Globally, about 350 million people suffer from major depression(1). Depression, the most common of all the psychiatric disorders, caused high rates of morbidity and mortality and was reported as the second leading cause of years lived with disability(Reference Ferrari, Charlson and Norman2). Moreover, negative influences have been occurring in individuals with depressive symptoms such as attempting suicide(Reference Chen, Bennett and Clarke3) and sleep disturbance(Reference Seow, Subramaniam and Abdin4). Meanwhile, about 90 % of individuals with mental disorders in China had never sought any type of professional help(Reference Phillips, Zhang and Shi5). Women are nearly twice as likely, in contrast with men, to suffer from an episode of depression(Reference Kessler, McGonagle and Swartz6), with an increased prevalence among those postmenopausal(Reference Sassarini7). Thus, preventing high-risk population like postmenopausal women from depressive symptoms is urgent and significant.
Accumulated evidence suggested that dietary exposures are related to depressive symptoms with biological processes such as inflammation(Reference Liu, Manson and Buring8) and oxidative processes(Reference Black, Penninx and Bot9). The most frequently investigated nutrients and food are vitamins B (including folate, riboflavin, pyridoxine and cobalamin), C and D, n-3 fatty acids, fish, fruits and vegetables(Reference Murakami and Sasaki10). Moreover, reduced endogenous oestrogens are considered as a risk factor for postmenopausal depression(Reference Georgakis, Thomopoulos and Diamantaras11), and dietary phyto-oestrogens modulate sex hormone levels in postmenopausal women(Reference Low, Dunning and Dowsett12).
Considering the correlations and synergies between foods and nutrients, it is difficult to identify associations for single items(Reference Hodge and Bassett13). A dietary pattern is a compositive variable that integrates consumption of several foods or food groups and is expected to have a greater impact on disease risk compared with any single nutrient or food(Reference Hu14). Thus, we hypothesised that dietary patterns may have a potentially beneficial effect on the prevention of depressive symptoms in postmenopausal women. However, although a few studies have investigated the relationship between dietary patterns and depressive symptoms in middle-aged women(Reference Mihrshahi, Dobson and Mishra15,Reference Rienks, Dobson and Mishra16) , only one Chinese Hong Kong study has investigated the relationship between dietary patterns and depressive symptoms in postmenopausal women(Reference Liu, Ho and Xie17). However, previous study indicated that food culture and dietary habits are substantially different among various regions in China(Reference Song and Cho18). We therefore designed a cross-sectional study to investigate how dietary patterns are related to depressive symptoms among postmenopausal women living in northern China.
Materials and methods
Participants
The Tianjin Chronic Low-grade Systemic Inflammation and Health (TCLSI-Health or TCLSIH) Cohort Study is an ongoing large prospective dynamic cohort study concerning association between chronic low-grade systemic inflammation and health status of a population living in Tianjin, China(Reference Song, Du and Zhang19,Reference Sun, Wu and Zhang20) . Female participants in this cross-sectional study were recruited annually during their health examinations at the Health Management Centre of Tianjin Medical University General Hospital.
This cross-sectional study used the baseline data from the TCLSIH during 2013–2016. Participants completed an examination including evaluation of anthropometric parameters and biochemical blood examination. And they were asked to finish a self-administered questionnaire consisting of demographic and socioeconomic characteristics, physical health status, lifestyle and health habits, depressive symptoms and FFQ.
During the study period, a total of 2621 participants with absence of a period for at least 1 year were sampled. We excluded participants who did not complete data collection on FFQ or depression scale (n 516) or those with a history of CVD or cancer (n 54). Thus, 2051 participants (mean age 58·8, sd 7·4 years) were included in this analysis. The protocol of the present study has been approved by the Institutional Review Board of Tianjin Medical University, and each participant provided written informed consent for analysis of their data.
Identification of dietary patterns
The participants filled out a FFQ with guidance. The FFQ consists of 100 items, including seven frequency categories as follows: (1) almost never eat, (2) less than once per week, (3) once per week, (4) 2–3 times per week, (5) 4–6 times per week, (6) once per d and (7) twice or more per d for foods; and eight frequency categories are as follows: (1) almost never drink, (2) less than once per week, (3) once per week, (4) 2–3 times per week, (5) 4–6 times per week, (6) once per d, (7) twice or three times per d and (8) four or more times per d for beverages during the last month. Factor analysis was applied to find out major dietary patterns and factor loadings on all 100 food items and beverages. The reproducibility and validity of the questionnaire were assessed in a random sample of 150 participants living in Tianjin by comparing the data from repeat measure approximately 3 months apart and 4-d weighed dietary records (WDR). Spearman’s rank correlation coefficient for energy intake between two FFQ was 0·68 (P<0·0001), for food items (fruits, vegetables, fish, meat and beverages) ranged from 0·62 to 0·79, for energy intake by the WDR and the FFQ was 0·49 and for nutrients (vitamins C and E, polyunsaturated fats, saturated fats, carbohydrate and Ca) by the WDR and the FFQ ranged from 0·35 to 0·54.
The FFQ was designed to measure food intake of the participants in the last month. Because the reproducibility and validity of the questionnaire have been assessed, the FFQ represents the long-term food intake of the participants. Moreover, seasonal food intake in the questionnaire includes the intake in the last month and in natural mature season.
Factor analysis (principal components analysis) was used to derive dietary patterns and to determine factor loadings for each of food and beverage subgroups (in g/d). Factors were rotated with varimax rotation to maintain uncorrelated factors and enhance interpretability. A combined evaluation of the eigenvalues, scree plot test and factor interpretability was used in determining the number of retained factors. The distinctive dietary patterns of the study population were well described by the three factors. Factors were named descriptively based on the food items showing high loading (absolute value ≥ 0·30) with respect to each dietary pattern as follows: healthy dietary pattern (factor 1), sweets dietary pattern (factor 2) and traditional Tianjin dietary pattern (factor 3) (Table 1). In the present study, no component had a factor loading score ≤–0·30. We calculated a factor score by summing the consumption from each food item weighted by its factor loading. A higher factor score indicates greater conformity to the dietary pattern. Variables unrelated to a given dietary pattern are weighted close to zero. For further analysis, factor scores were categorised into four equal groups using quartile cut-offs.
Table 1. Factor loadings scores* of primary food groups of dietary patterns
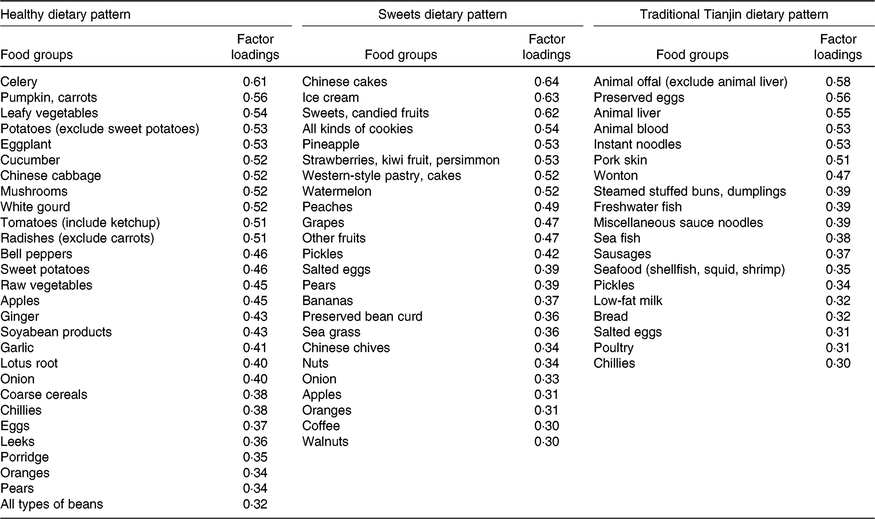
* The factor loading scores with an absolute value of more than 0·30 are shown.
Assessment of high depressive symptoms
Depressive symptoms were evaluated by the Chinese version of the Zung Self-Rating Depression Scale. There were twenty items on the scale, which was defined as either positive or negative. Participants were required to grade on 1–4 points for each item. Summary scores of twenty items ranged from 20 to 80, with higher values indicating greater depressive symptoms. Since a previous study indicated that a cut-off point at 48 best matched clinical judgement(Reference Gregory21), and a cut-off point of 48 was also used to define depressive symptoms in other studies(Reference Su, Yu and He22,Reference Aoki, Nagate and Utsumi23) , this cut-off point is used in the present study; scores higher than the cut-off indicate moderate depressive symptoms.
Assessment of other variables
All participants received standardised physical examination at the Health Management Centre. Waist circumference was measured in standing position at the level of the umbilicus. Blood pressure was measured twice on the upper left arm in a sitting position and the average used for analysis. Fasting blood sugar was measured using the glucose oxidase method. TAG were measured using the enzymatic colorimetric method. LDL and HDL were measured with an autoanalyzer (Roche Cobas 8000 modular analyzer). The metabolic syndrome was defined according to the criteria of the American Heart Association Scientific Statement(Reference Alberti, Eckel and Grundy24).
Anthropometric variables (e.g. height and weight) were measured using a standard protocol. BMI was calculated as weight in kilograms divided by height in square metres. As for socioeconomic variables, educational level was defined by the question ‘What is the highest degree you earned?’ and was divided into two categories: <college graduate or ≥college graduate. Marital status was classified as married or unmarried. The subjects were also classified as living alone or living with others. Occupation was classified as either senior officials and managers or professionals, while income was classified into two groups using the threshold of 10 000 yuan per month. ‘Visiting friends’ status was assessed by asking the question ‘Do you contact your friends and relatives often?’ and classified as ‘yes’ or ‘no’.
Information on the smoking (‘never’, ‘former’ and ‘current smoking’) and drinking (‘never’, ‘former’ and ‘current drinking’) status of the participants was obtained from a questionnaire survey. Physical activity in the most recent week was assessed using the short form of the International Physical Activity Questionnaire(Reference Craig, Marshall and Sjostrom25). The questionnaire asked whether subjects had performed any activities from the following categories during the previous week: walking; moderate activity (household activity or child care); and vigorous activity (running, swimming or other sports activities). Metabolic equivalent (MET) hours per week were calculated using the corresponding MET coefficients (3·3, 4·0 and 8·0, respectively) according to the MET coefficient of activity × duration (h/d) × frequency (d/week). Total physical activity levels were assessed in terms of weekly MET-h, which was calculated by combining separate hours for different activities. History of physical illness was evaluated on the basis of response (‘yes’ or ‘no’) to questions concerning a history of diseases (including liver diseases, gallstones, gastritis, chronic obstructive pulmonary diseases, pulmonary tuberculosis, gout, rheumatism, cataract, glaucoma, hearing disturbance, cervical spondylosis and lumbar spondylosis) and physician-diagnosed diseases (including diabetes, hypertension and the metabolic syndrome).
Statistical analysis
Descriptive data are presented as means with 95 % CI or as percentages and examined by ANOVA and χ 2 test for categorical variables. Quartiles were categorised across the scores of each dietary pattern based on the distribution of the scores for all the participants and used for further analysis. Association between quartile categories of dietary pattern scores and depressive symptoms was examined using multiple logistic regression analysis. Depressed status was used as dependent variable, and factor score was used as independent variable. OR and 95 % CI were calculated. A linear trend across increasing quartiles was tested using the median value of each quartile as a continuous variable based on linear regression. For model 1, the analysis was conducted without any adjustment; model 2 was adjusted for age and total energy intake; and model 3 was additionally adjusted to physical activity, smoking status, drinking status, education, job, income, marital status, visiting friends, living alone and metabolic symptom. Log transformation was used when the variables are not subject to normal distribution. P values <0·05 were considered statistically significant and all tests presented were two tailed. All statistical analyses were performed using the Statistical Analysis System 9.1 edition for Windows (SAS Institute Inc.).
Results
After a varimax rotation, a factor analysis revealed three dietary patterns and the main factor loadings of each pattern (Table 1). These three patterns explained 20·5 % of the variance in dietary consumption (i.e. 8·9 % for factor 1, 6·3 % for factor 2 and 5·3 % for factor 3). According to their contributions to total variation, factor 1 was identified as a healthy dietary pattern characterised by a high consumption of vegetables, fruits and soyabean products; factor 2 was identified as a sweets dietary pattern typified by a greater consumption of ice cream, desserts and fruits; and factor 3 was defined as the traditional Tianjin dietary pattern characterised by a greater consumption of grain, milk, meat, animal blood, animal offal, sausages, preserved eggs, seafood and pickle products.
The participant characteristics according to their depressed status are presented in Table 2. Compared with participants without depressive symptoms, participants who had depressive symptoms tended to be younger (P<0·01) and had lower plasma concentrations of TAG (P = 0·01), lower systolic blood pressure (P < 0·01), lower diastolic blood pressure (P < 0·05), decreased physical activity (P < 0·001), a lower prevalence of the metabolic syndrome (P < 0·05) and were less likely to be married (P < 0·05).
Table 2. Baseline participant characteristics by depressed status (yes or no) according to the Self-Rating Depression Scale (SDS; cut-off point of 48)
(Adjusted geometric mean values and 95 % confidence intervals; percentages)
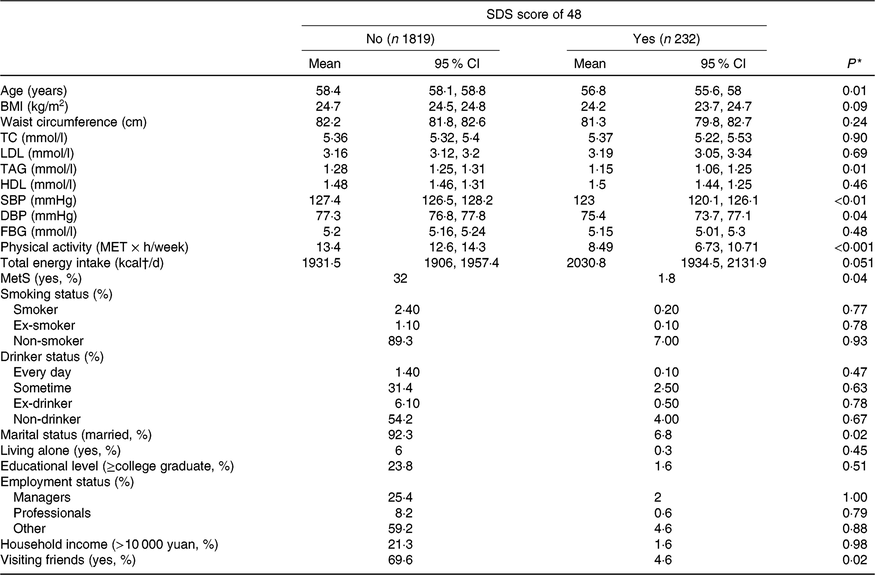
TC, total cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; MET, metabolic equivalents; MetS, metabolic syndrome.
* ANOVA or χ 2 test.
† To convert kcal to kJ, multiply by 4·184.
Associations between dietary patterns and depressive symptoms are shown in Table 3. The healthy dietary pattern was inversely associated with prevalence of depressive symptoms. The OR for the extreme quartile was 0·57 (95 % CI 0·33, 0·97) (P for trend = 0·03) after adjusting for all confounding factors. In contrast, participants with a high intake of sweets were more likely to report depressive symptoms. The OR across quartiles were 1·00 (reference), 0·75 (95 % CI 0·42, 1·3), 1·08 (95 % CI 0·64, 1·81) and 1·66 (95 % CI 1·03, 2·71) after adjustments (P for trend < 0·01). Participants in the highest quartile of the traditional Tianjin dietary pattern had a 153 % greater risk (OR 2·53; 95 % CI 1·58, 4·16; P for trend < 0·0001) of developing depressive symptoms than those in the lowest quartile after adjustments.
Table 3. Adjusted relationships between quartiles of dietary pattern factor scores and depressive symptoms
(Numbers of subjects; unadjusted and adjusted odds ratios and 95 % confidence intervals)
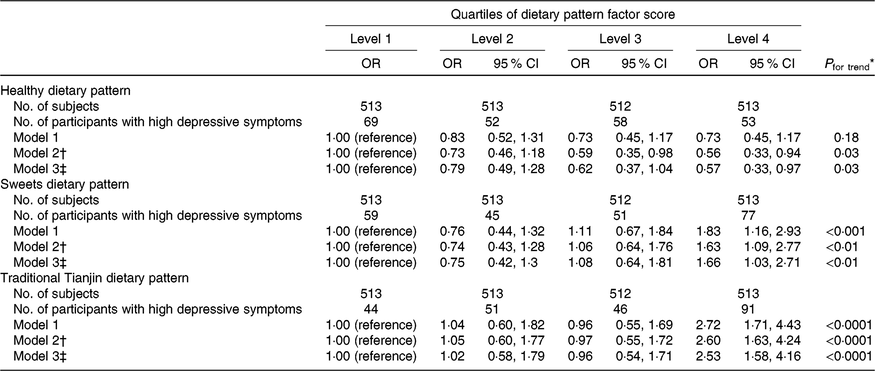
* Multiple logistic regression analysis.
† Adjusted for age and total energy intake.
‡ Adjusted for age, BMI, smoking status, drinking status, physical activity, marital status, total energy intake, household incomes, employment status, educational levels, visiting friends, living alone, metabolic syndrome.
Discussion
In this cross-sectional study, we identified three dietary patterns, and all these dietary patterns were associated with depressive symptoms after adjustments for confounding variables. A healthy dietary pattern (vegetables, fruits and soyabean products) was associated with the decreased prevalence of depressive symptoms, whereas both greater adherence to sweets (ice cream, desserts and fruits) and traditional Tianjin dietary patterns (animal blood, animal offal, sausages and preserved eggs) were associated with a higher prevalence of depressive symptoms. The present study examines the relationship between dietary patterns and depressive symptoms in postmenopausal women.
In the present study, we have hypothesised that dietary patterns may have a potential correlation with depressive symptoms in postmenopausal women. A number of studies(Reference Martinez-Gonzalez and Sanchez-Villegas26) have investigated the relationship between dietary patterns and depressive symptoms in both men and women. A cross-sectional study among postmenopausal women living in Hong Kong China showed that dietary patterns featuring a low intake of processed foods (rich in refined cereals, sweets, preserved food, fried food and semi-lean animal meat) and/or a high intake of whole plant foods (rich in whole grains, fruits and vegetables), that were similar to the healthy dietary pattern in the present study, were associated with a reduced risk of depression(Reference Liu, Ho and Xie17). To the best of our knowledge, no previous studies have assessed an association between dietary patterns and depressive symptoms in non-Chinese postmenopausal women. Only one Australian prospective study examined this association among middle-aged women(Reference Rienks, Dobson and Mishra16). This prospective study suggested that a Mediterranean-style dietary pattern, that was similar to our healthy dietary pattern and was characterised by garlic, peppers, mushrooms, lettuce, onion or leeks, cucumber, pasta, rice, tomatoes, red wine, celery, other beans, avocado, bean sprouts and zucchini, had protective influences against the depressive symptoms(Reference Rienks, Dobson and Mishra16). However, no significant association was observed between the meat and processed meat dietary pattern that was similar to the traditional Tianjin dietary pattern in the present study and was characterised by pork, bacon, sausages and lamb and depressive symptoms. Moreover, we also found that a sweets dietary pattern, which had not been identified in other non-Chinese population, was positively associated with depressive symptoms. Thus, further studies are warranted to illustrate the associations between common and region-specific dietary patterns and depressive symptoms in postmenopausal women.
In the present study, the association between a healthy dietary pattern and depressive symptoms has been suggested by a number of plausible mechanisms in recent years. First, the high content of antioxidants such as carotenoids from vegetables and fruits may be an explanation for the protection(Reference Beydoun, Beydoun and Boueiz27). Second, the potential protective effect of healthy dietary patterns could also come from large amounts of folate found in cruciferous vegetables, leafy vegetables and dried legumes(Reference Akbaraly, Brunner and Ferrie28). Folate is hypothesised to protect brain function by decreasing homocysteine, which has a neurotoxic effect including impaired methylation, excitotoxicity, oxidative stress and hypoxia in the central nervous system(Reference Bottiglieri29). Finally, Chinese are known to consume relatively high intake of soyabean and soya products(Reference Mei, Yeung and Kung30). Isoflavones, rich in soyabeans and soyabean products, are effective in reducing depressive and anxiety symptoms among postmenopausal women(Reference Lipovac, Chedraui and Gruenhut31) through selective β oestrogenic receptor binding, and interactions with the dopaminergic, serotonergic and cholinergic systems, and brain regions crucial to higher cognitive function and mood(Reference Craig and Murphy32).
The present study also suggests that the sweets dietary pattern and traditional Tianjin dietary pattern are positively associated with depressive symptoms. The mechanisms that have been suggested to explain the positive association between the sweets dietary pattern and depressive symptoms are probably related to the high glycaemic load of the consumed items. The intake of a diet with a high glycaemic load has been associated in the short-term with rapid and immediate changes in serotonin levels and consequently with a relief of some psychological symptoms(Reference Murakami, Sasaki and Takahashi33). Increased inflammation and circulating cytokines have already been proven to be associated with depressive symptoms by extensive studies(Reference Berk, Williams and Jacka34). In fact, previous research shows that a higher intake of refined carbohydrates was associated with a higher level of C-reactive protein, a marker of proinflammatory cytokines(Reference Liu, Manson and Buring8), which have already been proven to be possible mediators of known environmental risk factors in depression(Reference Berk, Williams and Jacka34,Reference Miller, Maletic and Raison35) . The traditional Tianjin dietary pattern characterised by preserved eggs, animal blood, animal offal, sausages and seafood contains a lot of fat. Also, consumption of a high-fat diet also leads to chronic systemic inflammation(Reference Schachter, Martel and Lin36). Another plausible mechanism relates to the consumption of a high-Pb-containing food, preserved eggs. Previous study suggested that dietary Pb exposure may increase the risk of mental health problems including depression(Reference Yu, Shao and Yu37). This confirmed our previous studies that the sweets dietary pattern and traditional Tianjin dietary pattern were associated with high depressive symptoms among Chinese adults(Reference Xia, Wang and Yu38).
Although this large population-based study considered many confounding factors, there are several limitations to our study. First, to confirm the level of depressive symptoms depends on not only total scores on the Self-Rating Depression Scale but also diagnostic interviews. Total scores do not correspond with a clinical diagnosis of depression but rather indicate the level of high depressive symptoms that may be of clinical relevance(Reference Zung39). Therefore, further studies should conduct a standardised comprehensive structured diagnostic interview in order to measure the depressive symptoms more persuasively. Second, due to the nature of the self-reporting questionnaire, food intake may not be exact with recall bias. Finally, since this is a cross-sectional study, reverse causation cannot examine in the present study. Depressive symptoms may have effect on dietary patterns that remains an alternative interpretation of the observed associations. Therefore, a prospective study or an intervention trial should be undertaken to confirm the existence of a relationship between the healthy dietary pattern and depressive symptoms.
Conclusion
In the present study, a higher score of the healthy dietary pattern characterised by vegetables, fruits, whole-grain food and soyabean products was inversely associated with depressive symptoms. On the contrary, adherence to the sweets dietary pattern characterised by ice cream, desserts and fruits and to the traditional Tianjin dietary pattern characterised by preserved eggs, animal blood, animal offal, sausages and seafood was positively associated with depressive symptoms. The findings suggest that dietary patterns appeared to be related to postmenopausal depressive symptoms. A long-term prospective study or randomised trials are required to clarify this causality.
Acknowledgements
We gratefully acknowledge the participants of the study and Tianjin Medical University General Hospital-Health Management Centre for the possibility to perform the study.
All data included in the present study are available upon request by contact with the corresponding author.
The present study was supported by grants from the National Natural Science Foundation of China (no. 81872611, 91746205, 81673166, 81372118, 81372467 and 81302422), the Key Technologies R&D programme of Tianjin (Key Project: no. 11ZCGYSY05700, 12ZCZDSY20400, 13ZCZDSY20200, and 15YFYZSY00020), the National Science and Technology Support Programme (no. 2012BAI02B02), 2012 and 2016 Chinese Nutrition Society (CNS) Nutrition Research Foundation – DSM Research Fund (no. 2014-071, 2016-046 and 2016-023), the Technologies development programme of Beichen District of Tianjin (no. bcws2013-21, bcws2014-05 and 2015-SHGY-02), the technologies project of Tianjin Binhai New Area (no. 2013-02-04 and 2013-02-06), the Science Foundation of Tianjin Medical University (no. 2010KY28 and 2013KYQ24), the Key Laboratory of Public Health Safety (Fudan University), Ministry of Education (no. GW2014-5) and the National Training Programmes of Innovation and Entrepreneurship for Undergraduates (no. 201510062013), China.
K. L. and Y. G. analysed data and wrote the manuscript. M. L., J. F., X. W., G. Y., Q. Z., L. L., G. M. and Z. Y. contributed to the discussion and edited the manuscript. H. W., Y. X., X. B., S. Z., S. S., X. W., M. Z. and H. J. contributed to collect the data and interpreted the results. Q. J. and K. S. revised the manuscript for important intellectual contents. Y. W. and K. N. designed the study, reviewed and edited the manuscript. K. N. had full access to all the data and took responsibility for the integrity of the data.
The authors declare that there are no conflicts of interest regarding the publication of this paper.






