285 results
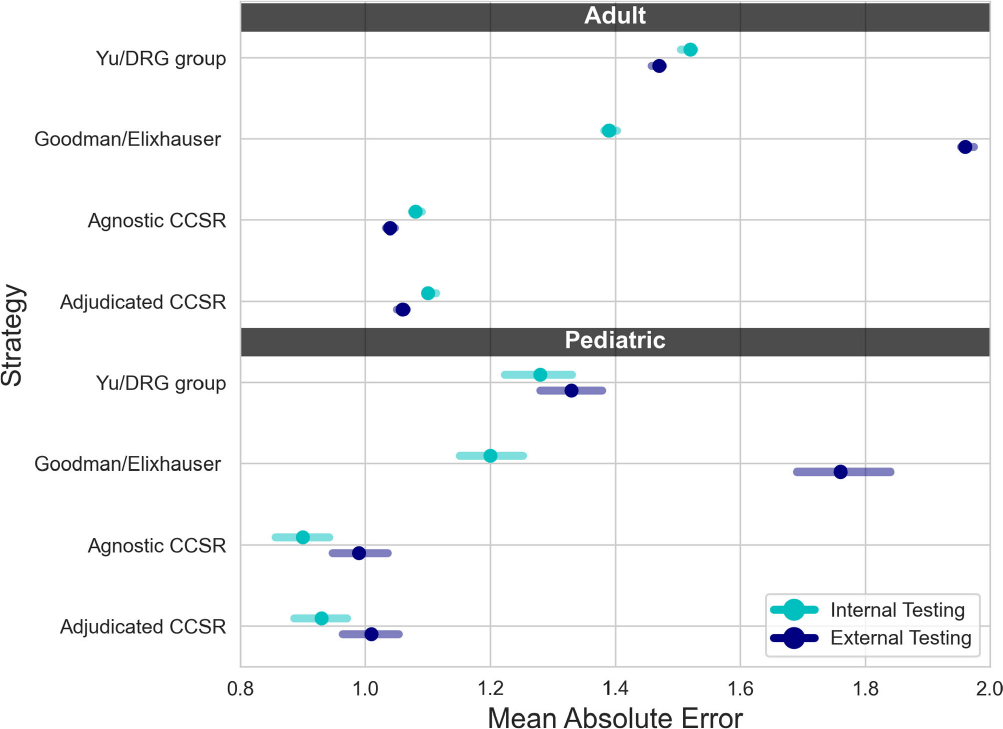
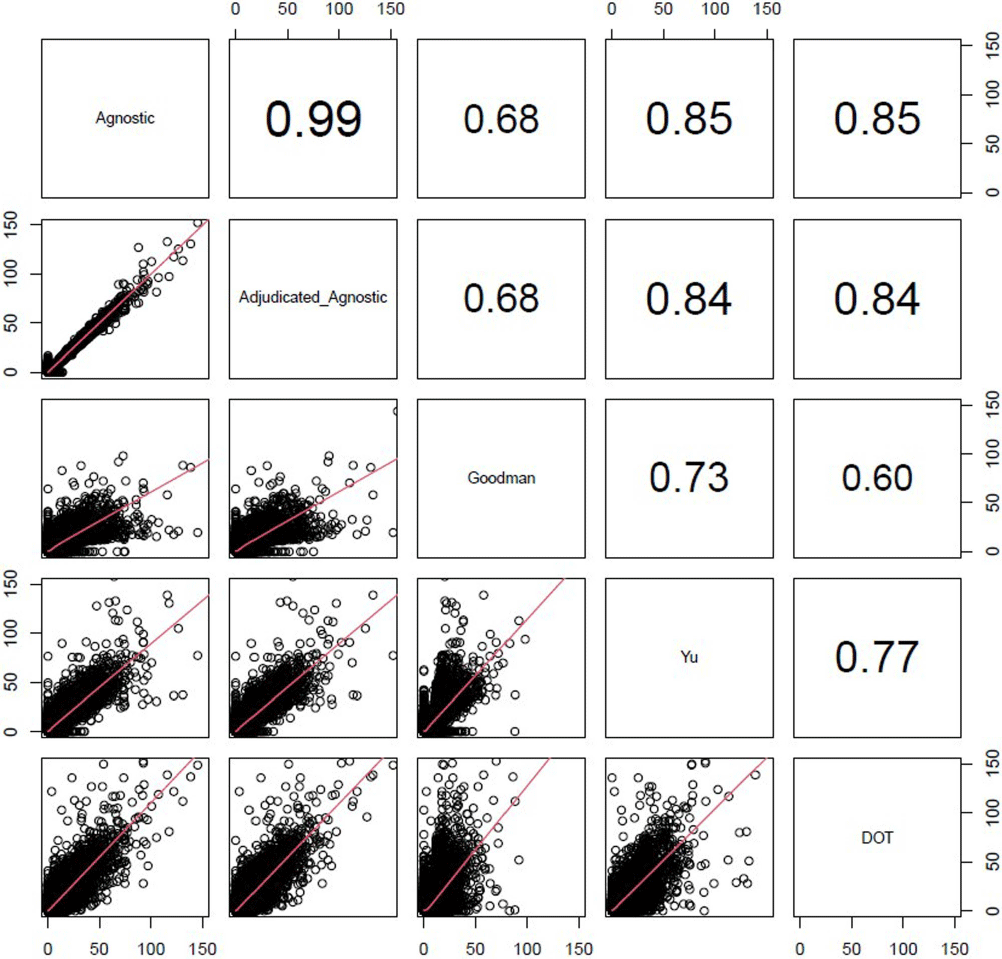
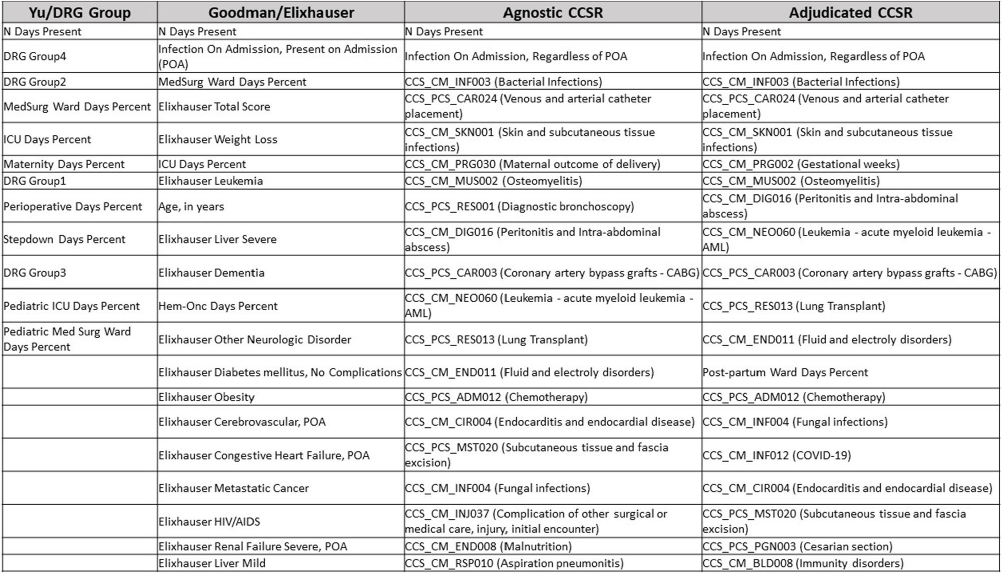
A Comparison of Variable Input Strategies used for Risk-adjustment Models of Antimicrobial Use
-
- Journal:
- Antimicrobial Stewardship & Healthcare Epidemiology / Volume 4 / Issue S1 / July 2024
- Published online by Cambridge University Press:
- 16 September 2024, pp. s30-s31
-
- Article
-
- You have access
- Open access
- Export citation
Building a Special Pathogen Response Center from the Ground Up
-
- Journal:
- Antimicrobial Stewardship & Healthcare Epidemiology / Volume 4 / Issue S1 / July 2024
- Published online by Cambridge University Press:
- 16 September 2024, p. s95
-
- Article
-
- You have access
- Open access
- Export citation
SHEA position statement on pandemic preparedness for policymakers: emerging infectious threats
-
- Journal:
- Infection Control & Hospital Epidemiology , First View
- Published online by Cambridge University Press:
- 19 July 2024, pp. 1-3
-
- Article
- Export citation
SHEA position statement on pandemic preparedness for policymakers: the role of healthcare epidemiologists in communicating during infectious diseases outbreaks
-
- Journal:
- Infection Control & Hospital Epidemiology , First View
- Published online by Cambridge University Press:
- 05 June 2024, pp. 1-5
-
- Article
- Export citation
SHEA position statement on pandemic preparedness for policymakers: building a strong and resilient healthcare workforce
-
- Journal:
- Infection Control & Hospital Epidemiology , First View
- Published online by Cambridge University Press:
- 05 June 2024, pp. 1-4
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
SHEA position statement on pandemic preparedness for policymakers: introduction and overview
-
- Journal:
- Infection Control & Hospital Epidemiology , First View
- Published online by Cambridge University Press:
- 05 June 2024, pp. 1-3
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
SHEA position statement on pandemic preparedness for policymakers: pandemic data collection, maintenance, and release
-
- Journal:
- Infection Control & Hospital Epidemiology , First View
- Published online by Cambridge University Press:
- 05 June 2024, pp. 1-5
-
- Article
- Export citation
Testing residual chloramine levels in tap water across sink locations in a US academic hospital setting
-
- Journal:
- Infection Control & Hospital Epidemiology , First View
- Published online by Cambridge University Press:
- 20 March 2024, pp. 1-2
-
- Article
- Export citation
The impact of minimally invasive surgical approaches on surgical-site infections
-
- Journal:
- Infection Control & Hospital Epidemiology / Volume 45 / Issue 5 / May 2024
- Published online by Cambridge University Press:
- 03 January 2024, pp. 557-561
- Print publication:
- May 2024
-
- Article
- Export citation
Strategies to maintain an N95 respirator supply during a pandemic supply-chain shortage
-
- Journal:
- Infection Control & Hospital Epidemiology / Volume 45 / Issue 5 / May 2024
- Published online by Cambridge University Press:
- 13 December 2023, pp. 688-689
- Print publication:
- May 2024
-
- Article
-
- You have access
- HTML
- Export citation
The impact of environmental cleaning protocol featuring PX-UV in reducing the incidence of multidrug-resistant gram-negative healthcare-associated infection and colonization in intensive care units in Thailand
-
- Journal:
- Infection Control & Hospital Epidemiology / Volume 45 / Issue 5 / May 2024
- Published online by Cambridge University Press:
- 13 December 2023, pp. 684-687
- Print publication:
- May 2024
-
- Article
- Export citation
Bacille Calmette-Guérin preparation and intravesical administration to patients with bladder cancer: Risks to healthcare personnel and patients, and mitigation strategies
-
- Journal:
- Infection Control & Hospital Epidemiology / Volume 45 / Issue 4 / April 2024
- Published online by Cambridge University Press:
- 11 December 2023, pp. 520-525
- Print publication:
- April 2024
-
- Article
- Export citation
Approaching coronavirus disease 2019 (COVID-19) vaccine hesitancy among healthcare personnel: The importance of cultural competency
-
- Journal:
- Infection Control & Hospital Epidemiology / Volume 44 / Issue 9 / September 2023
- Published online by Cambridge University Press:
- 18 July 2023, pp. 1371-1372
- Print publication:
- September 2023
-
- Article
- Export citation
The role of environmental and healthcare-associated infections in Asia: Lessons learned from the coronavirus disease 2019 (COVID-19) pandemic
-
- Journal:
- Antimicrobial Stewardship & Healthcare Epidemiology / Volume 3 / Issue 1 / 2023
- Published online by Cambridge University Press:
- 15 June 2023, e100
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Strategies to prevent Clostridioides difficile infections in acute-care hospitals: 2022 Update
- Part of
-
- Journal:
- Infection Control & Hospital Epidemiology / Volume 44 / Issue 4 / April 2023
- Published online by Cambridge University Press:
- 12 April 2023, pp. 527-549
- Print publication:
- April 2023
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Implementation should be a standard component of practice guidelines and guidance documents
-
- Journal:
- Infection Control & Hospital Epidemiology / Volume 44 / Issue 9 / September 2023
- Published online by Cambridge University Press:
- 02 March 2023, pp. 1365-1368
- Print publication:
- September 2023
-
- Article
- Export citation
Back to the future: Redefining “universal precautions” to include masking for all patient encounters
-
- Journal:
- Infection Control & Hospital Epidemiology / Volume 44 / Issue 9 / September 2023
- Published online by Cambridge University Press:
- 10 February 2023, pp. 1373-1374
- Print publication:
- September 2023
-
- Article
- Export citation
Increase in the incidence of Candida parapsilosis and Candida tropicalis bloodstream infections during the coronavirus disease 2019 (COVID-19) pandemic
-
- Journal:
- Antimicrobial Stewardship & Healthcare Epidemiology / Volume 3 / Issue 1 / 2023
- Published online by Cambridge University Press:
- 10 January 2023, e2
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
How to measure time preferences: An experimental comparison of three methods
-
- Journal:
- Judgment and Decision Making / Volume 8 / Issue 3 / May 2013
- Published online by Cambridge University Press:
- 01 January 2023, pp. 236-249
-
- Article
-
- You have access
- Open access
- HTML
- Export citation
Asymptomatic screening for severe acute respiratory coronavirus virus 2 (SARS-CoV-2) as an infection prevention measure in healthcare facilities: Challenges and considerations
- Part of
-
- Journal:
- Infection Control & Hospital Epidemiology / Volume 44 / Issue 1 / January 2023
- Published online by Cambridge University Press:
- 21 December 2022, pp. 2-7
- Print publication:
- January 2023
-
- Article
-
- You have access
- HTML
- Export citation