Implications
Some earlier research suggests that exposing pigs to high concentrations of atmospheric ammonia (⩾20 ppm) compromises their health. However, this speculation has been based upon extremely brief, unrealistic exposures. Recently, we kept pigs at either ∼20 ppm ammonia or in ‘fresh’ air for 15 weeks (i.e. an entire production cycle) but did not find any evidence of a change in liver function. This implies that pigs can be kept at ∼20 ppm ammonia without affecting either their health or metabolism.
Introduction
Housed pigs are routinely exposed to atmospheric ammonia at concentrations that some have long considered are hazardous to their health and/or productivity (e.g. Drummond et al., Reference Drummond, Curtis, Simon and Norton1980; Groot Koerkamp et al., Reference Groot Koerkamp, Metz, Uenk, Phillips, Holden, Sneath, Short, White, Hartung, Seedorf, Schröder, Linkert, Pedersen, Takai, Johnsen and Wathes1998). Furthermore, ammonia is considered a risk factor for the development of lung disease in humans, in particular agricultural workers and cleaners (Omland, Reference Omland2002; Anderson et al., Reference Anderson, Strader and Davidson2003; Vizcaya et al., Reference Vizcaya, Mirabelli, Antó, Orriols, Burgos, Arjona and Zock2011). Although some studies have found effects of elevated ammonia on growth and feed conversion efficiency in pigs, the experimental concentrations used often exceeded those found in commercial piggeries, sometimes excessively so (up to 150 ppm Stombaugh et al., Reference Stombaugh, Teague and Roller1969; Drummond et al., 1980). With respect to health, experiments have often failed to show changes in lung tissue or growth when pigs were co-exposed to both ammonia and an infectious agent (aerosols of Escherichia coli, toxigenic Pasteurella multocida or natural exposure to mycoplasma and bacteria; Drummond et al., Reference Drummond, Curtis and Simon1978; Diekman et al., Reference Diekman, Scheidt, Suttonn, Green, Clapper, Kelly and Van Alstine1993). The long-standing belief that exposure to ammonia is harmful was tested recently in a large experiment involving about 1000 weaner pigs; it showed that exposure for 5½ weeks post-weaning to concentrations of atmospheric ammonia up to ∼40 ppm had no effect on either productivity (Wathes et al., Reference Wathes, Demmers, Teer, White, Taylor, Bland, Jones, Armstrong, Gresham, Hartung, Chennells and Done2004) or respiratory disease (Done et al., Reference Done, Chennells, Gresham, Williamson, Hunt, Taylor, Bland, Jones, Armstrong, White, Demmers, Teer and Wathes2005), although weaner pigs were shown to find concentrations >20 ppm aversive when given a choice of environments using preference testing (Jones et al., Reference Jones, Webster and Wathes1999). The former findings are counter-intuitive and imply that there is little reason for farmers or others to worry about atmospheric ammonia in terms of pig production, other than any concerns about the pig’s preferences for fresh air.
Recently, we have studied the behavioural and physiological responses of growing pigs exposed continuously to atmospheric ammonia for 15 weeks (O’Connor et al., Reference O’Connor, Parker, McLeman, Demmers, Lowe, Cui, Davey, Owen, Wathes and Abeyesinghe2010; Parker et al., Reference Parker, O’Connor, McLeman, Demmers, Lowe, Owen, Davey, Wathes and Abeyesinghe2010). Here protracted, controlled exposure mimics environmental management in pig farming. Although there was evidence that the pigs found 20 ppm of ammonia exposure stressful (they exhibited less play behaviour and appeared to have down-regulated hypothalamic-pituitary axes), there were few effects on important production parameters such as growth and food conversion efficiency as well as general health scores. The higher concentration of ammonia used in this experiment (nominally 20 ppm) is commercially relevant as the current occupational exposure standard in humans is 8 h of exposure to 25 ppm ammonia in any 24 h period (Health and Safety Executive, 2011). This is often taken as a guideline for ammonia exposure of pigs and other housed livestock in the absence of evidence to the contrary: there is currently no legislation regarding maximum atmospheric ammonia in pig housing (Groot Koerkamp et al., Reference Groot Koerkamp, Metz, Uenk, Phillips, Holden, Sneath, Short, White, Hartung, Seedorf, Schröder, Linkert, Pedersen, Takai, Johnsen and Wathes1998).
The liver is an organ, which has a central role in the regulation of carbohydrate, protein and lipid metabolism, immune function, inflammation, hormone metabolism and removal of waste products from the blood (Nemeth et al., Reference Nemeth, Baird and O'Farrelly2009). Changes in circulating glucocorticoid levels and cytokines associated with stress and disease are known to influence hepatic function (Ingenbleek and Bernstein, Reference Ingenbleek and Bernstein1999; Chida et al., Reference Chida, Sudo and Kubo2006; Marelli et al., Reference Marelli, Terova, Cozzi, Lasagna, Sarti and Cavalchini2010). The liver therefore plays a sentinel role in detecting and responding to factors affecting normal homoeostasis. The aim of this experiment was to determine any changes in hepatic gene expression in growing pigs, which were chronically exposed to atmospheric ammonia at a nominal concentration of 20 ppm.
Material and methods
Experimental design
This work was regulated under the United Kingdom Animals (Scientific Procedures) Act 1986. Full details of the experiment can be found in O’Connor et al. (Reference O’Connor, Parker, McLeman, Demmers, Lowe, Cui, Davey, Owen, Wathes and Abeyesinghe2010). Briefly, two batches (N batch=112) of 4-week-old hybrid gilts (50% White synthetic Pietrain – 25% white Duroc, 12.5% Landrace, 12.5% Large White; PIC, Carlisle, Cumbria, UK) were obtained at weaning from the same commercial indoor pig farm. The first batch was obtained in May and the second in September 2008. On arrival, they were allocated randomly to groups of 14, kept in one of eight experimental rooms (∼19.6 m2) in a 23 fully factorial design and maintained under a combination of either low (control) or high ammonia (nominally 5 or 20 ppm of atmospheric ammonia; low or high mechanical noise (nominally <60 v. 80 dB(A)) and low or high light intensity (nominally 40 v. 200 lux for 12 h). Only liver samples from the pigs in the control or single-treatment ammonia rooms were used for the current study, so they were also exposed to control levels of light intensity and noise, that is, nominally 200 lux and <60 dB(A). Exposure to the environmental stressors began at the start of the experiment (i.e. on arrival of the pigs at the experimental site) and continued to its conclusion 15 weeks later. Growth parameters were monitored by manually weighing all the pigs once per week using calibrated scales (Pharmweigh Junior, Bury St Edmunds, Suffolk, UK). Health was monitored weekly with respect to nasal and/or ocular discharge, respiratory difficulty, diarrhoea and lameness as described previously (O’Connor et al., Reference O’Connor, Parker, McLeman, Demmers, Lowe, Cui, Davey, Owen, Wathes and Abeyesinghe2010).
The pigs were transported to a commercial abattoir at the end of the experiment for slaughter and removal of liver tissues. Small sections (∼1 cm2) of each liver were dissected, wrapped in foil, frozen in liquid nitrogen, transferred to pre-labeled bags, transported back to the laboratory on dry ice and stored at −80°C before RNA extraction.
RNA extraction
Total RNA was prepared from 100 mg of fragmented frozen liver tissue of randomly selected pigs from one control room and one ammoniated room in each batch (eight pigs per treatment in Batch 1, seven per treatment in Batch 2, total n=30). Extraction of total RNA was carried out following an ARK Genomics Standard Operating Procedure (http://www.ark-genomics.org/protocols IGF100.00: Isolation of total RNA). A two-step extraction method was used, first with Trizol then with a RNeasy mini kit (QIAGEN 74104, Hilden, Germany) for further purification. RNA concentration and purity were determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies Inc., Wilmington, DE, USA), where all samples had a 260/280 ratio of absorbance between 1.8 and 2.1. Samples from Batch 2 (n=14) were used for microarray analysis followed by quantitative real-time PCR (qPCR) on selected genes. Samples from Batch 1 were only used for qPCR.
Microarray hybridisation
All total RNA samples were checked for the concentration, integrity and purity using an Agilent Bioanalyzer according to the manufacturer’s instructions (Agilent Technologies Inc., Santa Clara, CA, USA). Microarray hybridisation and data acquisition were carried out by ARK-Genomics (Roslin Institute, University of Edinburgh) using 23 K Affymetrix GeneChip Porcine Genome Arrays based on established ARK-Genomics protocols (http://www.ark-genomics.org/protocols).
Microarray data analysis
Data were analysed using an S+ArrayAnalyzer 2.1 built in S-Plus Enterprise Developer 7.0 software package (Insightful Corp., Seattle, Washington, USA). Probe-level expression data generated by the scanner (.CEL files) were imported into the S+ArrayAnalyzer. Each probe set on the Affymetrix array contains 11 pairs of primers to target one gene: as an initial quality control step any probes with readings for less than seven detected pairs were filtered out. Quality control diagnostics were performed using plots of MvA, Box, RNA degradation and principal component analysis for expression intensity, which confirmed the good quality of all slides. The probe pairs were summarised into a single value per gene using Robust Multichip Analysis and normalised with a median inter-quartile range method.
Principle component analysis of the array data was performed using the BioConductor 2.4 in R-Package. Differences of probes/genes expression between control and ammonia-treated groups were compared using an unpaired t-test with Benjamini–Hochberg adjustment for a false discovery rate (FDR). In addition, differences of the normalised expression values between treatment groups were tested using a Significant Analysis of Microarray package (SAM, Stanford University, USA) at a FDR rate of α=0.05.
qPCR
Four of the most highly down-regulated genes were selected for follow-up studies by qPCR: lipin 1 (LPIN1), Chemokine (C-X-C motif) ligand 14 (CXCL14), serine dehydratase (SDS) and hepcidin antimicrobial peptide (HAMP) plus actin beta (ACTB; as a reference gene; see Table 1 for details). These genes were chosen based on the functional information available at the time for the pig genome, suggesting that they might play a role in metabolism and diseases affecting liver. All chemicals and reagents were purchased from Sigma-Aldrich Company Ltd. (Poole, Dorset, UK) or VWR International Ltd. (Poole, Dorset, UK) unless otherwise specified. Optimised qPCR assays were developed, as described previously (Fenwick et al., Reference Fenwick, Fitzpatrick, Kenny, Diskin, Patton, Murphy and Wathes2008). Total RNA from each sample was treated for potential genomic DNA carryover in a single reaction in accordance with guidelines supplied by Promega (Promega Corporation, Madison, WI, USA). From this reaction, precisely 1 µg of DNase-treated RNA was reverse transcribed using random hexamer primers and processed accordingly (Reverse Transcription System Kit; Promega). A mastermix of reagents was prepared for the above reaction to minimise potential variation. A negative control included all reagents as above, minus the cDNA template. Gene symbols, sequence information, accession numbers and expected product lengths are provided in Supplementary Table S1.
Table 1 Top eight hepatic genes up- or down-regulated after 15 weeks exposure of growing pigs to atmospheric ammonia (either <5 ppm (control) or ∼20 ppm) according to fold changes in expression
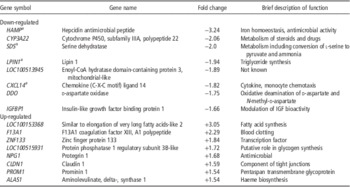
a Used in follow-up study by qPCR.
Gene transcripts were quantified as described in detail previously (Fenwick et al., Reference Fenwick, Fitzpatrick, Kenny, Diskin, Patton, Murphy and Wathes2008). Standards for qPCR were prepared from purified PCR products that were quantified by spectroscopy (NanoDrop) and diluted over at least eight orders of magnitude. Briefly, for each assay a mastermix was prepared that contained a final concentration of 10 µl Kapa qPCR SYBR Green Mix (Anachem Ltd, Luton, UK), 500 nM forward and reverse primers and nuclease-free water. Primer annealing and amplicon-specific melting temperatures were determined using the gradient function of CFX-96 Real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA. USA). Equivalent amounts of sample cDNA were added to each reaction in duplicate. To minimise variation, all samples included in each analysis were derived from the same reverse transcription (RT) batch, prepared under the same conditions and were analysed on a single plate. Thermal cycling conditions applied to each assay consisted of an initial Taq activation step at 95°C for 15 min followed by 38 cycles of denaturation (95°C), annealing (range 50.0°C to 64.2°C, see Supplementary Table S1), extension (72°C) and an amplicon-specific fluorescence acquisition reading (range 74°C to 84°C). A melting curve analysis was performed for each amplicon between 50°C and 95°C and as such any smaller non-specific products such as dimers were melted (if present) before fluorescence acquisition. All qPCR results were recorded with the Bio-Rad CFX Manager Software (V1.6, Bio-Rad Laboratories). For comparison of expression data, absolute values were derived from standard curves generated from purified cDNAs identical to amplified products and expressed as fg/μg reverse-transcribed RNA.
Data analysis
Data from the qPCR analysis were expressed as the mean and root mean square error in fg/1 µg RNA and subjected to ANOVA using SPSS V19 software package (Chicago, IL, USA), including treatment (Control or High Ammonia) and Batch (1 or 2) as factors. Fold changes between groups were calculated as Ammonia/Control when the value of the ammonia group was greater than that of control group and as −Control/Ammonia when the value of ammonia group was less than that of control group using the values without logarithmic transform. Differences were considered significant at P<0.05.
Results
Array analysis
Out of 23 K probes/genes included on the array, around 15 K currently have gene symbol annotations. Overall, only 21 of these genes were down-regulated >1.5 fold and nine were up-regulated >1.5 fold in the 20 ppm ammonia-treated group in comparison with the controls The fold changes for the top 8 genes in each direction are shown in Table 1. Samples from all 14 pigs were analysed using principal component analysis. This identified two groups of animals, but pigs from the control and high ammonia treatments were equally divided between the two groups (Figure 1). Differences of probes/genes expression between control and ammonia-treated groups were next compared using standard array analysis methods: (i) an unpaired t-test with Benjamini–Hochberg adjustment for FDR; and (ii) normalised expression values between treatment groups were tested using SAM at an FDR rate of α=0.05. Neither method detected significant differential expression of hepatic genes between treatment groups.
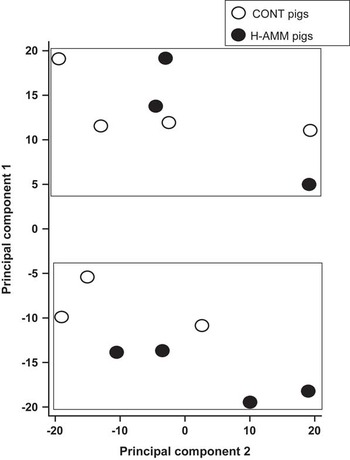
Figure 1 Principal component analysis of hepatic samples from growing pigs exposed to either nominally 5 ppm (CONT) or 20 ppm ammonia (H-AMM), n=7 pigs per treatment. Affymetrix GeneChip porcine arrays. Samples were analysed by 23 K Affymetrix porcine arrays. Two clusters were identified but they did not relate to the treatment.
qPCR
Five genes were selected for follow-up studies by qPCR to validate the array results: ACTB (reference gene), LPIN1, CXCL14, SDS and HAMP. The samples from pigs in Batch 2 from the array analysis were repeated and eight additional pigs from each of the control and ammonia-treated groups in Batch 1 were also included. Results are summarised in Table 2. This showed good agreement in the fold changes in expression detected by array and qPCR analysis in Batch 2 but these fold change results were not consistent with those found using qPCR in Batch 1. ANOVA indicted that batch had a significant influence on expression of all of these genes at P⩽0.01 but only one (CXCL14) was significantly influenced by ammonia treatment (P=0.002). The reference gene ACTB was not altered in response to treatment. As a further check on the data, the expression of the four test genes was calculated as the ratio of expression to ACTB. Using this approach, there were no significant differences in expression according to treatment (P all >0.1).
Table 2 Hepatic gene expression measured by qPCR in the growing pig after 15 weeks exposure to atmospheric ammonia (either <5 ppm (control) or ∼20 ppm of atmospheric ammonia)

RMSE=root mean square error; qPCR=quantitative real-time PCR.
Gene expression was analysed using both qPCR and gene expression array.
a Genes analysed were: ACTB= actin beta; LPIN1=lipin 1; CXCL14=Chemokine (C-X-C motif) ligand 14; SDS=serine dehydratase; HAMP=hepcidin antimicrobial peptide.
b Values shown are the means of eight pigs per group in Batch 1 and seven pigs per group in Batch 2 expressed in fg/µg RNA×106.
Weight and growth rates
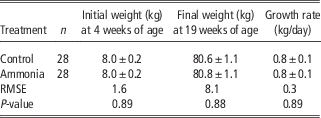
Data on weights of animals and growth rates have previously been reported for the complete experiment covering 224 pigs (O’Connor et al., Reference O’Connor, Parker, McLeman, Demmers, Lowe, Cui, Davey, Owen, Wathes and Abeyesinghe2010). In summary, the mean weight of the pigs increased from 7.8±0.1 kg in 4-week-old pigs at the start of the experiment to 81.9±0.7 kg in the 19-week-old pigs at the end of the experiment. There was a significant difference between batches in the mean weight over the course of the experiment: Batch 1, 36.0±0.2 kg; Batch 2 39.3±0.2 kg, (P<0.01). When batch was controlled for there was, however, no significant effect of any of the treatments on the mean weight or growth trajectories of the pigs. The average data for the animals in the control and ammonia-treated groups are summarised in Table 3.
Table 3 Comparison of weights and growth rates between growing pigs kept for 15 weeks in either control (<5 ppm) or high ammonia (∼20 ppm) atmosphere
RMSE=root mean square error.
Values are expressed as mean±s.e.m.
Discussion
The results of this study showed little or no significant hepatic response of growing pigs after 15 weeks of exposure to 20 ppm of atmospheric ammonia. Using standard analytical methods for gene expression arrays, there were no significant changes in expression. This was surprising for two reasons. First, array studies are able to detect small changes in gene expression across the whole genome. The experiment’s design meant that it was able to detect a fold change of 1.25 between two samples, at α=0.05 and a power >0.8 in a test of the experimental hypothesis with an unpaired t-test and making the usual assumptions about heteroscedastcity, random allocation of experimental subjects, etc. Our results showed an extremely good relationship between the fold changes in gene expression measured by the arrays and qPCR. This provides further confidence in the validity of the results.
Second, the liver is a central organ, which responds to stress, metabolic changes and disease, all of which might reasonably have been expected to change in response to the ammonia treatment (Berczi, Reference Berczi1998; Chida et al., Reference Chida, Sudo and Kubo2006). The biological functions of the liver include detoxification of harmful substances in the blood, glycogen storage, protein synthesis, bile secretion and hormone synthesis (Nemeth et al., Reference Nemeth, Baird and O'Farrelly2009). In the context of this experiment, it is unlikely that uptake of the atmospheric ammonia would have been sufficient to produce any measurable change in plasma ammonium levels. Instead it might have caused lung disease (Done et al., Reference Done, Chennells, Gresham, Williamson, Hunt, Taylor, Bland, Jones, Armstrong, White, Demmers, Teer and Wathes2005) or caused the animals stress through living in an environment which they could find aversive (Jones et al., Reference Jones, Webster and Wathes1999). Using array analysis, we have previously detected many significant changes in hepatic gene expression in response to negative energy balance in cows (McCarthy et al., Reference McCarthy, Waters, Kenny, Diskin, Fitzpatrick, Patton, Wathes and Morris2010) and transport stress in broiler chickens (Sherlock et al., Reference Sherlock, Wathes, Cheng and Wathes2012). Similarly, lesions of the foot and hock induced by ammonia treatment of the litter resulted in differential expression of 417 hepatic genes in broiler chickens (Sherlock et al., Reference Sherlock, Wathes, Cheng and Wathes2012). Others have successfully used expression profiling to monitor responses to calorie restriction in prepubertal pigs (Lkhagvadorj et al., Reference Lkhagvadorj, Qu, Cai, Couture, Barb, Hausman, Nettleton, Anderson, Dekkers and Tuggle2010). Therefore, the lack of differentially expressed genes in liver of pigs exposed to ammonia suggests that a myriad of metabolic processes were apparently unaffected by this treatment.
The pigs used in this experiment were part of a larger study into the effects of three environmental stressors (ammonia, noise and low light). This found no significant difference in the overall activity levels or growth rates of pigs housed in either high-ammoniated or low-ammoniated environments, although, in common with the results on gene expression reported here, there was a significant batch effect (O’Connor et al., Reference O’Connor, Parker, McLeman, Demmers, Lowe, Cui, Davey, Owen, Wathes and Abeyesinghe2010; Parker et al., Reference Parker, O’Connor, McLeman, Demmers, Lowe, Owen, Davey, Wathes and Abeyesinghe2010). In particular, the mean weight of the pigs over the course of the experiment was higher in Batch 2, although both sets of animals came from the same source and genetic stock and were housed in the same building. After controlling for the batch effect, the pigs in the ammoniated rooms had slightly lower salivary cortisol, larger adrenal cortices and performed less play in week 3 of the experiment, although there were no differences in play behaviour in weeks 8 or 14. As liver samples were only collected at the end of the experiment, it remains possible that changes in hepatic gene expression may have occurred earlier but that the pigs adapted to cope with this environment over time. However, we were interested in chronic not acute changes and from both a commercial and welfare perspective the net effect, integrated over an animal’s lifetime, may be more important.
We selected four genes in addition to the reference gene ACTB for further analysis, which showed slightly lower hepatic expression in the ammonia-treated pigs: SDS, LPIN1, HAMP and CXCL14. Although their changes in expression were not statistically significant, the functions of the proteins derived from these genes suggested that they might plausibly have been influenced by the treatment. Serine dehydratase is one of three enzymes involved in metabolising serine and glycine. The protein is found mainly in liver where l-serine dehydratase converts l-serine to pyruvate and ammonia (Ogawa, Reference Ogawa2000). Lipin 1 is involved in adipose tissue development and triglyceride metabolism (Reue and Brindley, Reference Reue and Brindley2008). In humans, LPIN1 is a candidate gene for lipodystrophy, a disease that is characterised by loss of body fat, fatty liver, hypertriglyceridemia and insulin resistance. HAMP is produced by the liver and plays an important role in the maintenance of iron homoeostasis. The gene is up-regulated during inflammation, potentially resulting in anaemia (Deicher and Hörl, Reference Deicher and Hörl2006). Chemokine (C-X-C motif) ligand 14 is an immunoregulatory cytokine, which displays chemotactic activity for monocytes (Shurin et al., Reference Shurin, Ferris, Tourkova, Perez, Lokshin, Balkir, Collins, Chatta and Shurin2005).
Out of these genes, only CXCL14 showed a significant effect of ammonia treatment across both batches of pigs (P<0.001), although there was also a trend (P<0.054) for LPIN1. There has been limited previous work investigating hepatic CXCL14 expression using murine models. It is up-regulated in response to hepatic injury (De Minicis et al., Reference De Minicis, Sekim, Uchinami, Kluwe, Zhang, Brenner and Schwabesv2007; Li et al., Reference Li, Gao, Yan, Yuan, Sah, Satya, Liu, Han and Yu2011) and schistosome infection (Burke et al., Reference Burke, McManus, Ramm, Duke, Li, Jones and Gobert2010) and is thought to attract monocytes, promote fat deposition, inhibit hepatocyte proliferation and contribute to the formation of hepatic granulomas and fibrosis. Hepatic down-regulation, as seen here in response to atmospheric ammonia, would presumably decrease such responses, although the relevance of this observation is uncertain without further investigation.
Overall, our results did not support the hypothesis that chronic exposure to ammonia would be reflected in changes in hepatic gene expression. This suggests that growing pigs can adapt to this level of environmental exposure without affecting their production efficiency or health. Such levels of ammonia do, however, raise cause for concern with regard to welfare as pigs are known to find them aversive (Jones et al., Reference Jones, Webster and Wathes1999).
Conclusions
Despite traditional beliefs about the putative effects of exposure to atmospheric ammonia on the livestock performance, this experiment has not provided any scientific evidence that chronic exposure to a nominal concentration of 20 ppm affects hepatic gene expression in growing pigs. Other studies of respiratory disease have also failed to find an effect.
Acknowledgements
This work was funded by the BBSRC. The authors thank members of the Centre for Animal Welfare, RVC for assistance with tissue collection at the abattoir. They are also grateful to staff at the RVC Biological Services Unit for animal husbandry.






