Insulin resistance is a pathophysiological condition where the insulin receptor is less sensitive and therefore insulin in a natural dose is not enough to produce normal biological effects. It is recognised as the underlying cause of many adverse health events such as type 2 diabetes and the metabolic syndrome(Reference Lann and LeRoith1), and also it often occurs before the events. Cumulative studies have revealed that diet plays an important role in insulin resistance. Studies(Reference Spence, McKinley and Hunter2–Reference Isharwal, Misra and Wasir6) on the association between the intake of individual dietary components and insulin resistance have been reported. For instance, vegetables and fruit(Reference Spence, McKinley and Hunter2), low-fat dairy products(Reference Tremblay and Gilbert3) and regular alcohol consumption(Reference Gunji, Matsuhashi and Sato4, Reference Flanagan, Moore and Godsland5) were found to be inversely associated with insulin resistance, while dietary SFA intake(Reference Isharwal, Misra and Wasir6) has been reported to be positively associated with insulin resistance.
The traditional approach focusing on a single food or nutrient has its methodological and conceptual limitations(Reference Hu7, Reference Newby and Tucker8). It fails to take into account nutrient interaction and to detect small effects from single nutrients. More importantly in reality, no one consumes just one single food or nutrient. It cannot provide tangible dietary advice as well(Reference Newby and Tucker8). In nutritional epidemiology, dietary pattern analysis has emerged as an alternative method to assess dietary exposures. It has been shown to be a useful method to evaluate diet–disease relationships(Reference Hu7–Reference Kant9). Due to its ability to examine the effects of overall diet, dietary pattern analysis has been often used to identify the relationship between diet and insulin resistance/diabetes(Reference Esmaillzadeh, Kimiagar and Mehrabi10–Reference Odegaard, Koh and Butler13).
China has seen the fastest economic growth in the world in the context of its transition from a planned economy to a market economy. The economic development has a substantial effect on nutritional and lifestyle changes, which further contribute to increasing rates of obesity and diabetes in the population(Reference Wang, Mi and Shan14). Few studies have shown that dietary patterns were associated with diabetes and glucose tolerance abnormality in China(Reference He, Ma and Zhai11, Reference Villegas, Yang and Gao12) or among the Chinese population(Reference Odegaard, Koh and Butler13). Identifying the association between dietary patterns and insulin resistance would help prevent those health events at an earlier stage. However, no study in China can be found on such an association. The aim of the present study was to examine the association between dietary patterns identified by factor analysis and insulin resistance in Chinese adults without known diabetes.
Subjects and methods
Subjects and study design
Study subjects were the participants in the 2006 wave of the China Health and Nutrition Survey (CHNS) in Jiangsu Province. This study is a nationwide ongoing open cohort designed to evaluate the effects of health, nutrition and family planning policies on population health and nutritional status under economic transformation in China. More detailed information has been described elsewhere(Reference Popkin, Du and Zhai15). Jiangsu was the only province that collected blood samples in the CHNS project in 2006. The study sample was from six areas (Suzhou, Yangzhou, Shuyang, Taixing, Haimen and Jinhu) by a multistage random cluster process. In total, sixteen villages and townships within the counties and eight urban and suburban neighbourhoods within the cities were selected randomly. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the review board in the Jiangsu Provincial Center for Disease Control and Prevention. All participants provided written informed consent, with a response rate of 91·3 %.
For the present study, 1156 subjects aged 18 years and above with dietary survey and blood sample information were included. To improve the validity of the data, forty-six individuals were further excluded due to their implausible daily energy intake ( < 2092 kJ/d ( < 500 kcal/d), >16 736 kJ/d (>4000 kcal/d) for women, >17 573 kJ/d (>4200 kcal/d) for men) based on the Chinese context(Reference Willett16). Also, forty persons who had previously diagnosed diabetes were excluded, leaving 1070 participants (488 men and 582 women) for the present analysis. Among them, there were sixty-two previously undiagnosed cases with diabetes.
Assessment of dietary intake
A validated semi-quantitative FFQ was used to collect dietary intake information by a face-to-face interview, which was only used in Jiangsu in the 2006 CHNS(Reference Zhao, Hasegawa and Chen17, Reference Li, He and Zhai18). Participants were asked to recall their usual frequency and quantity of intakes of thirty-three food groups and beverages during the previous year with a series of detailed questions. Intake of each food item was calculated by multiplying the reported frequency of the food by estimated portion size of the food per time. Intakes of foods were converted into g/d for further analysis. Total energy and nutrients were computed by using the Chinese Food Composition Table(Reference Yang19).
Dietary patterns
Dietary patterns were identified by factor analysis using a principal component analysis method. Cheese was excluded due to no consumption among the present study subjects. Several food groups and beverages in the FFQ were aggregated into main food groups, mainly according to macronutrient composition and culinary use, i.e. tofu was a proportional aggregation of tofu, dried bean curd and soyabeans according to the protein content; pickled vegetables were the total of preserved vegetables, pickled vegetables, kimchi and Chinese sauerkraut; beverages represented juice and other soft drinks excluding water. As a result, twenty-six foods/food groups were entered into the analysis in absolute weight in grams.
The PROC FACTOR procedure in SAS (SAS Institute) was used to perform the analysis. The number of factors retained was determined by eigenvalue (>1·25), scree plot, factor interpretability and the variance explained (>5 %) by each factor. The factors retained were then rotated with an orthogonal rotation (‘Varimax’ option in SAS) to improve interpretability and minimise the correlation between the factors(Reference Kant9). From these analyses, a four-factor solution was selected. A factor score was then calculated for each subject for each of the four factors, as the sum of the products of the factor loading coefficient and standardised daily intake of each food/food group associated with that pattern. Labelling of the factors was primarily descriptive and based on our interpretation of the pattern structures.
Laboratory measurements
Venous blood samples were obtained in the morning after an overnight fast of at least 8–12 h. The fasting status was verbally confirmed by subjects before the blood sampling. All blood samples were collected in three vacuum tubes and processed within 3 h. Serum was stored at − 70°C until laboratory assays. Fasting glucose was assessed by an enzymology method using the OLYMPUS Chemistry Analyzer AU400 (Mishima Olympus Company Limited). Plasma insulin was measured by an ELISA Kit (Millipore Corporation). The homeostasis model assessment of insulin resistance (HOMA-IR) score was calculated as(Reference Matthews, Hosker and Rudenski20):
Its highest quartile was used to define insulin resistance, as published previously(Reference Isomaa, Almgren and Tuomi21, Reference Kalish22).
Covariates
Anthropometric data were measured directly by trained health workers following standard protocols. Weight in light clothing and without shoes was measured to the nearest 0·1 kg and height was measured to the nearest 0·1 cm. BMI was calculated as weight in kg over the square of height in m. We additionally added BMI into the logistic regression model to examine whether the association was mediated by obesity.
Income was estimated by family annual income per capita. It was categorised as low ( < 5000 Yuan), medium (5000–10 000 Yuan) and high (>10 000 Yuan). Physical activities including domestic, occupational, transportation and leisure-time physical activity were collected by staff-administered questionnaires. They were assessed in terms of metabolic equivalents × h/week to account for both intensity and time spent on activities. The level of physical activity was the product of time spent in each activity multiplied by specific metabolic equivalent values based on the ‘Compendium of Physical Activities’(Reference Ainsworth, Haskell and Whitt23, Reference Zuo, Shi and Yuan24). Other demographic and lifestyle covariates, such as sex, age and smoking status, were collected by a questionnaire-based interview. Current smoking status was coded as dichotomous variables (yes/no).
Statistical analysis
Factor scores for each pattern were categorised into quartiles (quartiles 1 and 4 represented low and high adherence, respectively, to each dietary pattern). Sample characteristics are presented as mean values and standard deviations for continuous variables, and percentages for categorical variables. Fasting glucose, insulin and HOMA-IR are expressed as medians (interquartile ranges) due to skewness and normalised by log transformation before fitting into linear regression models.
Dietary fibre, micronutrients, alcohol, beer and wine intakes were adjusted for sex, age and total energy intake. Linear trend was tested by the general linear model for continuous variables and the χ2 test for trend for categorical variables. Multivariate logistic regression was used to determine the association between food patterns and insulin resistance, adjusted for sociodemographic and lifestyle factors including sex, age, income, energy intake, physical activity and smoking status. In order to make full use of available information, HOMA-IR levels as a continuous variable across the quartiles of dietary patterns were also computed and tested for linear trend by the general linear model. All the analyses were performed using SAS software (version 8.1; SAS Institute). Statistical significance was considered when P< 0·05 (two-sided).
Results
We identified four major dietary patterns by factor analysis. Factor loadings for the four dietary patterns are shown in Table 1. The four dietary patterns were labelled as ‘Western’ (characterised by animal foods, milk, cake, etc.), ‘High-wheat’ (high in wheat instead of rice, whole grain and beef/lamb), ‘Traditional’ (high in eggs, tofu, organ meat, pickled vegetables, etc.) and ‘Hedonic’ (high in beer, wine and alcohols, and fresh vegetables). In total, the four factors explained 29·8 % of the variance in dietary intake (9·4, 8·5, 6·4 and 5·5 %, respectively).
Table 1 Rotated factor loading matrix for the four dietary patterns among 1070 Chinese adults*
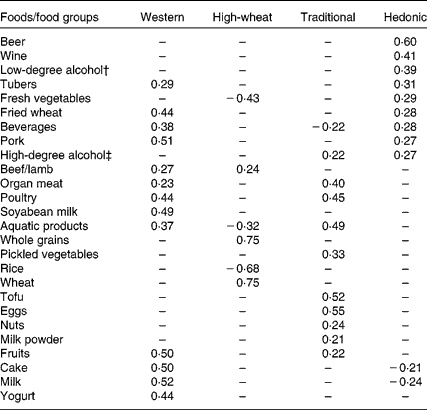
* Absolute values < 0·20 are indicated by a dash for simplicity.
† Alcohol content by volume ≤ 38 %.
‡ Alcohol content by volume >38 %.
The sample characteristics and dietary intakes of the study participants across the quartiles of the four dietary patterns are presented in Table 2. Those in the top quartile of all dietary patterns except the Traditional pattern were younger. Those in the top quartile of the Western and High-wheat patterns were more likely to be women, while the opposite was found in the other two patterns. A significant increasing trend of smoking status was observed in the Traditional and Hedonic patterns. A larger BMI was found with the increasing quartiles of the Traditional pattern, while the opposite trend was found in the Hedonic pattern. The physical activity level was increasing across the higher quartile of the Hedonic pattern, while the opposite trend was identified in the Western and High-wheat patterns.
Table 2 Characteristics and dietary intakes of the study participants for the lowest (Q1) and highest (Q4) quartiles of the four dietary patterns for 1070 adults in the China Health and Nutrition Survey study (Mean values and standard deviations or standard errors)

MET, metabolic equivalents.
* P values for linear trend for continuous variables (general linear model) and for categorical variables (χ2 test for trend).
† Mean values adjusted for sex, age and total energy intake.
There was a significant positive association between the total energy intake and the Western, Traditional and Hedonic patterns, while a negative association existed in the High-wheat pattern (P for trend < 0·001). The intake of protein, fat, Zn and Se increased, while that of carbohydrate, dietary fibre and Mg decreased except for vitamin C, across the quartiles of the Western pattern. All the nutrient intakes mentioned above increased except fat, vitamin C and Zn across the quartiles of the High-wheat pattern. There was a clear positive association between all the nutrient intakes except vitamin C and the Traditional pattern. In the Hedonic pattern, all intakes of macronutrients and micronutrients mentioned, except dietary fibre, increased across the quartiles (P for trend < 0·001). Alcohol intake decreased across the quartiles of the Western pattern, while it increased across the quartiles of the Hedonic pattern. Intakes of beer and wine increased across the quartiles of the Hedonic pattern also (P for trend < 0·001).
The associations between the dietary patterns and insulin resistance by using multivariate logistic regression are shown in Table 3. No significant interactions were found between dietary pattern and sex (data not shown). Therefore, sex was included as one of the covariates. The Western pattern was positively associated with insulin resistance both in the crude model (P for trend < 0·001) and in the model adjusted for potential confounders (P for trend = 0·009). Compared with the lowest quartile of the Western pattern, the highest quartile had higher odds of insulin resistance (adjusted OR 1·89, 95 % CI 1·12, 3·19). The High-wheat pattern was also positively associated with insulin resistance in the crude model (P for trend = 0·018) and in the model adjusted for all the confounders except BMI (P for trend = 0·033); however, the significance disappeared after plenary adjustment. No relationship was seen with the Traditional pattern. A significant negative association was found between the Hedonic pattern and insulin resistance (P for trend = 0·001 for the crude model and 0·013 for the further adjusted model). BMI attenuated this association slightly after additional adjustment but remained significant (P for trend = 0·035 for plenary adjustment). There was a 42 % decrease in the odds after adjustment for all covariates in the highest quartile of the Hedonic pattern, compared with the lowest quartile (adjusted OR 0·58, 95 % CI 0·34, 0·99).
Table 3 Multivariate models adjusted for insulin resistance across the quartile (Q) categories of the dietary patterns in Jiangsu Province, China (Odds ratios and 95 % confidence intervals)

* Adjusted for sex and age ( < 40, 40–49, 50–59 or ≥ 60 years).
† Further adjusted for income (low, medium or high), energy intake (kJ/d), physical activity (metabolic equivalents × h/week) and smoking status (yes/no).
‡ Additionally adjusted for BMI ( < 18·5, 18·5–23·9, 24–27·9 or >28 kg/m2).
In order to identify the association between the dietary patterns and insulin resistance more clearly, we performed further analyses. A significant negative correlation between logarithmically transformed HOMA-IR and the Hedonic pattern scores as continuous variables (Pearson's r − 0·161, P< 0·001) and a significant positive correlation between transformed HOMA-IR and the Western pattern (Pearson's r 0·142, P< 0·001) were found (data not shown).
As shown in Table 4, we observed that HOMA-IR levels as a continuous variable increased across the quartiles of the Western pattern and decreased across the quartiles of the Hedonic pattern. We also found that the shifts of HOMA-IR levels were mainly influenced by the significant change in fasting insulin level instead of glucose level. Finally, in an additional sensitivity analysis, we excluded those subjects with other known chronic diseases (hypertension, myocardial infarction or stroke); the results were consistent (data not shown).
Table 4 Fasting glucose, insulin, homeostasis model assessment for insulin resistance (HOMA-IR) levels and insulin resistance across the quartiles of the four dietary patterns (Medians and interquartile ranges (IQR))

* Log-transformed when testing for linear trends.
† Adjusted for sex and age ( < 40, 40–49, 50–59, or ≥ 60 years), income (low, medium or high), energy intake (kJ/d), physical activity (metabolic equivalents × h/week), smoking status (yes/no) and BMI ( < 18·5, 18·5–23·9, 24–27·9 or >28 kg/m2).
Discussion
We derived four dietary patterns in our population by using factor analysis: the Western, High-wheat, Traditional and Hedonic pattern. Further analysis indicated that the Western pattern was associated with greater odds of insulin resistance. In contrast, the Hedonic pattern was negatively associated with insulin resistance in Chinese adults. Both associations were independent of sex, age, BMI, income, energy intake, physical activity and smoking status.
A dietary pattern approach has been used to identify the association between holistic diet exposure and diabetes in China(Reference He, Ma and Zhai11, Reference Villegas, Yang and Gao12). In the present study, the positive association between the Western pattern and insulin resistance is similar to previous studies(Reference Esmaillzadeh, Kimiagar and Mehrabi10, Reference He, Ma and Zhai11).
Considering the background of an alarming increase in the prevalence of diabetes(Reference Yang, Lu and Weng25) and other non-communicable diseases, and nutrition transition(Reference Popkin and Du26, Reference Zhai, Wang and Du27) happening in China, we suggest avoiding such an eating pattern with high intakes of energy and fat and low intake of dietary fibre among the population. However, the food groups of the Western pattern identified in the present study cannot be comparable directly with others, especially those undertaken in Western countries(Reference Esmaillzadeh, Kimiagar and Mehrabi10). Pork, organ meat and soyabean milk are characterised in the ‘Western’ pattern of our population, while processed meats, beef and butter/cheese are featured in the ‘Western’ pattern of Western populations.
The negative association between the Hedonic pattern and insulin resistance is a new and important finding in the present study of the Chinese population. Such an association was independent of sex, age, BMI, income, energy intake, physical activity and smoking status. The present finding was somewhat consistent with others done in different populations(Reference Gunji, Matsuhashi and Sato4, Reference Flanagan, Moore and Godsland5, Reference Imamura, Lichtenstein and Dallal28–Reference Athyros, Liberopoulos and Mikhailidis30). The results from a meta-analysis in 2005 showed that a U-shaped relationship between alcohol consumption and type 2 diabetes was found. Moderate alcohol consumption was protective against type 2 diabetes in men and women(Reference Koppes, Dekker and Hendriks31). Another meta-analysis study completed in 2009 also confirmed these findings(Reference Baliunas, Taylor and Irving32). Such findings are supported in individual studies from different populations(Reference Sato, Hayashi and Harita29, Reference Athyros, Liberopoulos and Mikhailidis30).
Available epidemiological evidence showed that the negative association between the Hedonic pattern and insulin resistance could be attributable to the pattern's healthy components, which may include alcohol in a moderate amount(Reference Koppes, Dekker and Hendriks31, Reference Baliunas, Taylor and Irving32), fresh vegetables(Reference Spence, McKinley and Hunter2), tubers(Reference Oki, Nonaka and Ozaki33) and some micronutrients such as vitamin C(Reference Hercberg, Czernichow and Galan34), Mg(Reference Mooren, Kruger and Volker35, Reference Guerrero-Romero, Tamez-Perez and Gonzalez-Gonzalez36), Zn(Reference Kelishadi, Hashemipour and Adeli37) and Se(Reference Mueller, Mueller and Wolf38). Contribution of these components was supported by the present results. Reduced insulin resistance may be due to their synergistic effect. As mentioned above, moderate alcohol consumption was independently associated with insulin resistance or diabetes. The average intake of alcohol in the present study population was actually low (15·5 g/d for alcohol, 27·6 g/d for beer and 3·0 g/d for wine). Even the mean intake of alcohol in the highest quartile of the Hedonic pattern was 38·5 g/d, which was still lower than the upper limit of moderate alcohol consumption(Reference Koppes, Dekker and Hendriks31, Reference Baliunas, Taylor and Irving32). Therefore, the present findings correspond to the left side and bottom of the U-shaped relationship found in previous results(Reference Koppes, Dekker and Hendriks31, Reference Baliunas, Taylor and Irving32).
Research focusing on the health effects of alcohol drinking has increasingly emerged in recent years, but little can be found about its protective effects among the Chinese population. It remains unclear that whether the Hedonic pattern has a causal protective mechanism on insulin resistance; therefore, clinical and public health recommendations from such findings may be too early. However, advice on reducing heavy alcohol drinking is always appropriate. The Dietary Guidelines for Americans and Chinese consistently suggest ‘if alcohol is consumed, it should be consumed in moderation’.
As we mentioned previously, a dietary pattern approach overcomes the defects of an individual food analysis, which is mainly limited by biological interactions. Normally, alcohol is not consumed separately in China but is consumed as part of specified dietary patterns. Therefore, the present study adds new evidence to previous research findings that the Hedonic dietary pattern is inversely associated with insulin resistance.
The strengths of the present study include the use of a validated semi-quantitative FFQ by a face-to-face interview, which ensured that the data we collected were accurate. Further, the findings are reliable because we adjusted for potential known confounders in the models. Moreover, as we excluded the subjects with known diabetes, it is unlikely that the results are confounded by diabetic medication.
However, limitations also need to be considered in the interpretation of the present findings. The main limitation of the present study is its cross-sectional nature. Reverse causation would be a possibility if health outcomes influence the intake of alcohol rather than the reverse. Although we excluded subjects with other known chronic diseases in sensitivity analysis, such a possibility has not been fully addressed here. The present findings remain to be verified by future prospective studies. Furthermore, we cannot generalise the present findings to all Chinese populations because there are considerable differences in eating habit, food categories and even cooking methods given such a large geographical diversity. Participants in the present study were sampled from six socio-economically and geographically different areas. So these patterns can represent the situation among the population in Jiangsu Province, Eastern China. Even in the same province, due to different study samples, different dietary patterns could be identified(Reference Shi, Hu and Yuan39). However, the cluster of wheat and rice in the opposite direction is consistent in different studies in China. The factor loading for the High-wheat pattern was 0·75 for wheat and − 0·68 for rice in the present study, while the factor loading for the ‘traditional’ pattern was − 0·75 for wheat and 0·78 for rice in another study in the province(Reference Shi, Hu and Yuan39).
In conclusion, the present findings indicate that the Western pattern was associated with greater odds of insulin resistance. In contrast, the Hedonic pattern was associated with decreased odds of insulin resistance in Chinese adults. Such associations were independent of sex, age, BMI, income, energy intake, physical activity and smoking status.
Acknowledgements
The authors are sincerely grateful to the CHNS project initiated by the Carolina Population Center at the University of North Carolina at Chapel Hill and the National Institute of Nutrition and Food Safety at the Chinese Center for Disease Control and Prevention. Also, we thank the entire study team and all participants in the study. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. H. Z. designed and conducted the analysis, interpreted the data, and wrote and edited the manuscript. Z. S. contributed to the design, data analysis and discussion, and reviewed the manuscript. B. Y., Y. D. and G. W. researched the data. X. P. contributed to the data analysis. A. H. contributed to the design and data interpretation, and reviewed the manuscript. The authors declare that they have no conflicts of interest.






