Book contents
- Uncommon Causes of Stroke
- Uncommon Causes of Stroke
- Copyright page
- Contents
- Contributors
- Preface
- Section 1 Infectious Conditions
- Section 2 Inflammatory Conditions
- Section 3 Hereditary and Genetic Conditions and Malformations
- Section 4 Vascular Conditions of the Eyes, Ears, and Brain
- Section 5 Disorders Involving Abnormal Coagulation
- Section 6 Systemic Disorders That Also Involve the Cerebrovascular System
- Section 7 Non-Inflammatory Disorders of the Arterial Wall
- Section 8 Venous Occlusive Conditions
- Section 9 Vasospastic Conditions and Other Miscellaneous Vasculopathies
- Index
- Plate Section (PDF Only)
- References
Section 7 - Non-Inflammatory Disorders of the Arterial Wall
Published online by Cambridge University Press: 15 June 2018
- Uncommon Causes of Stroke
- Uncommon Causes of Stroke
- Copyright page
- Contents
- Contributors
- Preface
- Section 1 Infectious Conditions
- Section 2 Inflammatory Conditions
- Section 3 Hereditary and Genetic Conditions and Malformations
- Section 4 Vascular Conditions of the Eyes, Ears, and Brain
- Section 5 Disorders Involving Abnormal Coagulation
- Section 6 Systemic Disorders That Also Involve the Cerebrovascular System
- Section 7 Non-Inflammatory Disorders of the Arterial Wall
- Section 8 Venous Occlusive Conditions
- Section 9 Vasospastic Conditions and Other Miscellaneous Vasculopathies
- Index
- Plate Section (PDF Only)
- References
Summary
- Type
- Chapter
- Information
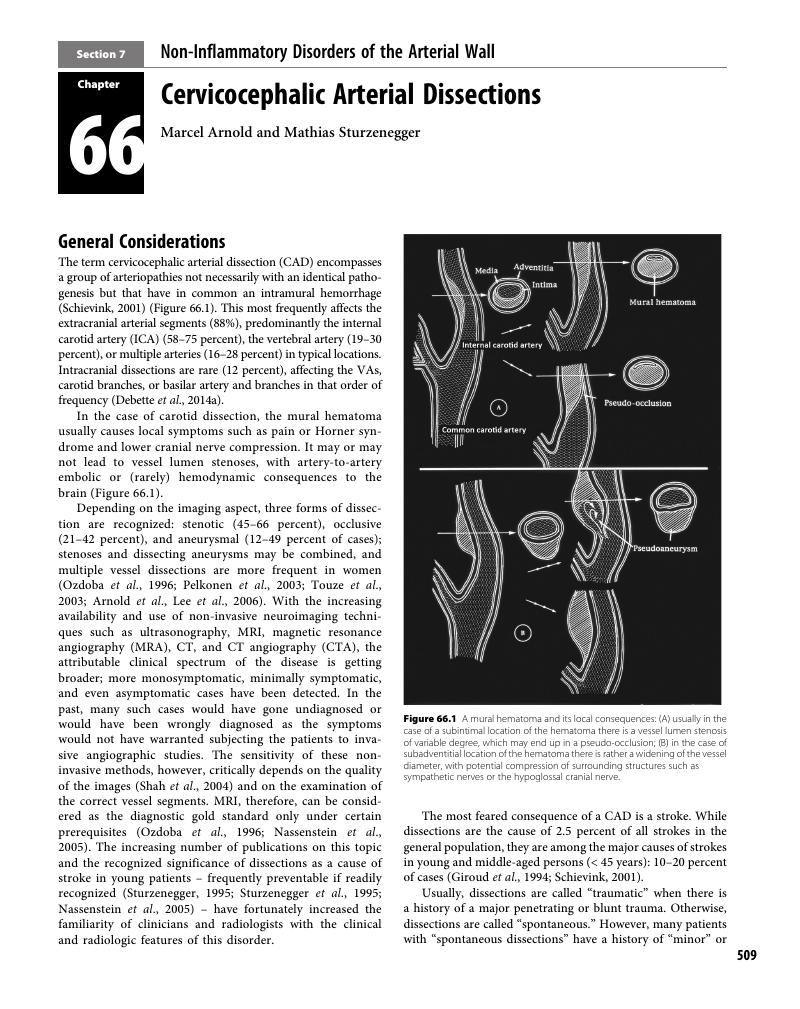
- Uncommon Causes of Stroke , pp. 509 - 588Publisher: Cambridge University PressPrint publication year: 2018



