Book contents
- The Epilepsy Prescriber’s Guide to Antiepileptic Drugs
- The Epilepsy Prescriber’s Guide to Antiepileptic Drugs
- Copyright page
- Contents
- Preface To The Second Edition
- Introduction
- Acetazolamide
- ACTH
- Carbamazepine
- Clobazam
- Clonazepam
- Diazepam
- Eslicarbazepine acetate
- Ethosuximide
- Felbamate
- Fosphenytoin
- Gabapentin
- Lacosamide
- Lamotrigine
- Levetiracetam
- Lorazepam
- Methsuximide
- Midazolam
- Oxcarbazepine
- Paraldehyde
- Perampanel
- Phenobarbital
- Phenytoin
- Piracetam
- Pregabalin
- Primidone
- Retigabine / ezogabine
- Rufinamide
- Stiripentol
- Sulthiame
- Tiagabine
- Topiramate
- Valproate
- Vigabatrin
- Zonisamide
- Abbreviations
- Interaction Table
- Index
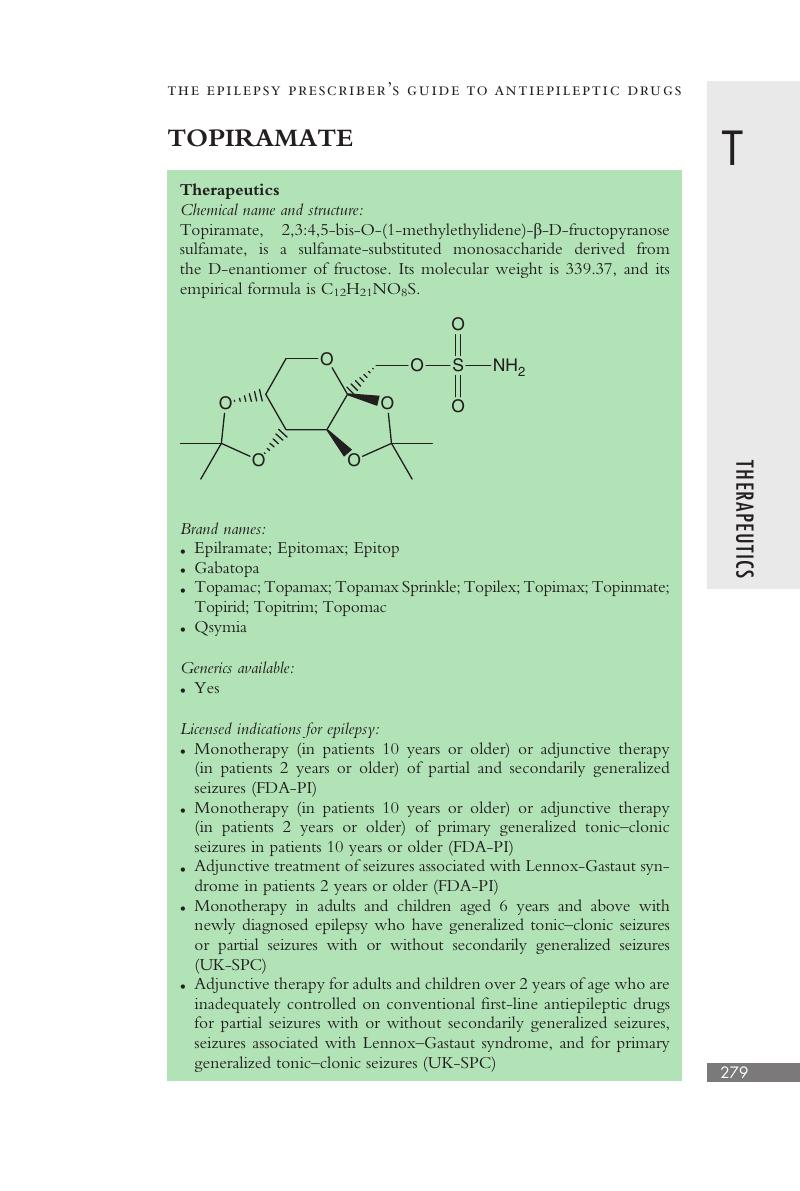
Topiramate
Published online by Cambridge University Press: 05 June 2014
- The Epilepsy Prescriber’s Guide to Antiepileptic Drugs
- The Epilepsy Prescriber’s Guide to Antiepileptic Drugs
- Copyright page
- Contents
- Preface To The Second Edition
- Introduction
- Acetazolamide
- ACTH
- Carbamazepine
- Clobazam
- Clonazepam
- Diazepam
- Eslicarbazepine acetate
- Ethosuximide
- Felbamate
- Fosphenytoin
- Gabapentin
- Lacosamide
- Lamotrigine
- Levetiracetam
- Lorazepam
- Methsuximide
- Midazolam
- Oxcarbazepine
- Paraldehyde
- Perampanel
- Phenobarbital
- Phenytoin
- Piracetam
- Pregabalin
- Primidone
- Retigabine / ezogabine
- Rufinamide
- Stiripentol
- Sulthiame
- Tiagabine
- Topiramate
- Valproate
- Vigabatrin
- Zonisamide
- Abbreviations
- Interaction Table
- Index
Summary
- Type
- Chapter
- Information
- The Epilepsy Prescriber's Guide to Antiepileptic Drugs , pp. 279 - 289Publisher: Cambridge University PressPrint publication year: 2013

