Bacterial lipopolysaccharide (LPS), a complex glycolipid, is the major component of the outer surface membrane in almost all gram-negative bacteria, and it can trigger inflammatory responses in diverse eukaryotic species ranging from insects to humans by activating signal pathways and promoting gene expressions, which are involved in the regulation of immune system and inflammation( Reference Alexander and Rietschel 1 , Reference Lu, Yeh and Ohashi 2 ). In broiler chickens, an enormous amount of research has shown that immunological stress mimicked by administrating LPS intraperitoneally or intravenously could induce acute inflammatory responses, stimulate synthesis of pro-inflammatory cytokines and impair nutrient adsorption, which ultimately contribute to compromised growth performance of LPS-challenged broilers( Reference Tan, Liu and Guo 3 – Reference Kaiser, Block and Ciraci 8 ). Aside from inducing inflammation, LPS challenge would also damage intestinal barrier function( Reference Gilani, Howarth and Kitessa 9 ), a key factor associated with intestinal and body’s health maintenance( Reference Groschwitz and Hogan 10 ). Wu et al.( Reference Wu, Zhou and Wu 11 ) have reported that LPS challenge to young broiler chickens impairs growth performance and increases circulating d-lactic acid (D-LA) level and diamine oxidase (DAO) activity, which are two sensitive biomarkers reflecting gut permeability. Similarly, our previous results reveal that LPS-challenged broilers exhibit impaired intestinal morphology, increased plasma DAO activity and altered mRNA expressions of tight-junction proteins in both jejunum and ileum, when compared with their unchallenged counterparts( Reference Li, Zhang and Chen 4 ). To maintain growth performance and welfare of broilers, it is therefore necessary to apply nutritional interventions in broiler production in order to alleviate the harmful consequences of bacterial LPS challenge.
Threonine (Thr), an indispensable amino acid for humans and animals, plays a vital role in the synthesis of Ig and gut mucosal proteins( Reference Tenenhouse and Deutsch 12 – Reference van der Schoor, Wattimena and Huijmans 14 ). In mammals, about 50 % of dietary Thr is utilised by gut to synthesise various mucosal proteins, particularly mucins, and this ratio would increase for young animals( Reference van der Schoor, Wattimena and Huijmans 14 – Reference van der Sluis, Schaart and de Koning 16 ). Dietary Thr restriction could reduce mucin synthesis, inhibit goblet cell differentiation and finally impair intestinal barrier function of pigs( Reference Wang, Zeng and Mao 17 – Reference Hamard, Mazurais and Boudry 19 ) or broilers( Reference Chee, Iji and Choct 20 – Reference Zhang, Chen and Eicher 22 ). Moreover, Thr deficiency could exacerbate inflammatory responses and worsen the gut barrier impairment of broilers under the combined feed withdrawal and coccidial infection challenge( Reference Zhang, Chen and Eicher 22 ) or coccidiosis challenge( Reference Wils-Plotz, Jenkins and Dilger 23 ). Usually, Thr level in the maize–soyabean meal diet ranges from 7·5 to 8·3 g/kg, which can almost meet the requirement of National Research Council (NRC)( 24 ) for broiler chickens. Previous studies have proven that Thr requirement of broilers increases when reared under stress and non-hygienic conditions( Reference Corzo, Kidd and Dozier 25 , Reference Star, Rovers and Corrent 26 ), and it is beyond the NRC recommendation to acquire the optimal immune response( Reference Taghinejad-Roudbaneh, Babaee and Afrooziyeh 27 ). Recently, Min et al.( Reference Min, Liu and Qu 28 ) have concluded that Thr requirement of broilers for the optimum gut morphology and antioxidant capacity is 125 % of NRC recommendation during a 42-d assay. Similarly, our recent results have revealed that the supplementation of l-Thr to a basal diet that could meet NRC recommendation of Thr, especially at a dosage of 3·0 g/kg, can improve intestinal morphology and caecal microflora composition, promote intestinal goblet cell density and beneficially modulate expressions of genes related to intestinal immunity and barrier function of broilers at an early age( Reference Chen, Cheng and Li 29 ). A higher level of dietary Thr level would also relieve inflammatory responses and intestinal barrier damage of broilers under pathogenic bacteria challenge( Reference Zhang, Chen and Eicher 22 , Reference Wils-Plotz, Jenkins and Dilger 23 ). In a chicken intestinal ex vivo model with LPS challenge, Zhang et al.( Reference Zhang, Chen and Eicher 30 ) have recently reported that Thr deprivation in culture medium impairs inflammatory and secretory immune responses, and culture of explants from birds fed a Thr-adequate diet is more responsive to LPS challenge than explants from birds fed a Thr-deficient diet, as evidenced by increased mRNA abundances of mucin 2 (MUC2), IL-8 and IgA. These results in turn suggest that a higher level of dietary Thr is required for LPS-challenged broilers. However, in vivo studies investigating the protective effects of dietary l-Thr supplementation on broilers challenged with LPS are absent. According to our previous results( Reference Chen, Cheng and Li 29 ) and the finding of other researchers( Reference Min, Liu and Qu 28 ), we supplemented 3·0 g/kg l-Thr to a control diet that met the NRC requirement of Thr for broilers, and then investigated whether this supplementation would bring beneficial consequences on the growth performance, inflammatory responses and intestinal barrier function of LPS-challenged broiler chickens at an early age.
Methods
Animals and treatment
The experimental protocols were in accordance with guidelines set by Nanjing Agricultural University Institutional Animal Care and Use Committee. A total of 144 1-d-old male broiler chicks (Arbor Acres Plus) were purchased from a local hatchery. After weighing, chicks were allocated to one of three treatments, with each treatment being composed of six replicates (cages) of eight birds each. The initial body weight of chicks was similar among cages. The three experimental treatments were as follows: (1) non-challenged broilers given a basal diet (control group); (2) LPS-challenged broilers given a basal diet (LPS challenge group); and (3) LPS-challenged broilers fed a basal diet supplemented with 3·0 g/kg l-Thr crystals (l-Thr administration group; the purity of l-Thr crystals is 98·5 %; Meihua Holdings Group Co., Ltd). The supplemental l-Thr level was optimised according to previous results( Reference Min, Liu and Qu 28 , Reference Chen, Cheng and Li 29 ). This experiment lasted for a total of 21 d (from 1 to 21 d of age). LPS from Escherichia coli (serotype O55.B5; Sigma-Aldrich) was dissolved in 0·86 % (w/v) sterile saline solution to prepare 1 mg/ml LPS solution. At 17, 19 and 21 d of age, broilers were intraperitoneally injected with LPS solution at a dosage of 1 ml/kg of body weight or equivalent volume of 0·86 % sterile saline solution (control treatment). The dosage of LPS and its administration routine adopted in this study were referred to available findings( Reference Tan, Liu and Guo 3 , Reference Gonzalez, Mustacich and Traber 31 , Reference Wang, Li and Shen 32 ). Ingredient composition and nutrient level of the basal diet are presented in the Table 1. The calculated Thr level (8·00 g/kg) in the basal diet 100 % met the NRC recommendation for broilers during 1 to 21 d of age( 24 ). The analysed Thr concentration that was measured by an automatic Hitachi L-8900 amino acid analyzer (Hitachi) in the control group, LPS challenge group and the l-Thr administration group was 7·84, 7·76 and 10·43 g/kg, respectively. The diets were prepared isonitrogenously by supplementing the basal diet (control group and LPS challenge group) with approximate 2·3 g/kg l-alanine (98·5 %; Sigma-Aldrich). Birds were housed in three-level cages (120×60×50 cm), and had ad libitum access to mash feed and water in a temperature- and light-controlled facility with a light schedule of 23 h light and 1 h darkness. The temperature of the chicken house was maintained between 32 and 34°C for the initial 3 d, and it was then decreased by 2 to 3°C each week until a final temperature of 26°C was achieved. Mean relative humidity was kept approximately at 70 % for the first 3 d, and maintained at 60–65 % thereafter.
Table 1 Composition and nutrient level of the basal diet (g/kg, as fed basis unless otherwise stated)

* Premix provided per kg of diet: vitamin A (transretinyl acetate), 3·44 mg; vitamin D3 (cholecalciferol), 0·075 mg; vitamin E (all-rac-α-tocopheryl acetate), 30 mg; menadione, 1·3 mg; thiamin, 2·2 mg; riboflavin, 8 mg; nicotinamide, 40 mg; choline chloride, 600 mg; calcium pantothenate, 10 mg; pyridoxine·HCl, 4 mg; biotin, 0·04 mg; folic acid, 1 mg; vitamin B12 (cobalamin), 0·013 mg; Fe (from ferrous sulphate), 80 mg; Cu (from copper sulphate), 8·0 mg; Mn (from manganese sulphate), 110 mg; Zn (from zinc oxide), 60 mg; iodine (from calcium iodate), 1·1 mg; Se (from sodium selenite), 0·3 mg.
Sample collection
At 21 d of age, one bird in each cage (replicate) with a body weight close to the mean body weight in the respective cage was selected and weighed immediately after LPS challenge (six birds per treatment, and eighteen birds in total). Whole-blood samples were collected in both glass anti-coagulant tubes coated with EDTA and tubes without anti-coagulant by wing vein puncture, and kept refrigerated until analysis. Serum was then separated from blood samples collected from wing vein after centrifugation at 4450 g for 15 min at 4°C, and it was immediately frozen at −20°C until subsequent analysis. After blood collection, the birds were euthanised by cervical dislocation, and necropsy was carried out. The jejunum (from the end of the duodenum to the Meckel’s diverticulum) and ileum (from Meckel’s diverticulum to the ileocecal junction) samples were immediately dissected free from mesentery and the connective tissues, and placed on a chilled stainless steel tray. About 2-cm segments of mid-jejunum and mid-ileum were then excised, flushed gently and repeatedly with ice-cold PBS (pH 7·4) and immediately immersed in 10 % fresh (w/v), chilled neutral-buffered formalin solution for subsequent histological measurement. The remaining jejunal and ileal segments were opened longitudinally, and washed with ice-cold PBS to remove digesta. The jejunal and ileal mucosa was then scratched carefully using a sterile glass microscope slide and collected into sterile frozen tubes, which was snapped in liquid N2 and stored at −80°C for further analysis. Longitudinal sections of spleen (about 0·5 g) were also collected and store at −80°C after freezing in liquid N2.
Growth performance determination
At 16 and 21 d of age, birds were weighed after a 12-h feed deprivation period (the last LPS injection and birds selection for sampling at 21 d of age were performed at early morning (approximately 05.30 hours) before the 12-h feed withdrawal of broilers to avoid intestinal barrier impairment induced by feed deprivation), and the total feed consumption by the birds on a cage (replicate) basis was recorded to calculate average daily gain (ADG), average daily feed intake (ADFI) and feed:gain ratio (F:G). The spilled feed in the steel tray placed under the cage was carefully removed and weighed, which was considered as feed wastage. Birds that died during the experiment were weighed, and the data were included when calculating F:G.
Blood cell count assay
The whole-blood samples collected in the EDTA anti-coagulant tubes were used to determine the numbers of leucocytes, lymphocytes, mononuclear cells, neutrophils, eosinophils and erythrocytes, using an automatic blood counter (KT6180; Genrui Biotech Inc.) with adapted dilutions in the Animal Hospital of Nanjing Agricultural University.
Measurement of serum d-lactic acid level and diamine oxidase activity
The serum D-LA concentration was quantified using a D-LA colorimetric assay kit (catalogue no. K667-100; BioVision Inc.) according to the manufacturer’s protocols, and its determination range was 0·01–10 mmol/l. The DAO activity in the serum was measured according to the spectrophotometric method described by Hosoda et al.( Reference Hosoda, Nishi and Nakagawa 33 ). In brief, a 0·5-ml serum sample was added to the assay mixture containing 3·0 ml of PBS (0·2 mol/l, pH 7·2), 0·1 ml of horseradish peroxidase solution (0·04 g/l), 0·1 ml of o-dianisidine-methanol solution (5 g/l odianisidine in methanol) and 0·1 ml of substrate solution (1·75 g/l cadaverine dihydrochloride). The absorbance of reaction mixture at 436 nm was measured spectrophotometrically after incubation at 37°C for 0·5 h to calculate serum DAO activity. The reagents used in DAO activity assay were sourced from Sigma-Aldrich.
Determination of pro-inflammatory cytokines
Before measurement, approximately 0·3 g of tissue samples (spleen, and jejunal and ileal mucosa) were homogenised in an ice-cold bath with a chilled 154 mmol/l sterile sodium chloride solution (1:9, w/v) for 30 s, using a PRO-PK-02200D homogenizer (Pro Scientific, Inc.). After that, the homogenisation was centrifuged at 4450 g for 15 min at 4°C, and the resulting supernatant was collected in five aliquots, which were immediately stored at −20°C until the subsequent analysis. The levels of interferon-γ (IFN-γ), IL-1β (IL-1β) and TNF-α (TNF-α) in the serum, spleen and intestinal mucosa samples were measured by ELISA using chicken-specific IFN-γ (catalogue no. H025), IL-1β (catalogue no. H002) and TNF-α (catalogue no. H052) quantification kits (Nanjing Jiancheng Bioengineering Institute) based on the manufacturer’s instructions. The inter- and intra-assay CV were <8 and 10 %, respectively. All results were normalised against total protein level in each sample for inter-sample comparison. The total protein content in the spleen and mucosa samples was measured by Bradford method, using crystalline bovine serum albumin (Sigma-Aldrich) as the standard protein.
Histological measurement
Collected intestinal segments were transferred to 70 % ethanol after a 24-h fixation in buffered formalin. Fixed tissues were then dehydrated in ethanol, cleared in xylene and embedded in paraffin blocks. Serial paraffin sections were cut from each block at 5 μm, deparaffinised with xylene, rehydrated through graded alcohols and stained with haematoxylin and eosin. Photographs of the stained sections were taken through a microscope and digitalised with a Nikon ECLIPSE 80i light microscope equipped with a computer-assisted morphometric system (Nikon Corporation). Villus height and crypt depth of ten well-preserved villi and crypts in each section were measured. The combined Alcian Blue-periodic acid–Schiff stain technique( Reference Horn, Donkin and Applegate 34 ) was used to measure the intestinal goblet cell density. In detail, deparaffinised and rehydrated sections were stained with 1·0 % Alcian Blue solution (Alcian Blue in 3 % acetic acid solution), gently washed in double-distilled water for 10 min, oxidised in 1·0 % periodic acid solution for 15 min, rinsed again in double-distilled water for 10 min and then placed in periodic acid–Schiff solution for 30 min. Goblet cells were counted in five well-oriented villi per section, using the above-mentioned Nikon ECLIPSE 80i light microscope. Goblet cell density was measured as the goblet cell count divided by the corresponding villus length, averaged and expressed as goblet cell number per 100 μm of villus length. All staining chemicals were sourced from Sigma-Aldrich Chemical.
RNA isolation and mRNA quantification
Total RNA in the snap-frozen mucosal samples (jejunal and ileal mucosa) were isolated using the TRIzol reagent kit (TaKaRa Biotechnology), according to the instructions of the manufacturer. The integrity of isolated RNA was examined by electrophoresis on a 1 % agarose gel containing 0·5 mg/ml ethidium bromide, whereas the purity and concentration of RNA were quantified with a NanoDrop ND-1000 UV spectrophotometer (NanoDrop Technologies) according to OD260/280 readings. RNA samples were then diluted with diethyl pyrocarbonate-treated water (Biosharp) to a final concentration of 0·5 μg/μl. Thereafter, 1 μg of total RNA was reverse-transcribed into complementary DNA using the PrimeScriptTM RT reagent kit (TaKaRa Biotechnology) in the presence of random and oligo-dT primers, according to the manufacturer’s protocol. The reverse transcription condition was set up as follows: 15 min at 37°C and 5 s at 85°C. The primer sequences for the target and housekeeping genes (toll-like receptor 4 (TLR4), myeloid differentiation factor 88 (MyD88), IFN- γ, IL-1 β, IL-4, IL-10, zonula occludens-1 (ZO-1), occludin (OCLN), claudin-2 (CLDN2), claudin-3 (CLDN3), trefoil factor 2 (TFF2), MUC2 and β -actin) are available in the Table 2. Each primer was tested for PCR amplification efficiency by determining a standard curve with complementary DNA dilutions, and the efficiency values were between 97 and 102 % (Table 2). Reverse transcription products (complementary DNA) were diluted 10× before real-time PCR assay. Real-time PCR was performed on an ABI StepOnePlusTM Real-Time PCR System (Applied Biosystems). The reaction mixture was composed of 2 μl of complementary DNA, 0·4 μl each of the forward and reverse primers, 10 μl of SYBR Premix Ex TaqTM (TaKaRa Biotechnology), 0·4 μl of ROX reference dye (TaKaRa Biotechnology) and 6·8 μl of double-distilled water. PCR procedures consisted of a pre-run at 95°C for 30 s, forty cycles of denaturation at 95°C for 5 s, followed by a 60°C annealing step for 30 s. Melting curve conditions were as follows: one cycle of denaturation at 95°C for 10 s, followed by an increase in temperature from 65 to 95°C with temperature change velocity at 0·5°C/s. The 2-ΔΔCT method( Reference Livak and Schmittgen 35 ) was used to analyse the relative mRNA expression levels (fold changes), after normalisation against the reference gene β -actin, with the mRNA level of each target gene of broiler chickens in the control group being assigned as a value of 1.
Table 2 Sequences for real-time PCR primers
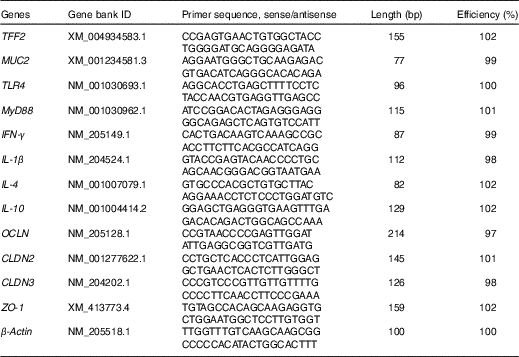
TFF2, trefoil factor 2; MUC2, mucin 2; TLR4, toll-like receptor 4; MyD88, myeloid differentiation factor 88; IFN-γ, interferon-γ; OCLN, occludin; CLDN2, claudin-2; CLDN3, claudin-3; ZO-1, zonula occludens-1.
Statistical analysis
Data were analysed by one-way ANOVA using SPSS statistical software (version 16.0 for windows; SPSS Inc.) with pen (cage) as the experimental unit. Differences among treatments were examined using Tukey’s multiple-range tests, and were considered to be significant when P<0·05. Data were presented as means with their pooled standard errors.
Results
Growth performance
Before LPS challenge (1–16 d of age), broilers exhibited (Table 3) similar growth performance (ADG, ADFI and F:G) among groups (P>0·05). Compared with the control group, LPS challenge reduced ADG and ADFI, whereas it increased F:G of broilers during 17–21 d of age (P<0·05). In contrast, the F:G of LPS-challenged broilers was decreased by l-Thr administration (P<0·05), and its level in LPS-challenged broilers receiving l-Thr administration was comparable with the control group (P>0·05). The values of ADG and ADFI in LPS-challenged broilers fed a basal diet supplemented with l-Thr were intermediate, when compared with the other two groups (P>0·05).
Table 3 Effects of dietary l-threonine (l-Thr) supplementation on the growth performance of lipopolysaccharide (LPS)-challenged broilers
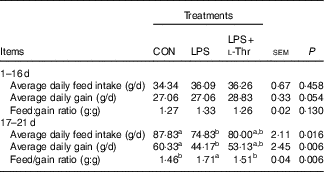
CON, non-challenged broilers fed a basal diet; LPS, LPS-challenged broilers fed a basal diet; LPS+l-Thr, LPS-challenged broilers fed a basal diet supplemented with 3·0 g/kg l-thr.
a,b Mean values within a row with unlike superscripts letters are significantly different (P<0·05).
Blood cell count
LPS challenge resulted in increases in the numbers of leucocytes, mononuclear cells and neutrophils in the blood of broilers when compared with their unchallenged counterparts (Table 4, P<0·05), with the counts of these aforementioned blood cells being intermediate in the l-Thr treatment group (P>0·05). However, the counts of lymphocytes, eosinophils and erythrocytes in the blood were similar among treatments (P>0·05).
Table 4 Effects of dietary l-threonine (l-Thr) supplementation on blood cell composition of lipopolysaccharide (LPS)-challenged broilers
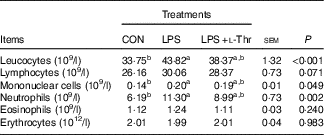
CON, non-challenged broilers fed a basal diet; LPS, LPS-challenged broilers fed a basal diet; LPS+l-Thr, LPS-challenged broilers fed a basal diet supplemented with 3·0 g/kg l-thr.
a,b Mean values within a row with unlike superscript letters are significantly different (P<0·05).
Inflammatory cytokine concentrations
LPS challenge elevated concentrations of IL-1β and TNF-α in both serum and spleen, IFN-γ in the jejunal mucosa and IL-1β in the ileal mucosa of broilers, when compared with the control group (Table 5, P<0·05). Supplementing l-Thr normalised the alteration in IL-1β concentration in the ileal mucosa of LPS-challenged broilers (P<0·05). Similarly, IFN-γ level in the jejunal mucosa of LPS-challenged broilers was reduced by l-Thr supplementation (P<0·05), but its value in the l-Thr treatment group was still higher than that in the control group (P<0·05). The values of serum IL-1β and serum and splenic TNF-α in the LPS-challenged broilers receiving l-Thr administration were intermediate among groups (P>0·05). Treatments did not alter the levels of IFN-γ in the serum and spleen, IL-1β and TNF-α in the jejunal mucosa or IFN-γ and TNF-α in the ileal mucosa (P>0·05).
Table 5 Effects of dietary l-threonine (l-Thr) supplementation on cytokines concentrations in the serum and tissues of lipopolysaccharide (LPS)-challenged broilers
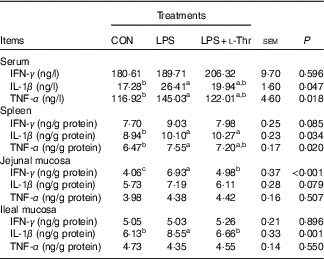
IFN-γ, interferon-γ; CON, non-challenged broilers fed a basal diet; LPS, LPS-challenged broilers fed a basal diet; LPS+l-Thr, LPS-challenged broilers fed a basal diet supplemented with 3·0 g/kg l-thr.
a,b,c Mean values within a row with unlike superscripts are significantly different (P<0·05).
Intestinal permeability, morphology and goblet cell density
As shown in the Table 6, LPS injection increased circulating D-LA concentration of broilers when compared with the control group (P<0·05), with the value being intermediate in the l-Thr administration group in comparison with the other two groups (P>0·05). However, treatments did not affect serum DAO activity (P>0·05), even though it was numerically higher in the LPS-challenged broilers fed a basal diet. LPS challenge resulted in shorter villus height, lower ratio between villus height and crypt depth and lower goblet density in both jejunum and ileum (P<0·05). In contrast, the supplementation of l-Thr increased jejunal villus height, villus:crypt depth ratio in both jejunum and ileum and ileal goblet cell density of LPS-challenged broilers (P<0·05); in addition, the levels of those parameters in the l-Thr treatment group were similar to those in the control group (P>0·05). Villus height in the ileum of LPS-challenged broilers receiving l-Thr was intermediate among the three groups (P>0·05). However, birds exhibited similar crypt depth in the jejunum and ileum among treatments (P>0·05).
Table 6 Effects of dietary l-threonine (l-Thr) supplementation on serum diamine oxidase activity and d-lactic acid concentration, intestinal morphology and goblet cell density of lipopolysaccharide (LPS)-challenged broilers
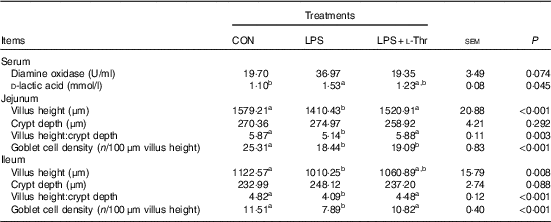
CON, non-challenged broilers fed a basal diet; LPS, LPS-challenged broilers fed a basal diet; LPS+l-Thr, LPS-challenged broilers fed a basal diet supplemented with 3·0 g/kg l-thr.
a,b Mean values within a row with unlike superscripts are significantly different (P<0·05).
Gene expressions
Compared with the control group (Table 7), LPS challenge increased mRNA abundances of MUC2, CLDN3, TLR4 and IFN- γ in the jejunal mucosa, and IL-1 β mRNA expression level in the ileal mucosa, whereas it decreased the mRNA expression abundance of ZO-1 in the ileal mucosa (P<0·05). The altered mRNA expressions of these aforementioned genes were reversed with l-Thr administration (P<0·05). However, treatments did not alter mRNA expression level of TFF2, OCLN, CLDN2, MyD88, IL-4 or IL-10 in the intestinal mucosa (P>0·05).
Table 7 Effects of dietary l-threonine (l-Thr) supplementation on the gene expressions in the intestinal mucosa of lipopolysaccharide (LPS)-challenged broilers
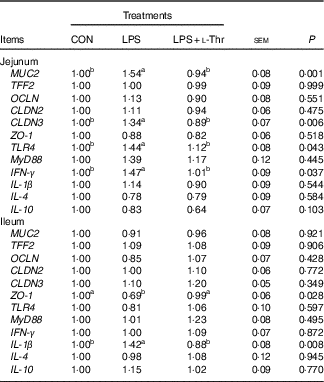
CON, non-challenged broilers fed a basal diet; LPS, LPS-challenged broilers fed a basal diet; LPS+l-Thr, LPS-challenged broilers fed a basal diet supplemented with 3·0 g/kg l-thr; MUC2, mucin 2; TFF2, trefoil factor 2; OCLN, occludin; CLDN2, claudin-2; CLDN3, claudin-3; ZO-1, zonula occludens-1; TLR4, toll-like receptor 4; MyD88, myeloid differentiation factor 88; IFN- γ, interferon-γ.
a,b Mean values within a row with unlike superscripts are significantly different (P<0·05).
Discussion
Numerous studies have proven that LPS challenge would lead to compromised growth performance of broilers( Reference Tan, Liu and Guo 3 , Reference Li, Zhang and Chen 5 , Reference Wang, Li and Shen 32 , Reference Zheng, Wu and Song 36 , Reference Zhang, Zhong and Zhou 37 ), and we once again confirmed its harmful consequences on growth performance of broilers in this study, as evidenced by reduced weight gain and feed conversion efficiency. The impaired growth performance of LPS-challenged broilers is attributable to reduced appetite( Reference Vichaya, Hunt and Dantzer 38 ), disrupted gut barrier function( Reference Li, Zhang and Chen 4 , Reference Groschwitz and Hogan 10 ), impaired nutrient adsorption( Reference Zhang, Zhao and Cao 7 ) and especially the diversion of nutrients away from growth in support of various immune-related processes as the synthesis of various acute proteins and cytokines( Reference Spurlock 39 ). Thr requirement of broilers would increase when exposed to pathogenic bacterial challenges. Star et al.( Reference Star, Rovers and Corrent 26 ) have shown that a higher dietary Thr:Lys ratio improves growth performance of broilers during subclinical infection with Clostridium perfringens. Dietary Thr supplementation also improves growth performance of broilers challenged with Eimeria maxima ( Reference Wils-Plotz, Jenkins and Dilger 23 ). In this study, the compromised growth performance of LPS-challenged broilers was alleviated with the supplementation of l-Thr, when referring to the improved feed utilisation efficiency of LPS-challenged broilers after l-Thr administration. These results, in turn, indicated that the requirement of Thr in the LPS-challenged broilers also elevated.
Immune cell distribution in the blood is sensitive to immunological stress( Reference Dhabhar, Miller and McEwen 40 ). Shen et al.( Reference Shen, Piao and Kim 41 ) have observed higher counts of leucocytes and lymphocytes in blood of LPS-treated broilers. Similarly, Li et al.( Reference Li, Zhang and Chen 5 ) have reported that LPS challenge elevates the numbers of leucocytes in broilers. LPS injection increased the number of leucocytes, mononuclear cells and neutrophils, suggesting that LPS challenge induced inflammatory response in blood in this study. In this experiment, LPS challenge also increased pro-inflammatory cytokine levels in serum, spleen and intestinal mucosa, with the protein concentrations of mucosal cytokines being in parallel with their mRNA expression levels. The similar results are also observed by Wang et al.( Reference Wang, Shen and Li 42 ) and Wu et al.( Reference Wu, Zhou and Wu 11 ) in LPS-challenged broilers. TLR4 serves as a signalling receptor for LPS( Reference Beutler 43 , Reference Miller, Ernst and Bader 44 ), and its activation by LPS results in the release of critical pro-inflammatory cytokines( Reference Lu, Yeh and Ohashi 2 ). In our study, the mRNA abundance of jejunal TLR4 was promoted with LPS challenge, implying that the activation of TLR4 was also involved in the regulation of pro-inflammatory cytokine synthesis in this study. Mucins such as MUC2 have immunoregulatory effects on the gut, and it could interfere with antigen-sampling dendritic cell expression of inflammatory cytokine by inhibiting gene transcription through NF-κB( Reference Shan, Gentile and Yeiser 45 ). In mammals, studies have shown that intestinal inflammation would increase gastrointestinal Thr uptake and mucin synthesis, and luminal Thr concentration acutely affects mucin synthesis( Reference Nichols and Bertolo 46 – Reference Rémond, Buffière and Godin 48 ). These findings together suggest that Thr could modulate gut inflammatory reaction by regulating mucin synthesis. In addition, the key role that Thr plays in the synthesis of Ig, antibodies and other immune-related proteins also enables Thr to regulate immune system( Reference Taghinejad-Roudbaneh, Babaee and Afrooziyeh 27 , Reference Li, Yin and Li 49 – Reference Defa, Changting and Shiyan 51 ). In a chicken intestinal ex vivo model, Zhang et al.( Reference Zhang, Chen and Eicher 30 ) have shown that a 2-h Thr withdrawal in the culture medium induces compensatory increases in the mRNA abundances of IL-8 and IgA when exposed to LPS challenge, and these increases are inhibited with Thr supplementation. Similarly, we showed that dietary l-Thr supplementation alleviated the inflammatory responses in LPS-challenged broilers, as evidenced by the decreases in pro-inflammatory cytokines (IL-1β and IFN-γ) at both protein and transcription levels in the intestinal mucosa of LPS-challenged broilers receiving l-Thr. The inhibitory effects of dietary l-Thr supplementation on pro-inflammatory cytokine synthesis have also been reported in the intestine of broilers challenged with coccidiosis( Reference Wils-Plotz, Jenkins and Dilger 23 ), and weaned piglets challenged with Escherichia coli K88+ ( Reference Ren, Liu and Wang 52 ). In our study, the decreases in protein and transcription levels of pro-inflammatory cytokines in the intestine of broilers receiving l-Thr administration was in accordance with their simultaneously reduced intestinal TLR4 mRNA abundance, implying that l-Thr may inhibit the synthesis of cytokines by inactivating TLR4.
Plasma D-LA, a product of bacterial fermentation of carbohydrates, is a sensitive marker to reflect intestinal injury and to monitor intestinal permeability( Reference Sun, Fu and Rong 53 ). In this study, LPS challenge increased circulating D-LA level, impaired intestinal morphology and disrupted the mRNA expressions of intestinal tight junctions (increased mRNA expression of CLDN3, whereas reduced ZO-1 mRNA level), indicating that LPS challenge impaired intestinal barrier function. Similarly, oral administration of LPS impairs gut barrier function of broilers at an early age, as demonstrated by higher circulating D-LA level and DAO activity( Reference Wu, Zhou and Wu 11 ). Our previous study has also shown that intraperitoneal LPS injection damages intestinal barrier function of young broilers, according to the elevated plasma DAO activity, impaired intestinal morphology and disordered expressions of intestinal tight-junction proteins( Reference Li, Zhang and Chen 4 ). The damaged intestinal barrier function of LPS-challenged broilers in this study is supposed to be associated with the simultaneous activation of TLR4 and promoted pro-inflammatory cytokine synthesis. LPS could result in an increase in intestinal permeability in vitro and in vivo by inducing enterocyte membrane expression and localisation of TLR4( Reference Guo, Al-Sadi and Said 54 ). In addition, previous studies have evidenced that the pro-inflammatory cytokines such as IL-1β and IFN-γ would impair intestinal tight junction and increase gut permeability( Reference Capaldo and Nusrat 55 , Reference Al-Sadi, Ye and Dokladny 56 ). It is interesting to observe that the intestinal tight-junction proteins CLDN3 and ZO-1 exhibit opposite response to LPS challenge in this study. Tight junctions, the multi-protein complexes, are composed of transmembrane proteins, peripheral membrane (scaffolding) proteins and regulatory molecules including kinases, among which CLDN family proteins are the most important of the transmembrane proteins, whereas ZO family proteins are peripheral membrane proteins and are crucial to tight-junction assembly( Reference Turner 57 ). The increased CLDN3 mRNA expression is therefore thought to be associated with compensatory reaction, whereas the decreased mRNA level of ZO-1 is likely owing to the possible loss of tight junctions. Similar results are also reported previously in vivo ( Reference Li, Zhang and Chen 4 ) and in vitro ( Reference Bein, Zilbershtein and Golosovsky 58 ). In an in vitro study, Smirnova et al.( Reference Smirnova, Guo and Birchall 59 ) have found that LPS up-regulates mRNA expression and secretion of mucin and cytokine in goblet cells in a concentration- and time-dependent manner. In this study, LPS challenge also elevated mRNA expression level of MUC2 in jejunal mucosa, which was in agreement with the finding of Zhang et al.( Reference Zhang, Chen and Eicher 30 ). The increased MUC2 mRNA level may be due to a kappa B site in the 5'-flanking region of the MUC2 gene that can activate MUC2 transcription, and the elevated pro-inflammatory cytokine levels, as summarised by Andrianifahanana et al.( Reference Andrianifahanana, Moniaux and Batra 60 ). In our study, the intestinal goblet cell density of broiler chickens was reduced by LPS challenge, suggesting that LPS challenge interfered with the differentiation of goblet cells. Studies have shown that LPS-induced matrix metalloproteinase-9 and TLR4 activation are involved in the inhibition of goblet cell differentiation( Reference Garg, Ravi and Patel 61 , Reference Sodhi, Neal and Siggers 62 ). Considering the key roles goblet cell and MUC2 play in the gut barrier maintenance( Reference Kim and Ho 63 ), the regulatory effects of LPS on goblet cell density and MUC2 transcription may also contribute to the impaired intestinal barrier function of LPS-challenged broilers in this study. It has been reported that dietary Thr requirement for broilers to acquire the optimal intestinal morphology is 125 % of NRC recommendation( Reference Min, Liu and Qu 28 ). Zhang et al.( Reference Zhang, Chen and Eicher 22 ) observed that a higher level of Thr diet decreased gut permeability and improved intestinal morphology of broilers exposed to the combined feed withdrawal and coccidial vaccine challenge. Similarly, Wils-Plotz et al.( Reference Wils-Plotz, Jenkins and Dilger 23 ) reported that dietary Thr addition ameliorated the impaired barrier function of broilers challenged with E. maxima. In our study, we found that l-Thr supplementation improved intestinal morphology and normalised mRNA expressions of intestinal tight-junction proteins in LPS-challenged broilers, suggesting that l-Thr supplementation improved intestinal barrier function of broilers challenged with LPS. The improvements in the intestinal barrier function of LPS-challenged broilers receiving the l-Thr were in parallel with their reduced gut pro-inflammatory cytokines at both protein and transcription levels, as well as the decreased intestinal TLR4 mRNA expression level, which in turn revealed that l-Thr supplementation may improve the gut barrier function by inhibiting the synthesis of pro-inflammatory cytokines, and by inactivating TLR4 signal. Dietary Thr level is extremely vital for goblet cell differentiation and mucin synthesis, which are two critical components of the intestinal barrier( Reference Wang, Zeng and Mao 17 , Reference Law, Bertolo and Adjiri-Awere 18 , Reference Chee, Iji and Choct 20 , Reference Chee, Iji and Choct 21 ). In this study, l-Thr supplementation increased the ileal goblet cell density of LPS-challenged broilers, and this may be owing to a higher level of Thr requirement for the differentiation of goblet cell in LPS-challenged broilers. Zhang et al.( Reference Zhang, Chen and Eicher 30 ) have found that Thr addition normalises the mRNA expression level of MUC2 in an ex vivo chicken ileal explant under LPS exposure. The alteration in the jejunal MUC2 mRNA abundance of LPS-challenged broilers was also reversed when supplementing l-Thr in this study. In this way, the regulatory effects of l-Thr on intestinal goblet cell density and MUC2 mRNA expression would be therefore closely associated with the improved barrier function of LPS-challenged broilers receiving l-Thr administration.
In conclusion, our results suggested that the dietary l-Thr supplementation at a dosage of 3·0 g/kg could alleviate the harmful consequences of LPS challenge on growth performance, inflammatory responses and intestinal barrier function of broilers receiving a basal diet, in which Thr level met NRC requirement of broiler chickens.
Acknowledgements
The technical assistance of the labmates is gratefully acknowledged.
This study was funded by the China Postdoctoral Science Foundation (grant no. 2017M621765).
The contributions of the authors are as follows: Y. Chen and Y. Z. designed the study. Y. Chen, H. Z. and Y. Cheng participated in the animal experiment. Y. Chen, H. Z., Y. Cheng, Y. L. and C. W. conducted the research. Y. Chen, H. Z., Y. Cheng and Y. L. analysed the data. Y. Chen discussed the results and wrote the paper. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.









