China is the world’s largest contributor to duck production, accounting for three-fourth of the global duck husbandry. Meat ducks are highly sensitive to pathogens and toxins in feed, such as antinutritional factors as well as environmental stress, such as heat and immune stress(Reference Qin, Tian and Zhang1–Reference Bi, Nan and Zheng3). Research has verified that toxins and stresses usually lead to the damage of intestinal morphology and an increased inflammatory response(Reference He, Zhang and Chen4,Reference Bai, Zhang and Ding5) , which generally result in the occurrence of low-grade inflammation of the intestine in ducks in the production field. The general growth and well-being of animals are related to the health status of the intestine(Reference Ducatelle, Goossens and De Meyer6). Thus, it is still a big challenge for poultry nutritionists to maintain the gut health of ducks through nutritional strategy.
The intestinal epithelium is part of the mucosa layer that forms a physical barrier to allow the absorption of nutrients and protect against invading pathogens and toxins(Reference De Medina, Romerocalvo and Mascaraque7). In addition, the gut microbiota is an essential component of the barrier system and is highly related to epithelial metabolism, proliferation and survival(Reference Anderson, Dalziel and Gopal8). Emerging evidence indicates that these commensal microbes suppress pathogens by competing for access to the epithelial surface and sustain barrier integrity(Reference Neish9,Reference Trachsel, Briggs and Gabler10) . Furthermore, some specific bacteria can also release SCFA to modulate multiple physiological processes, especially butyrate, which plays a specific role in the maintenance of gut health(Reference Leonel and Alvarezleite11). However, defective barrier function is associated with a variety of intestinal disorders and diseases(Reference Groschwitz and Hogan12). Therefore, enhancing the intestinal barrier has become a way for prevention and therapy.
Resistant starch (RS), a type of prebiotic, is defined as a starch that escapes digestion in the small intestine and reaches the large intestine(Reference Keenan, Zhou and Hegsted13). Raw potato starch (RPS) is classified as a type 2 RS (RS2, starch granules). Type 2 resistant starch starches are resistant to digestion due to their compact structure that limits the ability of digestive enzymes to access the starch(Reference Sajilata, Singhal and Kulkarni14). Currently, there is a growing interest in supplementing RS in human and animal diets due to its beneficial effects on improved gut health(Reference Keenan, Zhou and Hegsted13). Results from previous studies in pigs have suggested that RPS could improve intestinal morphology in the colon and increase the expression of genes responsible for barrier function(Reference Trachsel, Briggs and Gabler10,Reference Nofrarías, Martínez-Puig and Pujols15) . Meanwhile, RS is also a fermentable substrate for caecal and colonic microbes to produce SCFA, such as acetate, propionate and butyrate, and reduce pH in the caecum(Reference Fuentes-Zaragoza, Riquelme-Navarrete and Sánchez-Zapata16). Nofrarías et al.(Reference Nofrarías, Martínez-Puig and Pujols15) found that long-term intake of 25 or 35 % RPS could increase butyrate production and improve colonic mucosal integrity in pigs. In accordance, several studies have shown that mouse or rat diets supplemented with RS could promote gut health, which is likely attributable to an alteration in the microbial community characterised by increased butyrate-producing bacteria, thereby reducing inflammation and maintaining physiologic balance(Reference Jiminez, Uwiera and Abbott17). Moreover, Keenan et al.(Reference Keenan, Zhou and Mccutcheon18) found that rats fed RS had increased gene transcription of proglucagon (Gcg) in the caecum. An in vitro study also showed that butyrate could increase Gcg gene expression in a dose-dependent manner(Reference Zhou, Hegsted and Mccutcheon19). Collectively, these observations suggest an important correlation between dietary RS and intestinal barrier health. Although a considerable amount of research has explored the health-promoting facets of feeding RS(Reference Keenan, Zhou and Hegsted13), the effects of RS on the gut are extremely complex and much remains to be learned. In the light of this, the aim of the present study was to investigate whether RPS could modulate barrier function and induce shifts in the microbial composition of the caecum in meat ducks.
Methods
Animals and diets
All experimental procedures were approved by the Animal Care and Use Committee of Sichuan Agricultural University. A total of 360 Cherry Valley meat-type male ducks of 1-d-old (54·92 (sd 0·23) g; Sichuan Mianying Breeding Duck Co. Ltd) were allocated to three dietary treatments with eight replicates (fifteen birds each) following a completely randomised design: three isoenergetic and isonitrogenous diets with 0 (control), 12 or 24 % purified RPS (AVEBE Ltd). RPS used in the present study contained 54·72 % RS (DM basis). Table 1 shows the diets that were formulated in line with National Research Council guidelines(20). Birds were offered starter (1–14 d) and finisher (15–35 d) diets as mash form. All ducks were reared in cages (2·2 × 1·2 × 0·9 m) in a temperature- and humidity-controlled room with feed and water ad libitum throughout the experimental period. Body weight and feed intake per pen were recorded at 1 and 35 d of age.
Table 1. Composition and nutrient levels in the basal diets (DM basis)
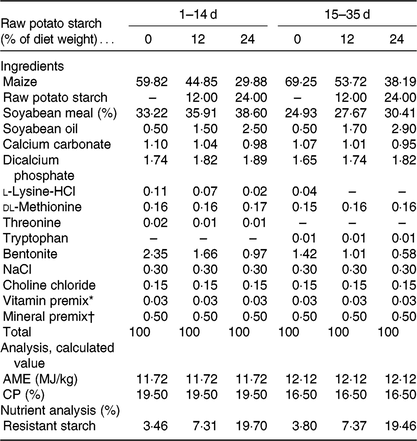
AME, apparent metabolism energy; CP, crude protein.
* Provided per kg of diet: vitamin A, 2·06 mg; vitamin D3, 0·04 mg; vitamin E, 30·01 mg; thiamine, 1 mg; riboflavin, 3·9 mg; pyridoxine, 3·375 mg; vitamin B12, 0·01 mg; calcium pantothenate, 8·85 mg; folate, 0·5 mg; biotin, 0·1 mg; niacin, 49·25 mg.
† Provided per kg of diet: Cu (CuSO4·5H2O), 8 mg; Fe (FeSO4·7H2O), 80 mg; Zn (ZnSO4·7H2O), 90 mg; Mn (MnSO4·H2O), 70 mg; Se (NaSeO3), 0·3 mg; iodine (KI), 0·4 mg.
Data collection and sampling
Two birds per pen were sampled at 35 d. One was killed by cervical dislocation, and the length and weight of the full caeca were measured, then the pH of the caecum was registered (pH-STAR, Matthuas, Inc.), and the contents of caeca were removed and weighed. Another bird was anaesthetised, and blood was taken by jugular vein to harvest plasma for endotoxin and cytokines determination. A portion of the caecum was flash-frozen in liquid N2 and stored at –80°C until gene expression analysis, and the remaining caecum were collected for histological determination. Digesta samples were randomly divided into two parts. One portion was stored at –20°C for SCFA determination, and the other was kept in liquid N2 for microbial community analysis.
Resistant starch assay
RS in RPS and diets was analysed using an RS assay kit (K-RSTAR; Megazyme Ltd).
Plasma endotoxin and cytokine assay
Plasma concentrations of endotoxins were determined using a commercially available ELISA kit, according to the manufacturer’s protocol (Mosak Biotechnology Co., Ltd). This kit was sensitive to 5-pg/ml endotoxins. Plasma TNF-α, IL-1β and IL-10 concentrations were measured using ELISA commercial kits (Mosak Biotechnology Co., Ltd). Cytokines were measured as described by the manufacturer’s instructions. All assays were done in duplicate.
Histology
Segments of the mid-caecum (about 1·0 cm) were removed, flushed gently with ice-cold physiological saline solution and then fixed in 4 % (w/v) paraformaldehyde, embedded in paraffin, sectioned at 5 μm and stained with haematoxylin and eosin by following the standard procedures. The photomicrographs (BA400Digital; Mike Audi Industrial Group Co., Ltd) were obtained to measure villus height (VH), crypt depth (CD) and mucosal thickness using an image analyser (Image Pro-Plus) at 100× magnification. VH was measured from the tip to the crypt–villus junction, and CD from the crypt–villus junction to the base(Reference Nabuurs, Hoogendoorn and Der Molen21). The mucosal thickness was defined as the distance from the base of muscularis mucosa to the tip of the villus(Reference Ki, Kim and Cho22).
RNA extraction and real-time PCR
Total RNA was isolated from frozen caecal samples using trizol reagent (Takara), according to the manufacturer’s specifications. Primers were designed using Primer 3 (http://bioinfo.ut.ee/primer3-0.4.0/primer3/) and are shown in online Supplementary Table S1. Real-time quantitative PCR was carried out using the ABI 7500 real-time PCR detection system (Applied Biosystems). All PCR contained 3 μl of 10-fold diluted complementary DNA, 1 μl each of 5 μm forward and reverse primers and 5 μl of 1× SYBR Green Master Mix (Takara). Amplification program was 95°C/15 min, followed by forty cycles of 95°C/5 s and 60°C/30 s, and a final melting curve analysis. Glyceraldehyde-3-phosphate dehydrogenase and β-actin were selected as the reference genes, and a normalisation factor was obtained by calculating the geometric mean of the values of the selected reference genes, which was subsequently used to normalise the relative amounts of RNA of interest(Reference Vandesompele, De Preter and Pattyn23).
SCFA analysis
Approximately 0·5 g of caecal digesta was diluted with 2-ml ultrapure water, mixed and centrifuged (3000 g , 15 min). Supernatant (1 ml) was mixed with 0·2-ml ice-cold 25 % (w/v) metaphosphoric acid solution at 4°C for 30 min and then centrifuged. Concentrations of acetate, propionate and butyrate were measured by GC system (Varian CP-3800).
DNA extraction and sequencing of 16S rRNA
Total genomic DNA from samples was extracted using QIAamp PowerFecal DNA kit (Qiagen), according to the manufacturer’s instructions. DNA concentration and quality were checked using a NanoDrop Spectrophotometer. DNA was diluted to 10 ng/μl using sterile ultrapure water and stored at –80°C for downstream use. The V4 region of the 16S rRNA genes (Primer-515F: 5′-GTGCCAGCMGCCGCGGTAA-3′; -806R: 5′-GGACTACHVGGGTWTCTAAT-3′) was amplified using the specific primer with 12 nucleotide (nt) unique barcode (25). The PCR mixture (25 μl) contained 1× PCR buffer, 1·5-mm MgCl2, each deoxynucleoside triphosphate at 0·4 μm, each primer at 1·0 μm, 0·5 U of KOD-Plus-Neo (TOYOBO) and 10-ng template DNA. The PCR amplification program consisted of initial denaturation at 94°C for 1 min, followed by thirty cycles (denaturation at 94°C for 20 s, annealing at 54°C for 30 s and elongation at 72°C for 30 s) and a final extension at 72°C for 5 min. Three replicates of PCR for each sample were pooled. PCR products mixed with one-sixth volume of 6× loading buffer and then loaded on 2 % agarose gels and purified using QIAquick Gel Extraction kit (Qiagen) following the manufacturer’s recommendations and quantified using Qubit@ 2.0 Fluorometer (Thermo Scientific) and pooled with an equal molar amount. The library was applied to paired-end sequencing (2 × 250 bp) with the Illumina Hiseq apparatus at Rhonin Biosciences Co., Ltd.
Sequence processing
The sequences were analysed according to Usearch (http://drive5.com/uparse/) and QIIME pipeline(Reference Caporaso, Kuczynski and Stombaugh24). Reads from the original DNA fragments were merged using FLASH(Reference Magoč and Salzberg25), low-quality reads were filtered (q < 30) and potential chimeric sequences were removed using the Uchime algorithm(Reference Edgar, Haas and Clemente26). After finding duplicate sequences, all the singletons were discarded due to their possible bad amplicons (http://www.drive5.com/usearch/manual/singletons.html), which may lead to an overestimation of diversity. Sequences were clustered into operational taxonomic units at 97 % identity threshold based on the UPARSE algorithm in Usearch(Reference Edgar27). Taxonomy were assigned using the SILVA database (Release_123)(Reference Quast, Pruesse and Yilmaz28) and uclust classifier in QIIME with default parameters. Representative sequences were aligned using PyNAST(Reference Caporaso, Bittinger and Bushman29). A phylogenetic tree was constructed using the generalised time-reversible model in FastTree(Reference Price, Dehal and Arkin30). The relative abundance of the taxon at each taxonomic level was produced based on the operational taxonomic unit abundance and taxonomic annotation. α-Diversity and β-diversity metrics were calculated using R Vegan package(Reference Oksanen, Blanchet and Friendly31). Weighted and unweighted Unifrac distances were calculated in R GUniFrac package to determine the degree of similarity between samples and used to perform principal coordinate analysis. Random forest analysis was applied to obtain the important indicator taxa using R random Forest package with 1000 trees and other default parameters.
Statistical analysis
Data were analysed using one-way ANOVA of the mixed model procedure of SAS 9.4 software (SAS Institute, Inc.). The independent variable was the RPS treatment. When significant, post hoc comparisons of treatment means were made using Tukey’s test. Statistical significance was detected at P < 0·05. Figures were generated by Prism 7.0 (GraphPad Software, Inc.). All data were expressed as means and standard errors.
Results
Caecal parameters and morphology
Neither final body weight nor daily feed intake was affected by the dietary RPS during the 35-d study period (online Supplementary Table S2). The length and weight of caecum increased with dietary RPS supplementation and significantly increased in the 24 % RPS diet when compared with the control diet (P < 0·05). The pH of caecal contents was notably lower in ducks fed the 12 % RPS diet compared with the control diet. The caecal digesta contents did not differ among groups (Table 2). Ducks fed the RPS-containing diets had increased VH and ratio of VH:CD and decreased CD in the caecum compared with those fed the 0 % RPS diet (P < 0·05). The mucosal thickness was significantly increased by the 12 % RPS diet in the present study (P < 0·05).
Table 2. Effect of dietary raw potato starch (RPS) concentration on caecal parameters and morphology in meat ducks
(Mean values with their standard errors (n 8))
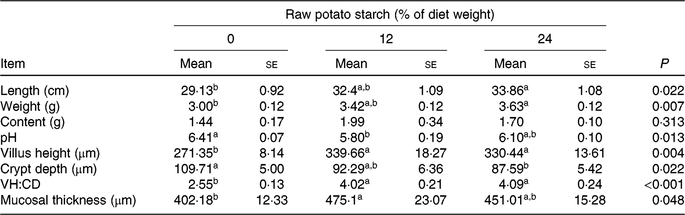
VH:CD, villus height:crypt depth ratio.
a,b Mean values in a row with unlike superscript letters were significantly different (P < 0·05; one-way ANOVA and Tukey's multiple range test).
Diet-related differences in microbial community
Illumina Hiseq high-throughput sequencing was performed to compare the microbial communities in the caecum among the three dietary groups. A total of 665 392 sequencing reads were obtained from the caecal digesta samples, and 5139 operational taxonomic units were identified in the caecum. There were no differences in richness estimators (Chao 1 and Ace) or diversity indices (Simpson and Shannon) of the caecal microbiota between the RPS diets and the control group (Table 3). Microbial distribution was then examined by principal coordinate analysis clustering using weighted Unifrac distance (β-diversity), which indicated separate clustering of caecal bacterial communities by diets (Fig. 2(A)). The overall commensal structures of the 0, 12 and 24 % groups were different because of the influence of the diets. Additionally, pairwise variation among samples was evaluated based on their treatments by weighted Unifrac distances (Fig. 2(B)). Pairwise distance between the 12 and 24 % RPS groups (12_24) was significantly greater than intra-group (0_0, 12_12, and 24_24) and inter-group (0_12 and 0_24) β-diversity, 0 and 24 % RPS groups (0_24) was significantly higher than intra-group (0_0 and 12_12), and the 0 and 12 % RPS groups (0_12) was significantly greater than intra-group (12_12). This difference in pairwise difference indicates a distinct role of RPS in altering the microbial structures.
Table 3. Effect of dietary raw potato starch on the diversity of caecal digesta microbiota in ducks
(Mean values with their standard errors (n 8))
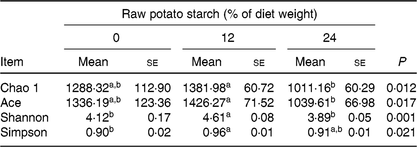
a,b Mean values in a row with unlike superscript letters were significantly different (P < 0·05; one-way ANOVA and Tukey's multiple range test).
The relative taxa abundance of bacteria was analysed from the phylum to genus level (Fig. 1 and online Supplementary Fig. S1). Bacteroidetes and Firmicutes were the two most dominant phyla in the duck’s caecal digesta (Fig. 1(A)). After supplementation with 12 % RPS in the diet, ducks had a distinctively higher abundance of Firmicutes compared with that of the control and 24 % RPS groups (P < 0·05). Additionally, Proteobacteria (P < 0·05) was significantly increased in the 24 % RPS group (Fig. 2(C)).
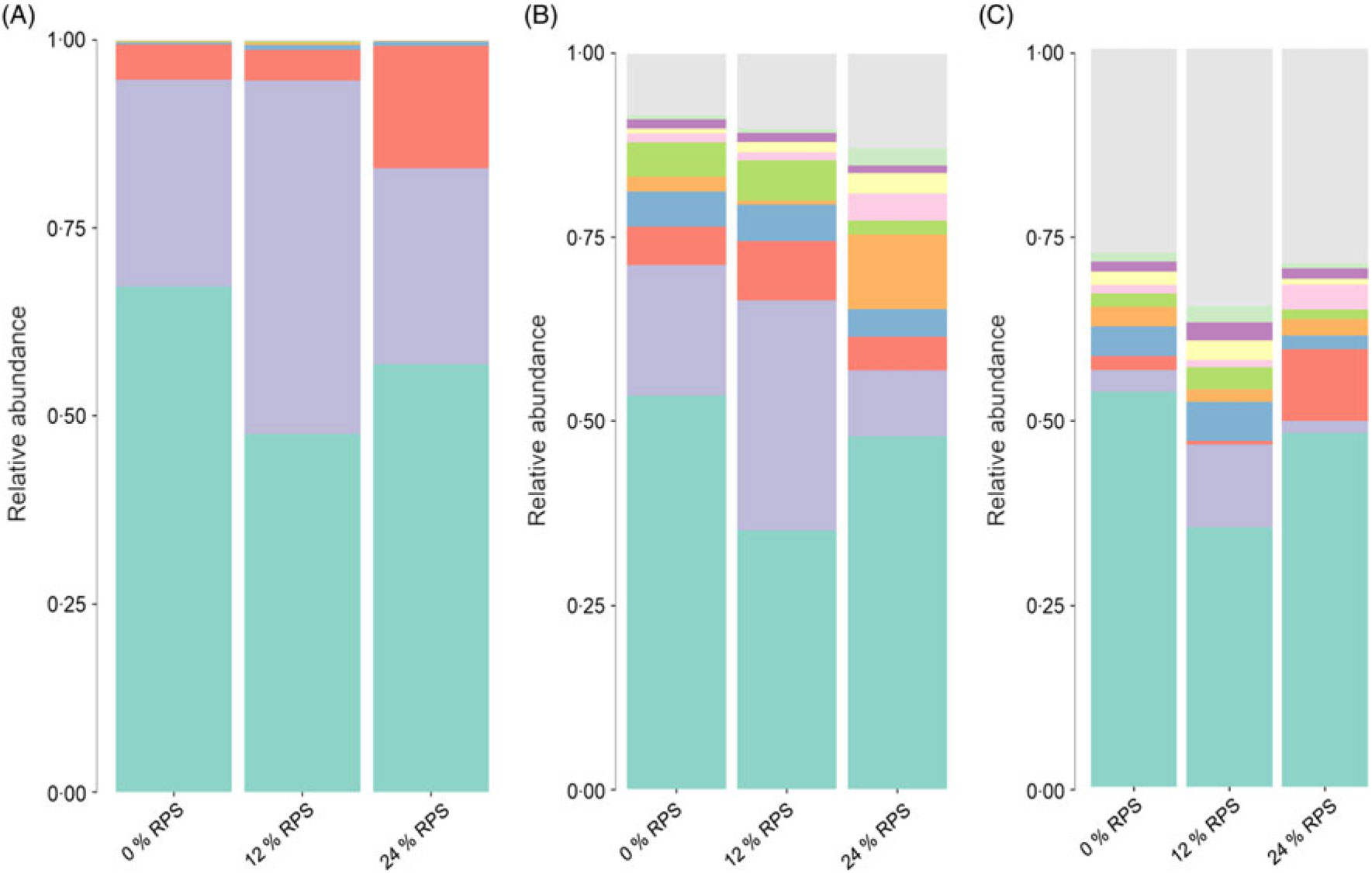
Fig. 1. Summary of bacterial taxa in duck caecal digesta observed by the concentration of raw potato starch (RPS) in the diet. The relative abundances of bacterial 97 % operational taxonomic units (OTU) are shown for duck caecal digesta samples grouped by RPS concentration. (A) Depiction of phylum-level classifications for observed OTU. Phylum: ![]() , others;
, others; ![]() , Acidobacteria;
, Acidobacteria; ![]() , Chlorobi;
, Chlorobi; ![]() , Fusobacteria;
, Fusobacteria; ![]() , Verrucomicrobia;
, Verrucomicrobia; ![]() , Deferribacteres;
, Deferribacteres; ![]() , Tenericutes;
, Tenericutes; ![]() , Actinobacteria;
, Actinobacteria; ![]() , Proteobacteria;
, Proteobacteria; ![]() , Firmicutes;
, Firmicutes; ![]() , Bacteroidetes. (B) Depiction of family-level classifications for observed OTU. Family:
, Bacteroidetes. (B) Depiction of family-level classifications for observed OTU. Family: ![]() , others;
, others; ![]() , Enterococcaceae;
, Enterococcaceae; ![]() , Bacteroidales S24-7 group;
, Bacteroidales S24-7 group; ![]() , Peptostreptococcaceae;
, Peptostreptococcaceae; ![]() , Desulfovibrionaceae;
, Desulfovibrionaceae; ![]() , Rikenellaceae;
, Rikenellaceae; ![]() , Enterobacteriaceae;
, Enterobacteriaceae; ![]() , Porphyromonadaceae;
, Porphyromonadaceae; ![]() , Lachnospiraceae;
, Lachnospiraceae; ![]() , Ruminococcaceae;
, Ruminococcaceae; ![]() , Bacteroidaceae. (C) Depiction of genus-level classifications for observed OTU for samples grouped by dietary RPS concentration. Genus:
, Bacteroidaceae. (C) Depiction of genus-level classifications for observed OTU for samples grouped by dietary RPS concentration. Genus: ![]() , others;
, others; ![]() , Subdoligranulum;
, Subdoligranulum; ![]() , Lachnoclostridium;
, Lachnoclostridium; ![]() , [Eubacterium] coprostanoligenes group;
, [Eubacterium] coprostanoligenes group; ![]() , Desulfovibrio;
, Desulfovibrio; ![]() , Barnesiella;
, Barnesiella; ![]() , Parabacteroides;
, Parabacteroides; ![]() , Alistipes;
, Alistipes; ![]() , Escherichia–Shigella;
, Escherichia–Shigella; ![]() , Faecalibacterium;
, Faecalibacterium; ![]() , Bacteroides.
, Bacteroides.
According to random forest analysis (Fig. 2(D)), the relative abundance of several genera within the phylum Firmicutes, including Faecalibacterium, Ruminococcaceae UCG-014, Subdoligranulum, Erysipelatoclostridium, Ruminococcaceae UCG-010 and Ruminiclostridium 5 was significantly higher in caecal digesta from birds fed the 12 % RPS compared with those fed the 0 % RPS diet. Furthermore, genera such as Lactobacillus and Bifidobacterium as well as Enterobacter were enriched in the digesta of birds fed the 24 % RPS diet, while Oscilospira and Subdoligranulum were relatively deficient compared with that of 0 % groups. Both 12 and 24 % RPS were lower in the Rikenellaceae RC9 gut group and Alloprevotella.
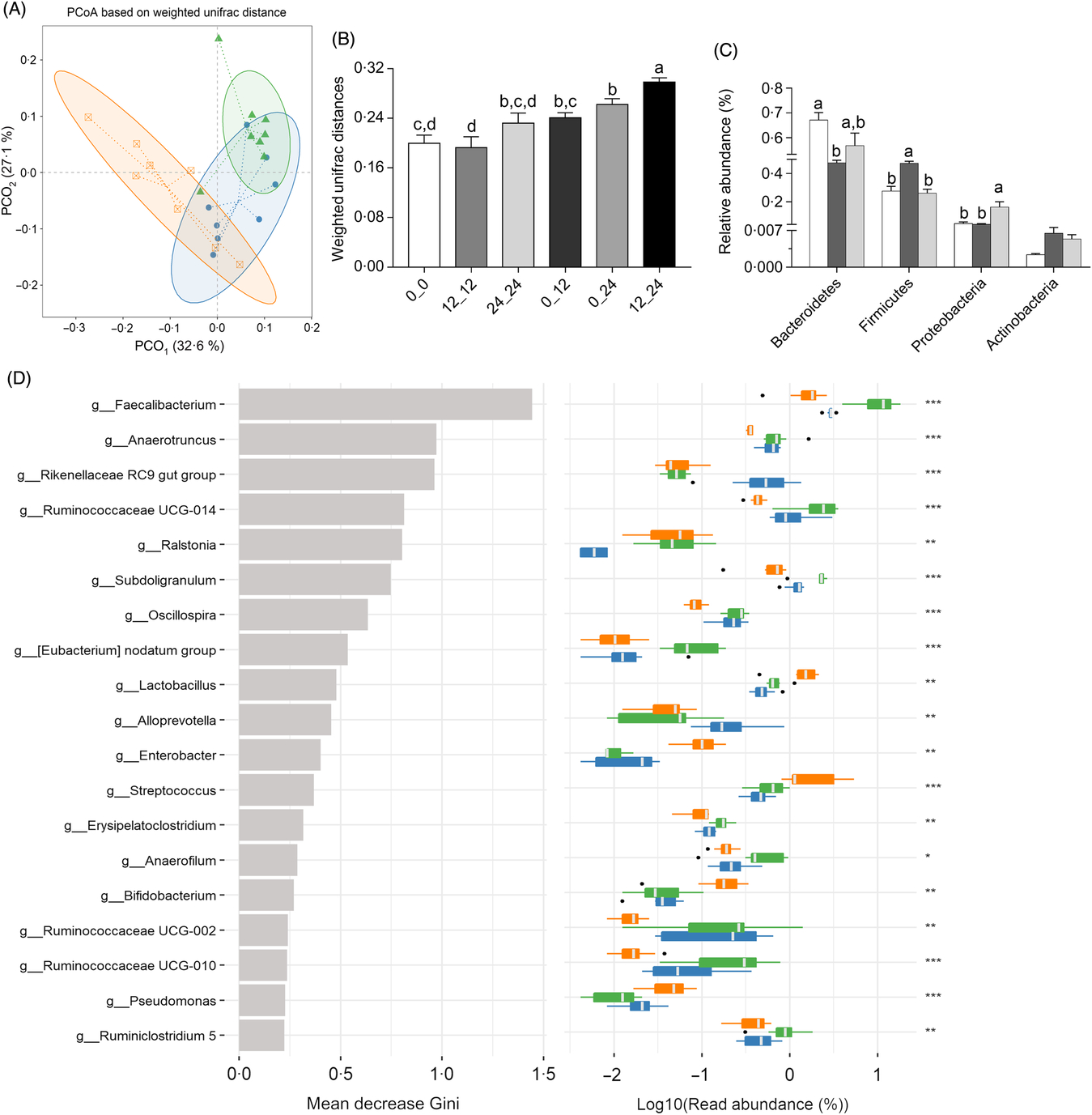
Fig. 2. Alteration of the microbial community of caecal digesta of ducks fed 0, 12, or 24 % raw potato starch (RPS). (A) Principal coordinate analysis (PCoA) among the three groups based on weighted UniFrac distances. Each point represented a sample. Group: ![]() , 0 % RPS,
, 0 % RPS, ![]() , 12 % RPS,
, 12 % RPS, ![]() , 24 % RPS. (B) Comparisons of pairwise weighted Unifrac distances. ANOVA was conducted for multiple comparisons with the least significant difference method for correction. 0: 0 % RPS; 12: 12 % RPS; 24: 24 % RPS. 0_0, 12_12 and 24_24 indicate intra-group distances; 0_12, 0_24 and 12_24 indicate inter-group distances. Data are means, with their standard errors represented by vertical bars. (C) Relative abundance of taxa at the phylum level in 0 % RPS (control), 12 % RPS and 24 % RPS. Significance was determined by one-way ANOVA. Data are means, with their standard errors represented by vertical bars.
, 24 % RPS. (B) Comparisons of pairwise weighted Unifrac distances. ANOVA was conducted for multiple comparisons with the least significant difference method for correction. 0: 0 % RPS; 12: 12 % RPS; 24: 24 % RPS. 0_0, 12_12 and 24_24 indicate intra-group distances; 0_12, 0_24 and 12_24 indicate inter-group distances. Data are means, with their standard errors represented by vertical bars. (C) Relative abundance of taxa at the phylum level in 0 % RPS (control), 12 % RPS and 24 % RPS. Significance was determined by one-way ANOVA. Data are means, with their standard errors represented by vertical bars. ![]() , 0 % RPS,
, 0 % RPS, ![]() , 12 % RPS,
, 12 % RPS, ![]() , 24 % RPS. (D) Gini index in random forest classification. The left panel represents the mean decreasing Gini index of each taxon in random forest analysis, and the right panel represents the relative abundance of bacteria in each group. Relative abundance was log10 transformed. Significance was determined by the Kruskal–Wallis rank sum test. * P < 0·05, ** P < 0·01 and *** P < 0·001. Group:
, 24 % RPS. (D) Gini index in random forest classification. The left panel represents the mean decreasing Gini index of each taxon in random forest analysis, and the right panel represents the relative abundance of bacteria in each group. Relative abundance was log10 transformed. Significance was determined by the Kruskal–Wallis rank sum test. * P < 0·05, ** P < 0·01 and *** P < 0·001. Group: ![]() , 24 % RPS;
, 24 % RPS; ![]() , 12 % RPS;
, 12 % RPS; ![]() , 0 % RPS.
, 0 % RPS.
Caecal digesta SCFA profiles
Acetate, propionate and butyrate contents were greater in ducks that were fed the 12 % RPS diet compared with the 0 % RPS diet (P < 0·05), and the SCFA contents of caecal digesta were inconspicuous when the RPS dose was added to 24 %, though the value in the 24 % RPS diet was still higher than that in the control diet (Fig. 3).
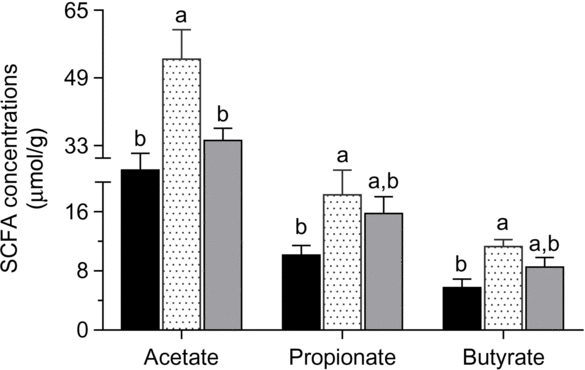
Fig. 3. SCFA concentrations of acetate, propionate and butyrate in ducks fed differing concentrations of raw potato starch (RPS). Values are means, with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P < 0·05; one-way ANOVA and Tukey’s multiple range test). ![]() , 0 % RPS;
, 0 % RPS; ![]() , 12 % RPS;
, 12 % RPS; ![]() , 24 % RPS.
, 24 % RPS.
Gene expression of barrier-related genes in the caecum
The caecal mucosal mucin (MUC)2, Gcg and tight junction-related gene mRNA expression levels of ducks are presented in Fig. 4. Supplementation with RPS-containing diets significantly up-regulated the expression of MUC2 and Gcg mRNA (Fig. 4(A) and (B)). When compared with the 0 % RPS diet, the 24 and 12 % RPS diets induced a significantly higher zonula occludens-1 and claudin 1 mRNA level, respectively (Fig. 4(C) and (E)). Dietary RPS administration did not alter the transcription level of occludin among groups (Fig. 4(D)).

Fig. 4. Expression of mRNA levels involved in caecal barrier function, including (A) mucin (MUC)-2, (B) proglucagon (Gcg), (C) zonula occludens (ZO)-1, (D) occludin and (E) claudin 1 of ducks fed differing concentrations of raw potato starch diets. Values are means, with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P < 0·05; one-way ANOVA and Tukey’s multiple range test).
Plasma endotoxin and cytokine contents
The concentrations of plasma endotoxin, TNF-α, IL-1β and IL-10 are shown in Fig. 5. Ducks fed either the 12 or the 24 % RPS diet showed reduced plasma levels of endotoxin, TNF-α and IL-1β when compared with the 0 % RPS diet, and no significant difference was observed for endotoxin and TNF-α, IL-1β between the RPS-containing diets (Fig. 5(A)–(C)). IL-10, however, was unaffected by dietary RPS contents (Fig. 5(D)).
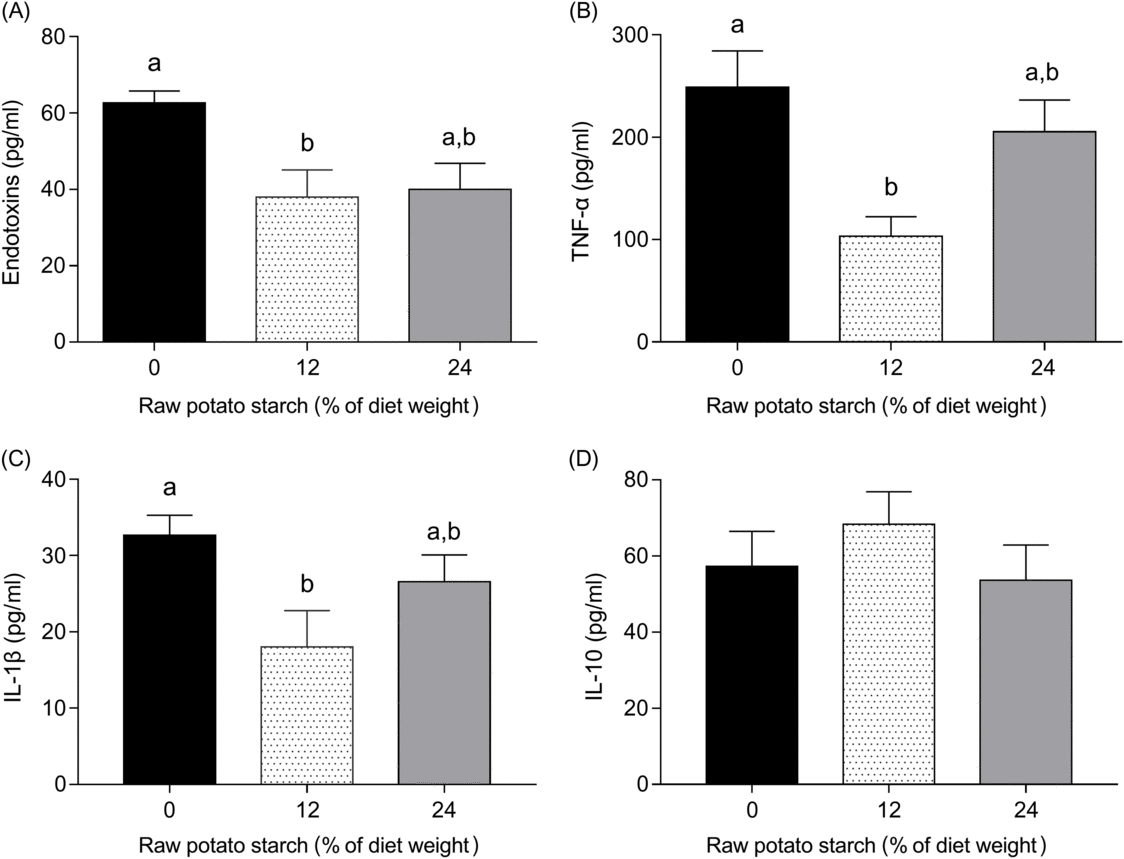
Fig. 5. Plasma (A) endotoxin, (B) TNF-α, (C) IL-1β and (D) IL-10 concentration of ducks consuming diets with different concentrations of raw potato starch. Values are means, with their standard errors represented by vertical bars. a,b Mean values with unlike letters were significantly different (P < 0·05; one-way ANOVA and Tukey’s multiple range test).
Discussion
The normal development of the hind gut is the foundation for maintaining barrier function and fermentability(Reference Whiteley, Higgins and Purdon32). Recent research showed that RPS could increase the weight of the large intestine and the mucosa thicknesses of the caecum in pigs(Reference Fang, Jiang and Su33). In our study, an increase in VH, VH:CD and mucosal thickness was observed following RPS supplements and indicated an enhanced absorptive capacity and intestinal health(Reference Ducatelle, Goossens and De Meyer6). The intestinal pH is largely affected by the amount of dietary fermentable carbohydrates entering the large intestine and has been used as an indicator for intestinal health(Reference Wang, Zhu and Li34). Our results showed that feeding the RPS-containing diets led to a lower pH in the caecal digesta. These results correspond with those of meta-regression analysis by Metzler-Zebeli et al.(Reference Metzler-Zebeli, Canibe and Montagne35) which found that, with a minimum amount of 10–15 % RS in the diet, type 2 resistant starch effectively decreased the pH in the hind gut of pigs. These results suggest that dietary RPS supplementation seems to have positive effects on the caecal development of ducks.
The health-promoting effects of RS may be mediated through improvement in microbiota composition. The microbiota within the hind gut plays a distinct role in defence against pathogens and maintenance of intestinal health(Reference Pourabedin and Zhao36). In the present study, diets did not affect the predominant phyla in the caecum, which is consistent with previous studies of ducks and chickens that Bacteroidetes and Firmcutes were the two dominant phyla in the caecum, with Bacteroidetes at a higher proportion(Reference Vasaï, Brugirard Ricaud and Bernadet37,Reference Xiao, Xiang and Zhou38) . However, several studies of prebiotics have shown expansion of Firmicutes(Reference Yacoubi, Saulnier and Bonnin39) or higher Firmicutes:Bacteroidetes ratio(Reference Molist, Manzanilla and Pérez40). It was demonstrated that the abundance of Firmicutes had a strong negative correlation with pathogenic bacterial populations in the intestine(Reference Mulder, Schmidt and Stokes41). Concomitant with these observations, RPS induced an increase in Firmicutes proportions mainly from a bloom in the relative abundance of families Lachnospiraceae and Ruminococcaceae. Both of these families are linked with gut health for their butyrate-producing properties(Reference Biddle, Stewart and Blanchard42). It is well established that changes in gut microbiota composition is highly associated with nutrient absorption and SCFA production. For example, members of Ruminococcaceae harbour a unique ability to increase the utilisation of starch(Reference Zeng, Huang and Lin43); Subdoligranulum, Faecalibacterium and Erysipelatoclostridium are butyrate producers(Reference Zhang, Dibaise and Zuccolo44,Reference Zhang, Guo and Xue45) , belonging to Firmicutes, with a higher abundance in the 12 % RPS group. Sun et al.(Reference Sun, Su and Zhu46) also reported that pigs fed an RPS diet increased the abundances of butyrate-producing bacteria. Research has also shown that dietary RPS can elevate butyrate concentration of the hind gut in pigs(Reference Trachsel, Briggs and Gabler10,Reference Fang, Jiang and Su33) . In the present study, it was evident that changes in butyrate content occurred after feeding the RPS-containing diets was concurrent with an increase in the abundance of butyrate-producing bacteria, as shown in our study. In addition, the abundance of Lactobacillus and Bifidobacterium, both well-known lactate-producing bacteria, was significantly enriched in ducks fed the 24 % RPS diet. Our observations are consistent with previous high throughput sequencing-based studies, with an increase in Bifidobacterium proportions following RS supplementation(Reference Martinez, Kim and Duffy47,Reference Walker, Ince and Duncan48) . It was suggested that the major end products of Bifidobacterium fermentation, acetate and lactate, can be co-metabolised by other bacterium to form butyrate(Reference Duncan, Louis and Flint49). The elevation in the abundance of butyrate-producing bacteria is further supported by increased butyrate and acetate concentrations in the caeca of ducks fed the 12 % RPS diet. However, it seems that the relative abundance of Lactobacillus and Bifidobacterium was too low to influence SCFA production, which resulted in the expected increase in acetate and butyrate concentrations as 12 % RPS not being reached after feeding 24 % RPS. These differences in SCFA stimulation may be partially explained by the lowered Oscillospira and Alloprevotella proportion in the 24 % RPS group. Since Oscillospira are butyrate producers, and Alloprevotella, a member of the Prevotellaceae family, also has the ability to produce SCFA in digestion of carbohydrates(Reference Gophna, Konikoff and Nielsen50,Reference Zhang, Wu and Lee51) . Another important observation in the present study was the increased abundance of Enterobacteriaceae in the caecum, due to the fact that SCFA could directly reduce the growth of Enterobacteriaceae by lowering the pH in the gut(Reference Zhang, Wu and Lee51). Nonetheless, given both microbiota composition and SCFA concentration observation, we believe that feeding RPS diets may have a favourable effect on barrier development in the caecum.
Butyrate is an important metabolite in the hind gut, arising from bacterial fermentation. Apart from providing energy for intestinal epithelial cell growth, butyrate also has been reported to improve gut barrier function(Reference Peng, Li and Green52). It has been reported that MUC2 synthesis is markedly elevated after butyrate stimulation(Reference Vieira, Leonel and Sad53). MUC2 is the main mucus barrier component that covers the intestinal mucosa to maintain an effective mucus layer(Reference Johansson54). Trachsel et al.(Reference Trachsel, Briggs and Gabler10) reported that an RPS diet could increase butyrate concentration and the expression of MUC2 in the caecum. Similar to previous work, we observed a higher MUC2 gene expression in RPS-containing diet groups, probably attributed to the elevation of butyrate production. Furthermore, tight junctions are primarily responsible for intestinal permeability(Reference Turner55). In the present study with meat ducks, feeding of 12 and 24 % RPS significantly increased the mRNA expression of claudin 1 and zonula occludens-1, respectively, which are the two main regulators of tight junction permeability. A promising candidate for promoting claudin 1 and zonula occludens-1 mRNA up-regulation was also the increased butyrate content when birds were fed RPS diets. Studies have shown that treatment with butyrate stimulated the expression of zonula occludens-1 and claudin 1(Reference Peng, Li and Green52,Reference Wang, Wang and Xin56) . Intriguingly, there was a significantly higher mRNA level of Gcg in the caecum from the RPS-fed groups in the present study. As known, Gcg is a precursor for glucagon-like peptide (GLP)-1 and GLP-2 that are secreted from enteroendocrine L cells, which have the ability to improve intestinal barrier function(Reference Drucker and Yusta57). It has been reported that butyrate stimulated the GLP-2 secretion through up-regulation of Gcg mRNA expression(Reference Tappenden, Albin and Bartholome58). GLP-2 stimulation has been shown to have a beneficial effect on intestinal morphology, VH and crypt cell proliferation in a study of broilers(Reference Hu, Guo and Huang59). At this point, this evidence provides an indirect but strong hint at the improvement of caecal morphology induced by RPS supplementation.
As a primary barrier between the external environment and gut internal milieu, the mucosa defends against numerous pathogen and noxious substances. A thinner and more permeable mucosal layer may increase endotoxin production and passage, thus causing defective barrier function(Reference De Medina, Romerocalvo and Mascaraque7,Reference Gil-Cardoso, Comitato and Gines60) . Disturbances in the intestinal barrier often trigger excessive release of pro-inflammatory cytokines, leading to an inflammatory response(Reference Groschwitz and Hogan12). TNF-α, one of the major pro-inflammatory cytokines, promotes claudin 1 removal from tight junctions, accelerates occludin degradation and enhances myosin light-chain kinase phosphorylation, thus augmenting paracellular permeability(Reference Amasheh, Fromm and Krug61). IL-1β, as a pro-inflammatory cytokine, also plays a crucial role in inflammatory reactions(Reference Nathalie, Cécile and Pascale62). The present data showed that ducks fed diets with RPS had reduced plasma endotoxin, TNF-α and IL-1β concentration in meat ducks when compared with ducks fed the 0 % RPS diet. Our observations are consistent with the previous studies that showed that RS in diets decreased serum TNF-α levels in mice(Reference Qian, Zhao and Song63). Knowledge of the mechanism by which RPS regulates inflammatory cytokines is still extremely limited. Our results demonstrated that enhanced caecal barrier function and elevated SCFA production may be achieved by feeding RPS, at least, play an impotent role in alleviating inflammation. A previous report reviewed altered intestinal permeability and bacterial translocation may result in intestinal infections and inflammation(Reference De Medina, Romerocalvo and Mascaraque7). Moreover, Zhou et al.(Reference Zhou, Packialakshmi and Makkar64) reported that butyrate can be an anti-inflammatory agent in the chicken. Collectively, our observations strongly provide an important link between the RPS-mediated changes in inflammatory responses to improve caecal barrier function of ducks.
The present study raises a new question about the dose of RPS used in meat duck for promoting caecal barrier function. The evidence of this work clearly demonstrates that diet supplemented with 12 % RPS partially improved caecal barrier function of meat ducks compared with 0 % RPS, but the positive role of RPS would be inconspicuous when the dose of RPS was added to 24 %, and it was not even an obvious difference between the 24 and 0 % RPS diets. For example, no statistical increases with the dietary increasing RPS in terms of SCFA contents. However, recent findings in mammals suggested that the contents of SCFA were also significantly increased when supplemented with more than 20 % RS(Reference Fang, Jiang and Su33,Reference Toden, Bird and Topping65) . It indicated that there might be an extremely complex mechanism underlying the relationship between the RPS and intestinal barrier function in meat ducks. Either specific structure of the digestive tract of poultry or difference in the sensitivity of ducks to RS could be possible factors. Thus, further studies are needed to validate this possibility.
In conclusion, we demonstrated that 12 % RPS supplement in the diet could enhance barrier function in the caecum of 35-d-old ducks. Significant biomarkers including improved caecal morphological parameters, altered microbial composition and increased expression of barrier markers as well as decreased plasma inflammatory markers were identified and modulated by supplementing dietary RPS. These results also strongly indicate that alterations in caecal microbial composition may result in an increase in butyrate concentration and subsequently impart positive effects on the intestinal barrier. Further studies are needed to elucidate the host response to dietary RPS supplementation in ducks undergoing intestinal challenges to further understand microbiota alterations and the effects of RPS on intestinal barrier function.
Acknowledgements
The authors thank Gregory Fraley for the revision of English. The raw pyrosequencing reads were submitted to Sequencing Read Archive (SRA) data base under the accession id: SRR7370079.
The research was supported by the National Natural Science Foundation of China (31772622), China Agriculture Research System (CARS-42-10), National Key R & D Program of China (2017YFD0502004), the 111 Project and Sichuan Agricultural University 211 Foundation of China.
S. Q., Q. Z. and K. Z. designed the experiment. S. Q., Z. S., Y. X., X. D. and H. P. conducted the experiment. S. Q., J. W., S. B. and Y. L. collected and analysed data. S. Q., T. J. A. and Q. Z. wrote the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114519002319











