Introduction
Seabird populations, considered good indicators of ecosystem health (Furness and Camphuysen Reference Furness and Camphuysen1997), are under severe pressure and have declined by 70% over the last 60 years (Croxall et al. Reference Croxall, Butchart, Lascelles, Stattersfield, Sullivan, Symes and Taylor2012, Paleczny et al. Reference Paleczny, Hammill, Karpouzi and Pauly2015). This is a concern, given that seabirds provide many important ecosystem services including nutrient cycling between pelagic and terrestrial ecosystems (Sekercioglu et al. Reference Sekercioglu, Daily and Ehrlich2004, Mulder et al. Reference Mulder, Jones, Kameda, Palmborg, Schmidt, Ellis, Orrock, Wait, Wardle, Young, Croll, Vidal, Mulder, Anderson, Towns and Bellingham2011). Seabirds face threats on two fronts: at sea (e.g. accidental by-catch) and at their onshore breeding colonies (e.g. predation by invasive species) (Jones et al. Reference Jones, Tershy, Zavaleta, Croll, Keitt, Finkelstein and Howald2008, Abraham and Thompson Reference Abraham and Thompson2011). Smaller species are often more threatened onshore (Jones et al. Reference Jones, Tershy, Zavaleta, Croll, Keitt, Finkelstein and Howald2008), while larger seabird species are more threatened at sea (Abraham and Thompson Reference Abraham and Thompson2011).
New Zealand is considered a world leader in mitigating the negative effects of invasive predators on (small) seabirds (e.g. Jones et al. Reference Jones, Holmes, Butchart, Tershy, Kappes, Corkery, Aguirre-Munoz, Armstrong, Bonnaud, Burbidge, Campbell, Courchamp, Cowan, Cuthbert, Ebbert, Genovesi, Howald, Keitt, Kress, Miskelly, Oppel, Poncet, Rauzon, Rocamora, Russel, Samaniego-Herrera, Seddon, Spatz, Towns and Croll2016). Numerous eradication programmes have been implemented to control invasive species and protect seabird populations (Towns and Broome Reference Towns and Broome2003). With increasingly better eradication techniques and protocols, larger islands have been successfully freed of their invasive species (Towns and Broome Reference Towns and Broome2003) and several seabird species appear to benefit from these eradications (Ismar et al. Reference Ismar, Baird, Gaskin, Taylor, Tennyson, Rayner, Bettesworth, Fitzgerald, Landers and Imber2014, Buxton et al. Reference Buxton, Taylor, Jones, Lyver, Moller, Cree and Towns2015).
Despite the efforts aimed at the mitigation and eradication of invasive predators other terrestrial threats, such as habitat loss, may also contribute to population declines or limit population recovery of seabirds (Taylor Reference Taylor2000). Habitat selection studies can be important to identify potential threats (e.g. Rayner et al. Reference Rayner, Hauber and Clout2007), for habitat selection is one of the key components of ecological research and fundamental to understanding ecological processes (Johnson Reference Johnson1980, Manly et al. Reference Manly, McDonald, Thomas, McDonald and Erickson2002). Ultimately, understanding the mechanisms dictating how and why species choose specific habitats will enhance the development of successful management strategies to preserve (seabird) species (Manly et al. Reference Manly, McDonald, Thomas, McDonald and Erickson2002, Cassini Reference Cassini2013).
The need for such studies is illustrated by the South Georgia Diving-petrel Pelecanoides georgicus (SGDP hereafter) in New Zealand, where it is listed as ‘Nationally Critical’ (Robertson et al. Reference Robertson, Dowding, Elliott, Hitchmough, Miskelly, O’Donnel, Powlesland, Sagar, Scofield and Taylor2013). The SGDP is a small burrow-breeding seabird, favouring barren habitats, with a circumpolar distribution across the southern oceans (Marchant and Higgins Reference Marchant and Higgins1990). The SGDP in New Zealand, however, has declined steeply and was subsequently extirpated across most of its historic range including the South Island, Stewart Island, Auckland Islands, and Chatham Islands (Taylor Reference Taylor2000, Holdaway et al. Reference Holdaway, Jones and Athfield2003, Wood and Briden Reference Wood and Briden2008). The only remaining SGDP colony in New Zealand currently persists in the dunes of the Sealers Bay on Codfish Island (Whenua Hou) with a total estimated population size of 150 adults (Taylor Reference Taylor and Miskelly2013). SGDPs in New Zealand differ from other remaining populations of the species in that they appear to specialize in breeding in coastal dunes (Marchant and Higgins 19990, Wood and Briden Reference Wood and Briden2008) rather than scree and scoria at higher altitudes (Marchant and Higgins Reference Marchant and Higgins1990, Taylor Reference Taylor2000, Holdaway et al. Reference Holdaway, Jones and Athfield2003, Wood and Briden Reference Wood and Briden2008).
Underlying causes of historic declines in New Zealand remain speculative but predation by introduced species such as Pacific rats Rattus exulans was most likely the main factor. The removal of introduced predators (brush-tailed possums Trichosurus vulpecula, Pacific rats, and Weka Gallirallus australis) from Codfish Island (Middleton Reference Middleton2007) initially resulted in an increased population trend of SGDPs between the 1980s and the late 1990s (Imber and Nilsson Reference Imber and Nilsson1980, West and Imber Reference West and Imber1989, Taylor Reference Taylor2000), but this increase appears to have halted since 2000 (Wood and Briden Reference Wood and Briden2008, Taylor Reference Taylor and Miskelly2013). The reason for this lack of population growth is unknown, but several contributing factors have been hypothesised. For example, given that SGDPs in New Zealand nest in coastal dunes, the impact of encroachment of the dunes by (invasive) vegetation is currently perceived as a threat (Taylor Reference Taylor and Miskelly2013). The apparent breeding habitat, coastal dunes, may also be at risk from stochastic events and catastrophes, such as storms (Cole Reference Cole2004). Furthermore, as Common Diving-petrels P. urinatrix (CDP hereafter) recently have started breeding in the same dunes (Taylor and Cole Reference Taylor and Cole2002), competition for nest sites might pose a threat to the SGDP. No pelagic threats have yet been identified for the Codfish Island population (Taylor Reference Taylor2000), though collision with vessels due to light attraction has been documented for other populations (Black Reference Black2005).
To better understand potential terrestrial threats to the SGDP on Codfish Island, we conducted burrow searches and recorded a range of physical and biological variables at both occupied SGDP burrows and random points between November 2015 and January 2016. We aimed to identify the most important dune characteristics influencing nest site selection in SGDPs. Such information is essential to identify conservation management options for the SGDP in New Zealand to ultimately achieve population growth.
Methods
Study area
We collected data on the nest site selection of SGDPs in the Sealers Bay dunes (-46.766, 167.645) on Codfish Island (Whenua Hou), c.3 km west of Stewart Island (Rakiura). We defined the exact study area using an aerial photograph of Codfish Island (G. Elliott unpubl. data 2004) geo-referenced to NZGD2000. The borders of the study area were defined by the Sealers Bay beach in the north, the forests of Codfish Island in the south and east and an unnamed stream in the west. The size of this area was 100 x 900 m, encompassing the entire Sealers Bay dunes and all SGDP burrows identified in previous surveys (Taylor and Cole Reference Taylor and Cole2002, Cole Reference Cole2004). While our study area included forest bordering dunes, we did not sample interior forest, as the SGDP in New Zealand appears to favour dune habitat (Marchant and Higgins Reference Marchant and Higgins1990, Taylor Reference Taylor2000, Holdaway et al. Reference Holdaway, Jones and Athfield2003, Wood and Briden Reference Wood and Briden2008).
Nest site selection
We assessed habitat selection within a use versus availability framework at the fourth scale (the selection of resources for one specific type of behaviour; Johnson Reference Johnson1980), i.e. nest site selection. We considered occupied SGDP burrows as used sites. To account for the available habitat, we created 150 random points within the entire study area using a random number generator in ArcGIS 10. At the study site, we marked random points with a bamboo/fibreglass pole and a track marker with an ID number. We discarded 19 random points, because they were located below the springtide line.
As previous surveys (Imber and Nilsson Reference Imber and Nilsson1980, Taylor and Cole Reference Taylor and Cole2002, Cole Reference Cole2004) indicated a strong dependency on foredunes, we assessed SGDP nest site selection using two approaches: 1) nest site selection in the whole dune system (all potential nesting habitat), and 2) nest site selection in the foredunes (core nesting habitat) (Pérez-Granados et al. Reference Pérez-Granados, López-Iborra and Seoane2016). For the second approach, we created a 20 m buffer around each detected burrow site in ArcGIS 10 and discarded all random points falling outside this buffer. The total number of remaining random points within these 20 m buffers was n = 45.
Burrow searches and occupancy assessment
We searched for burrows of diving-petrels Pelecanoides spp. and other Procellariiformes in the study area for 10 days in November 2015. Burrow searches were made by walking the length of the dunes in pairs with 10 m distance between observers. We marked every detected burrow with a bamboo/fibreglass pole, a track marker with an ID number and a reflector (to enable safe navigation of the colony at night with minimal impact on breeding birds). In addition, we took a GPS point for each burrow.
We used various techniques to determine burrow occupancy. In November and December 2015, we monitored the burrows with stick palisades (Johnston et al. Reference Johnston, Bettany, Ogle, Aikman, Taylor and Imber2003) and checked these palisades twice per week. To account for false positives (Taylor et al. Reference Taylor, Cockburn, Palmer and Liddy2012), we considered burrows with more than three records of activity as occupied. To identify the species present in the burrows, we used playback of calls of both SGDPs and CDPs in combination with hand capture of birds at night (Payne and Prince Reference Payne and Prince1979). In January 2016, we used burrow traps custom-made for Pelecanoides spp. (length = 30 cm, Ø 8 cm) at night to identify the occupants of remaining unidentified active burrows. Traps were checked every 45 to 60 minutes to prevent (heat) stress in these birds.
Variables affecting nest site selection
We recorded a range of physical and biological variables at occupied SGDP burrows and random points. We identified plant species within a circle with a surface area of 1 m2, using Wilson (Reference Wilson2009) and Wickes and Rance (Reference Wickes and Rance2010). We estimated the cover of each plant species as the vertical projection of all foliage onto a horizontal surface within this 1 m2 circle. To reduce the number of variables, the cover of all plant species per site was summed to create the explanatory variable plant cover. In addition, the cover of all invasive species was summed and divided through the sum of the cover of all plant species to form the explanatory variable invasive ratio. Due to the high vegetation density at some sites, we refrained from measuring maximum standing vegetation height and instead classified plant height into one of five classes (0–0.5 m, 0.5–1 m, 1–1.5 m, 1.5–2 m, and > 2 m). We measured slope with a handheld clinometer at the centre of the 1 m2 circle. We measured aspect using a handheld compass and transformed recorded measurements to values between 2 and 0 using the Beers et al. (Reference Beers, Dress and Wensel1966) transformation, in which we considered 45° the maximum aspect. North-east (seaward) facing slopes thus received a value of 2 and south-west (landward) facing slopes receiving a value of 0. We assessed soil compaction using a hand-held penetrometer (AMS Inc. G-281) with an adapter foot (AMS Inc. G-282) for sensitive soils (Ø 2.54 cm). With this penetrometer, we measured the force needed to penetrate the soil to a depth of 6.4 mm in kg/cm2. To account for micro-scale variation, we measured the soil compaction five times within a 1 m2 circle at each site (at the centre and on the edge in each wind direction) and averaged values per site. We investigated sand flux by measuring the accumulation or erosion of sand at the poles marking the sites over the course of two months (49–66 days). We accounted for the difference in exposure time between sites by dividing sand flux (mm) through the number of days. We measured the distance to the sea (defined by the springtide line), the distance to the closest occupied SGDP burrow (to assess the influence of social attraction or intraspecific competition), and the distance to the closest burrows occupied by other seabird species (to assess the influence of interspecific competition) in m in ArcGIS 10.
Data analysis
We constructed a priori models aligning with several biologically plausible hypotheses. We only included explanatory variables with Spearman correlation coefficients of r ≤ 0.6 in the same model. We then used generalised linear models (GLM) with a logit-link function (0 = random point, 1 = occupied SGDP burrow) to analyse nest site selection of the SGDP; inactive burrows and burrows occupied by other seabirds were not included in our analyses. We applied the Akaike Information Criterion corrected for small sample sizes (AICC) (Burnham and Anderson Reference Burnham and Anderson2002) to identify the relative importance of variables affecting nest site selection by SGDPs. We also generated a “full” model (a model that includes all uncorrelated variables with the indicative highest fit) and a null model. For each model, we generated the AICC, the difference in AICC values relative to the best model (ΔAICC) and Akaike weights (w i). We considered models with a ΔAICC < 4.0 to be supported by the data (Burnham and Anderson Reference Burnham and Anderson2002). We then summed the w i from all supported models to assess the relative importance of each variable (RVI).
The analysis of nest site selection by SGDPs was then repeated, as explained above, for the 20 m buffer created around each burrow with the remaining dataset. However, we also accounted for biologically plausible interactions between explanatory variables (i.e. the influence of the distance to sea on plant and physical variables, as well as the influence of plant variables on physical variables and vice versa) in this second layer of analysis, as biotic variables in foredunes are under higher pressure from abiotic influences than the more stable back dune habitats (Hesp Reference Hesp and Short1999). All statistical analyses were conducted in Program R 2.12.1 (R Development Core Team 2016) using the Hmisc (Harrel Reference Harrel2016) and MuMIn (Bartoń 2015) packages. We used Locally Estimated Scatterplot Smoothers (LOESS) of the ggplot2 package (Wickham Reference Wickham2009) for visual interpretation of the most important variables affecting nest site selection.
Results
Burrow searches and occupancy
We located 143 Pelecanoides spp. burrows in the study area, of which 109 showed signs of occupancy. Using playback, hand captures, and burrow traps, we identified 74 SGDP burrows, six CDP burrows, and four burrows with mixed occupancy (one burrow was inhabited by a SGDP x CDP pair, two SGDP burrows were taken over by CDPs, and one CDP burrow was taken over by SGDPs). The occupants of 25 Pelecanoides spp. burrows remained unidentified. In addition, seven larger burrows were found inhabited by Sooty Shearwaters Puffinus griseus.
Relative importance of variables in the whole dune system
Spearman’s correlation tests revealed that several explanatory variables were highly correlated (r ≥ 0.6; Appendix S1 in the online supplementary materials), and were therefore not included in the same models. Distance to sea, slope, aspect, sand flux, and sand penetration were all explanatory variables present in the best performing models (AICC < 4.0; Table 1). Distance to sea, slope, and aspect were the most important variables for SGDP nest site selection (RVI = 0.961; Table 2), followed by sand flux (RVI = 0.861) and sand penetration (RVI = 0.381). The relationship between SGDP nest site selection and the distance to sea was negative (Table 2 and Figure 1). The relationship between SGDP nest site selection and slope, aspect and sand flux was positive, while the relationship with sand penetrability was negative. SGDPs thus selected for dunes within 20 m distance from the sea with steep, NE (seaward) facing slopes, high sand flux, and loose soils. Competition/attraction and plant parameters were not included in the best models.
Table 1. Candidate models (including a “full” model that includes all uncorrelated variables with the indicative highest fit and a null model), number of explanatory variables included models (K), Akaike Information Criteria corrected for small sample sizes (AICC), the difference in AICC relative to the model most supported by the data (ΔAICC) and Akaike weights (w i) for nest site selection by South Georgia Diving-petrels in the whole dunes. Models with a ΔAICC <4 .0 (bold) are considered to be supported by the data.

Table 2. Regression coefficients (β), standard errors (SE) and relative variable importance (RVI) for explanatory variables included in the best preforming nest site selection models for South Georgia Diving-petrels in the whole dunes (AICC < 4.0). *indicates that β ± 2 *SE does not intersect 0).
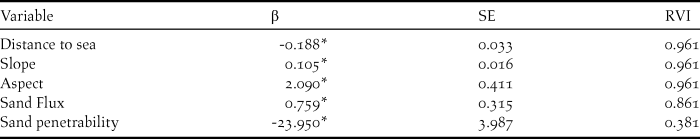
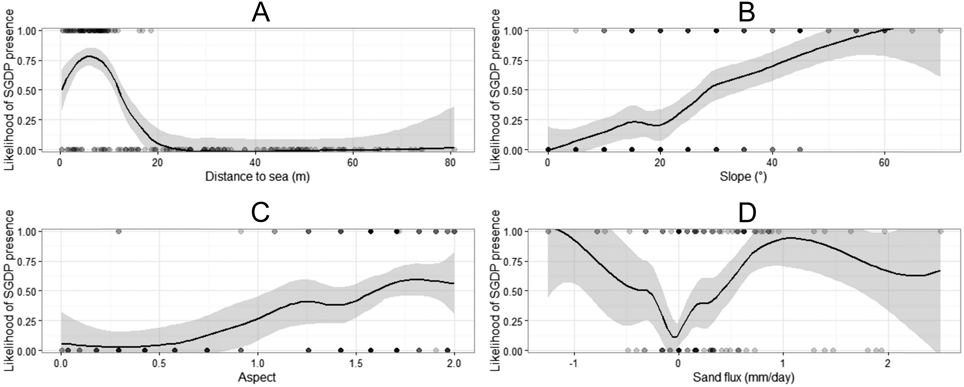
Figure 1. Scatterplots and Locally Estimated Scatterplot Smoothers (LOESS) including 95% confidence intervals for the four most important explanatory variables affecting nest site selection of South Georgia Diving-petrels in the whole dunes. A: distance to sea (as defined by the springtide line) in m. B: Slope in °. C: Aspect, transformed following Beers et al. (Reference Beers, Dress and Wensel1966), with NE (seaward) facing slopes receiving a value of 2 and SW (landward) facing slopes receiving a value of 0. D: sand flux in mm/day.
Relative importance of variables in the foredune
No explanatory variables were highly correlated in the foredune (Appendix S2). In the foredune, distance to sea, slope, aspect, sand flux, and plant cover were explanatory variables present in best performing models (Table 3). Distance to sea, slope, aspect, and plant cover were critical factors for SGDP nest site selection (RVI = 0.843). Sand flux appeared less important in the foredune (RVI = 0.317). There was a positive relationship between SGDP nest site selection and slope and aspect, while there was a negative relationship with the distance to sea (Table 4). Within the foredune, SGDPs thus also selected for sites close to the sea with steep seaward-facing slopes. Furthermore, the interactions between the distance to sea and physical dune variables proved influential as well as the interactions between plant cover and the physical dune variables (Table 4 and Figure 2). Within the foredune, no competition/attraction variables were included in the best performing models and neither were invasive ratio or plant height.
Table 3. Candidate models (including a full and a null model), number of explanatory variables included models (K), Akaike Information Criteria corrected for small sample sizes (AICC), the difference in AICC relative to the model most supported by the data (ΔAICC), and Akaike weights (w i) for nest site selection by South Georgia Diving-petrels in the foredune. Models with a ΔAICC < 4 .0 (bold) are considered to be supported by the data.
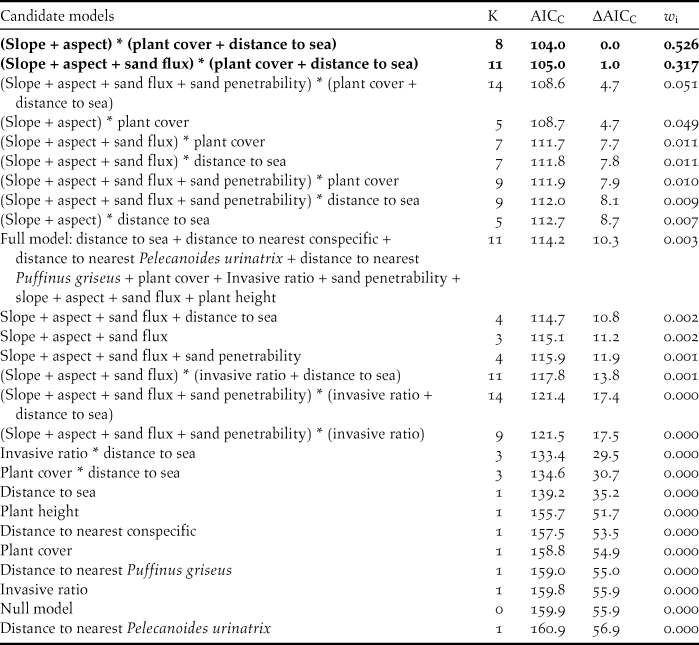
Table 4. Regression coefficients (β), standard errors (SE) and relative variable importance (RVI) for explanatory variables included in the best preforming nest site selection models for South Georgia Diving-petrels in the foredune (AICC < 4.0). * indicates that β ± 2 * SE does not intersect 0).
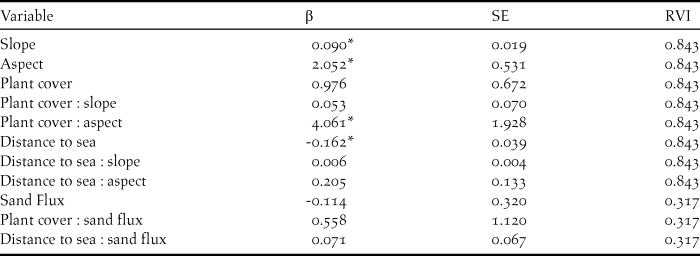
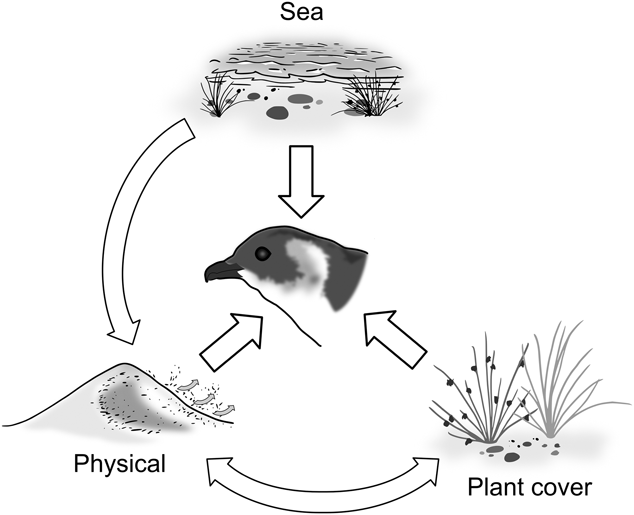
Figure 2. Explanatory variables and interactions affecting nest site selection in South Georgia Diving-petrels in the foredunes. Note that Physical is a cluster of slope, aspect and sand flux.
Discussion
Our results showed that SGDP nest site selection was dictated by the distance to sea, physical dune variables (slope, aspect, sand flux, and sand penetrability), and plant cover. SGDPs selected for foredunes with steep NE (seaward) facing slopes and mobile soils. Interactions between physical variables and the distance to sea as well as interactions between physical variables and plant cover also affected nest site selection in SGDPs. Inter- and intraspecific competition/attraction or invasive plants on the other hand did not appear to affect SGDP nest site selection on Codfish Island.
The strong preference of SGDPs to nest in steep, seaward-facing foredunes may be related to physical or ecological constraints. In particular, such slopes may provide easy take-off sites (Scott et al. Reference Scott, Moller, Fletcher, Newman, Aryal, Bragg and Charleton2009). SGDPs have short, paddle-like wings adapted to wing-propelled diving (Onley and Scofield Reference Onley and Scofield2007) and may therefore struggle to take off. Nesting in steep foredunes may thus help overcome the lack of potential “take-off trees”, as used by other Procellariiformes that breed in less barren habitat (Sullivan and Wilson Reference Sullivan and Wilson2001). Alternatively, the strong preference for foredunes may be caused by competition pressure outside these foredunes. SGDPs breeding in the Atlantic and Indian Ocean also favour barren habitats (albeit at much higher altitudes than SGDPs in New Zealand; Payne and Prince Reference Payne and Prince1979). This contrasts with the nest site selection of CDPs, which breed in a wider range of habitats (Payne and Prince Reference Payne and Prince1979, Marchant and Higgins Reference Marchant and Higgins1990). Thus, the preference for barren habitats by SGDPs globally (and foredunes in New Zealand specifically) may be an attempt to avoid competition with CDPs. While our results did not show a negative effect of CDP presence on SGDP nest site selection, additional observations suggest that this requires further investigation. Specifically, we found that three SGDP nests failed due to interactions with CDPs (including the mixed pair). The lack of more widespread competition with CDPs may currently be a consequence of their relative scarcity in the Sealers Bay dunes. Given that CDPs are generally more aggressive than SGDPs (S. Trainor pers. comm. 2016), the threats to the SGDP from competition may thus increase with an increasing population size of CDPs within the Sealers Bay Dunes.
Biotic and abiotic variables affect each other in dynamic ecosystems such as foredunes. However, the exact interactions between physical variables and overlaying plants remain poorly understood, as physical dune characteristics will influence plants (e.g. Sykes and Wilson Reference Sykes and Wilson1990, French Reference French2012, Murphy et al. Reference Murphy, Silberbauer, Streeter, Smiley, Smith, Darling, van Essen and Rapson2012), but plants can also shape dune profiles by changing physical variables (Hesp Reference Hesp and Short1999). Therefore, it is not surprising that SGDP nest site selection is affected by interactions between multiple variables. Further investigations will be necessary to unravel how different variables affect each other and consequently SGDP nest site selection.
Given the preference of SGDPs for fragile foredunes, this species is very susceptible to stochastic events and catastrophes during the breeding season. Storms are already impacting SGDPs on Codfish Island. In 2003, a storm extirpated at least 15% of the population, destroyed 40% of the nests, and removed the first 10 m of the dunes (estimated 23,377 m3 of sand; Cole Reference Cole2004). Unfortunately, such events are likely to increase in both intensity and severity in New Zealand due to human-induced climate change (Blair Reference Blair2007, Hennessy et al. Reference Hennessy, Fitzharris, Bates, Harvey, Howden, Hughes, Salinger, Warrick, Parry, Canziani, Palutikof, Hanson and van der Linden2007). Therefore, storms and storm surges during the breeding season are likely to be the most detrimental threat to SGDPs on Codfish Island.
The results showing preference for fragile foredunes provide an initial step towards understanding the exact mechanisms of nest site selection in the SGDP. Many Procellariiformes show strong nest site fidelity (Miskelly et al. Reference Miskelly, Taylor, Gummer and Williams2009). Foredunes are inherently mobile and dynamic (Hesp Reference Hesp and Short1999), therefore it is possible that dune variables dictating nest site selection may change over time. Given the short-term nature of our study, we thus recommend a long-term monitoring programme of the SGDP population, in combination with a study assessing nest site selection of new burrows. Furthermore, we suggest a bioacoustic attraction system (Miskelly and Taylor Reference Miskelly and Taylor2004) to be trialled at the Sealers Bay colony to test whether SGDP nest site selection can be altered to include sites farther from the foredunes. This would potentially render this population less susceptible to risks in fragile foredunes near the springtide line. Given the lack of social attraction explaining current nest selection patterns of SGDPs on Codfish Island, chances for success, however, appear limited. Therefore, we also suggest that a translocation of chicks, using similar techniques as developed for CDPs (Miskelly and Taylor Reference Miskelly and Taylor2004), to establish a new SGDP breeding colony should be considered in the future to render this species less vulnerable to storms and storm surges.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270917000041
Acknowledgements
We are indebted to David Young for providing the electronic artwork. We thank the Ngāi Tahu, Kakapo Recovery, and DOC Southern Islands for granting visits to Codfish Island. We would like to thank Stu Cockburn, Graeme Elliott, Stephen Hartley, Wei Ji Leong, Colin Miskelly, Matt Rayner, Rachael Sagar, and Alan Tennyson for their insights and advice. Furthermore, we are grateful to Graeme Miller and Juliet O’Connell for their support in the field. We thank Victor Anton, Vincent Bretagnolle, and Rachel Buxton for helpful comments that improved a previous version of our manuscript. This research was generously funded by the Ornithological Society of New Zealand (2015 Birds NZ Research Fund), the Centre for Biodiversity and Restoration Ecology (2015 CBRE Project Grant), and the Dune Restoration Trust of New Zealand (2016 Post Graduate Study Award). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals. All methods used were approved by an institutional animal ethics committee (VUW AEC 22252) and the DOC (45407-FAU and 47920-LND-1516/04).








