in spleen of mice fed a HFD for 5 weeks compared with the controls( Reference Lamas, Martinez and Marti 12 ). Interestingly, these findings in spleen are in contrast to a higher pro-inflammatory profile observed in the adipose tissue of obese mice and humans( Reference DeFuria, Bennett and Strissel 21 , Reference Ouchi, Parker and Lugus 39 ). These results may represent an impaired, acute innate immune response in the context of defence function rather than reduced chronic inflammatory state. Collectively, these data indicate that HFD-induced obesity impairs both T-cell proliferative response and their cytokine production induced by mitogens (Con A) or TCR antibodies (anti-CD3/C28), as well as production of pro-inflammatory cytokines induced by the bacterial endotoxin LPS, which targets accessory cells (macrophages and B cells). Furthermore, the reduced accessory cell response may also contribute to T-cell function because some of the cytokines they produce are essential for T-cell activation. These findings suggest that feeding a HFD, and the resulting obesity, may impair the ability of the peripheral immune system to appropriately defend against bacterial and antigenic challenges. These results are supported by the observation that impaired peripheral cytokine response is associated with increased morbidity and mortality in diet-induced obese mice following bacterial( Reference Strandberg, Verdrengh and Enge 40 ) or viral( Reference Smith, Sheridan and Harp 41 ) challenges. It is also consistent with previous studies reporting higher rates of infection (reviewed in Falagas & Kompoti( Reference Falagas and Kompoti 42 )) and impaired wound healing (reviewed in Pierpont et al.( Reference Pierpont, Dinh and Salas 43 )) within the obese human population, which may be partially attributed to impaired responses of the peripheral immune system.
The present study also demonstrates that the measurable effect of both HFD and blueberry supplementation on peripheral immune functions is time-dependent and non-uniform. Altered inflammatory state in adipose tissue was found at 8 weeks of HFD, with or without blueberry supplementation, as previously reported by DeFuria et al.( Reference DeFuria, Bennett and Strissel 21 ). In contrast, we observed little effect on the peripheral immune system after 8 weeks of dietary treatment. However, after 12 weeks of HFD, with or without blueberry supplementation, peripheral immune functions were impaired, suggesting that it may take longer for host defences to be altered by HFD and its resulting diet-induced obesity. Future studies should elucidate the heterogeneity and temporal sequence by which other immune functions and immune tissues are affected by HFD and obesity. As we did not have a group fed LFD supplemented with blueberry, we have no direct evidence to conclude whether or not the immune-modulating effects of blueberry would have been observed in LFD or normal chow-fed mice. However, our speculation is that this would be unlikely because blueberry may, similar to many other similar functional foods or nutrients, only prevent/reverse the impaired immune functions induced by consuming HFD and associated obesity, but without affecting the normal state of immune and inflammatory responses assumed to be the case with LFD or normal chow. The finding that blueberry had little effect on mice fed HFD for 8 weeks provided further support to this speculation.
Conclusion
In the present study, we observed that blueberry supplementation with a HFD may partially restore some of the HFD/obesity-induced immune dysfunction. To our knowledge, this is the first study to report that blueberry supplementation partially restores T-cell proliferative capacity and acute innate immune responses as indicated by inflammatory cytokine production in the context of obesity. The observed suppression of T-cell proliferation in HFD-fed mice may also be related to the reduced production of inflammatory cytokines (IL-6, TNF-α, IFN-γ). Decreased production of inflammatory cytokines by splenocytes from HFD-fed mice, in contrast to the reported inflammation in adipose tissue and peripheral blood of obese animals and humans, suggests that obesity-induced inflammation is tissue specific, but all related to a dysregulated inflammatory milieu. In addition to the adipose tissue, the systemic immune system may be an important site of action for the protective effects of blueberry on obesity and its associated morbidities. Further studies are needed to determine whether the observed changes in immune function are owing to a direct effect of HFD or a secondary effect owing to obesity induced by HFD.
Acknowledgements
The authors acknowledge Stephanie Marco for assistance with preparation of the manuscript.
This material is based upon work supported by the US Department of Agriculture – Agricultural Research Service, under agreement no. 58-1950-0-014. E. D. L. is the recipient of a Canadian Institutes of Health Research Postdoctoral Fellowship. The funders had no role in the design, analysis or writing of this article.
M. S. O., S. N. M. and D. W. formulated the research question and designed the study; Z. R., J. D. and D. W. conducted research; E. D. L. and D. W. analysed data and performed statistical analyses; E. D. L., D. W., M. O. and S. N. M. wrote the paper; and S. N. M. and D. W. have primary responsibility for final content. All authors have read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518001034







