Introduction
The superfamily Mactroidea currently includes four families: Mactridae Lamarck, Reference Lamarck1809, Anatinellidae Gray, Reference Gray1853, Cardiliidae Fischer, Reference Fischer1887, and Mesodesmatidae Gray, Reference Gray1840. The supra-generic classification of this superfamily was recently revised and rearranged (Signorelli and Carter, Reference Signorelli and Carter2016). Four subfamilies are currently included within the family Mactridae: Mactrinae Lamarck, Reference Lamarck1809; Lutrariinae Gray, Reference Gray1853; Darininae Signorelli in Carter et al., Reference Carter, Campbell, Altaba, Anderson and Araujo2011; and Tanysiphoninae Scarlato and Starobogatov in Nevesskaja et al., Reference Nevesskaja, Scarlato, Starobogatov and Eberzin1971. Mactridae is a globally distributed family of marine to estuarine species, mainly shallow subtidal, but occasionally occurring at depths down to 200 m. Mactridae species are commonly found burrowed in sandy and muddy bottoms. The diagnostic character of this group of bivalves is the hinge morphology with an inverted V-shaped cardinal tooth in the left valve. Currently, this family includes almost 300 extant and fossil species grouped in 90 genera.
The Cretaceous origin and early evolution of the family Mactridae have been studied by Saul (Reference Saul1973). Cretaceous descendants of Isocyprina (Eotrapezium) or Isocyprina (Venericyprina), such as the genera Priscomactra and Geltena, show striated lateral teeth, and their clade may be ancestral to Cenozoic Spisula (Mactrinae). It is possible that a clade with granulated lateral teeth, as in Mulinoides, independently derived from the same ancestral stock. This second clade may be ancestral to Cenozoic Mulinia and Pseudocardium, both within Mactrinae. On the other hand, Cretaceous descendants of Isocyprina sharpi Cox, Reference Cox1947, such as Aliomactra, Pteroluter, Willimactra, and Petromactra, constitute an ancestral clade of the Cenozoic Standella (Mactrinae) and Tresus (Lutrariinae). Dall (Reference Dall1898) proposed that the external ligament and resilium were not originally separated in Mactridae. Saul (Reference Saul1973) verified Dall's (Reference Dall1898) assumption that the ancestors of Mactridae lacked the resilium, but she indicated that the resilium did not arise from splitting of a submerged ligament. The earliest mactrids have an almost completely external ligament behind a moderate to high nymph (Vokes, Reference Vokes1946; Stephenson, Reference Stephenson1952).
Currently, Cretaceous mactrids are grouped in nine valid genera (Olsson, Reference Olsson1944; Vokes, Reference Vokes1946; Stephenson, Reference Stephenson1952; Saul, Reference Saul1973, Reference Saul1974) with short stratigraphic ranges. Morphological studies suggest that the chondrophore is enlarged and the external ligament is retained in Cenozoic mactrids (Saul, Reference Saul1973). The parallel development of granulate or striate lateral teeth plus the enlargement of the chondrophore suggests a polyphyletic condition of mactrids.
After the Cretaceous, the family Mactridae has been registered in several regions (Sacco, Reference Sacco, Bellardi and Sacco1901; Cossmann, Reference Cossmann1908; Stephenson, Reference Stephenson1952; Stenzel et al., Reference Stenzel, Krause and Twining1957; Belokrys, Reference Belokrys1964; Habe, Reference Habe1977; Woodring, Reference Woodring1982; Ionesi, Reference Ionesi1986; Ward, Reference Ward1992; Garvie, Reference Garvie1996; Nevesskaja et al., Reference Nevesskaja, Popov, Goncharova, Guzhov, Janin, Plubotko, Biakov and Gavrilova2013). Paleocene (Danian) Mactridae from the Montien deposits of Belgium, have been studied by Chavan (Reference Chavan1946) and Marlière (Reference Marlière1954). Praerangia is the only mactrid recorded from Paleocene deposits.
In the Eocene, members of Mactridae have been recorded from different deposits exposed in North America, Central America, South America, Antarctica, and western Africa. Currently, 10 valid genera have been described based on Eocene type species. The distribution range of Eocene mactrid genera shows an Atlantic pattern. Four genera were reported from North America, Central America, and South America, two from western Africa, three from Europe, and only one from Antarctica (Cossmann and Peyrot, Reference Cossmann and Peyrot1909; Böhm, Reference Böhm1929; Clark and Durham Reference Clark and Durham1946; Eames, Reference Eames1957; Woodring, Reference Woodring1982; Stilwell and Zinsmeister, Reference Stilwell and Zinsmeister1992; Garvie, Reference Garvie1996).
No mactrid genera with Oligocene type species were found in the literature. A different scenario is observed during the Miocene where members of Mactridae have been recorded in several regions. From the Burdigalian stage (Lower Miocene), mactrid genera have been recorded from different localities in western and southern Europe. From the Serravallian and Tortonian stages (upper Middle Miocene), the Eastern European clade, which commonly is known as Sarmatian Mactridae, includes nearly 70 specific names (Kolesnikov, Reference Kolesnikov1925, Reference Kolesnikov1935; Davitashvili, Reference Davitashvili, Archangelsky and Davitashvili1932; Zhizhchenko, Reference Zhizhchenko1934; Macarovici, Reference Macarovici1935, Reference Macarovici1969; Simionescu and Barbu, Reference Simionescu and Barbu1940; Papp, Reference Papp, Papp, Marinescu and Seneš1974). However, Sidorova (Reference Sidorova1959a, Reference Sidorovab, Reference Sidorova1960a, Reference Sidorovab) summarized all these previous works and reduced that number to seven valid species of Sarmatian Mactridae. Belokrys (Reference Belokrys1964), in a full revision of Sarmatian mactrids from Borisphen Gulf (southern Ukraine), recognized eight species and five subspecies. Some of these taxa are the type species of subsequently described genera (i.e., Chersonimactra, Planimactra, Podolimactra, Pseudomactra, Sarmatimactra). However, the number of valid taxa is far from being resolved and additional studies are required in order to understand the evolution of upper Middle Miocene mactrids.
Several taxa have been described from the Miocene–Pliocene of North and Central America. The North American fossil mactrids were first reported by Conrad (Reference Conrad1857, Reference Conrad1869) and subsequent works significantly contributed to the knowledge of fossil and extant taxa (Gould, Reference Gould1850, Reference Gould1852; Rémond, Reference Rémond1863; Gabb, Reference Gabb1866, Reference Gabb1869; Dall, Reference Dall1894; among others). In the early twentieth century, Packard (Reference Packard1916) summarized the knowledge of the group for the Pacific Coast of North America. Subsequently, Stewart (Reference Stewart1930) compiled the Mesozoic and Cenozoic bivalves described by Gabb. More recently, Ward (Reference Ward1992) contributed with the description of new fossil taxa from the east coast of North America. In Central America, Maury (Reference Maury1925, Reference Maury1928) studied several localities and described a new fossil genus. In the present study, six valid genera reported from Miocene–Pliocene deposits of North and Central America are listed. From the Miocene–Pleistocene of Oceania, mainly from New Zealand, several authors described new genera and species belonging to the family Mactridae (Marwick, Reference Marwick1952; Beu, Reference Beu1966, Reference Beu1968; among others). Recently, Beu (Reference Beu2004, Reference Beu2006) revised the taxonomy and biostratigraphy of Quaternary New Zealand fossil Mollusca, including Mactridae.
In Upper Pliocene–Lower Pleistocene deposits of the Central Paratethys, a significant radiation event in the Mactridae is associated with the Great Akschagylian transgression (Nevesskaja et al., Reference Nevesskaja, Gontsharova, Ilyina, Paramonova, Popov, Bogdanovich, Gabunia and Nosovsky1984). The Akschagylian Mactridae are currently represented by six genera with >20 included species. However, a full revision of Paratethys mactrids must include not only Akschagylian mactrids (Pliocene–Lower Pleistocene), but also Sarmatian (upper Middle Miocene) taxa. In several cases, the type series of type species of Akschagylian genera are lost. A complete revision, currently in progress, will reveal if designation of neotypes is necessary. Finally, although living Mactridae are well represented in Japanese waters (Habe, Reference Habe1977, Reference Habe, Kuroda and Habe1981), Rugosoxyperas, based on a fossil type species, was registered from Pleistocene deposits of Japan (Nomura and Zinbo, Reference Nomura and Zinbo1934).
Altogether, >100 genus-level names have been proposed within the family Mactridae, 79 of which are considered valid in the literature (Huber, Reference Huber2010, Reference Huber2015; Cosel and Gofas, Reference Cosel and Gofas2019; Signorelli, Reference Signorelli2019; Valentich-Scott et al., Reference Valentich-Scott, Coan and Zelaya2020; MolluscaBase, Reference MolluscaBase2022; among others). However, only 45 genera are based on fossil specimens as type species. This catalogue aims to give a synopsis of the current knowledge of those genera based on exclusively fossil type species.
Materials and methods
This study is based on an exhaustive literature search of described genera and species belonging to the family Mactridae. All original descriptions were checked. A new diagnosis for each genus is provided. The included characters in each diagnosis depend on the level of preservation of the fossil type species. The hinge was described with the method developed by Bernard and Munier-Chalmas (according to Cox in Moore, Reference Cox and Moore1969) where Arabic numbers are used to designate the cardinal teeth and roman numbers for the lateral teeth. This method uses odd numbers for all teeth in the right valve and even numbers for all teeth in the left valve. In addition, type species, type localities, and occurrences are included. For each genus, a remarks section includes the most recent published taxonomic opinions. However, in some cases, new taxonomic decisions have been taken based on morphological analysis of types. The type material of each type species was requested and photographically reproduced when possible. Genera based on extant type species were excluded from this work.
Repositories and institutional abbreviations
Academy of Natural Science of Drexel University, Philadelphia (ANSP); Museum für Naturkunde or Bundesanstalt für Geowissenschaften und Rohstoffe (BGR); Canterbury Museum, Christchurch, New Zealand (CMC); Institute of Geological & Nuclear Sciences, Lower Hutt, New Zealand, locality numbers (GS) and type Mollusca registration numbers (TM); Royal Belgian Institute of Natural Sciences, Belgium (IRSNB); Invertebrate Paleontology Department, Natural History Museum of Los Angeles County, Los Angeles, USA (LACMIP); Museum of Comparative Zoology, Harvard, University, Cambridge, USA (MCZ); Muséum National d'Histoire Naturelle, Paris (MNHN); Museo Regionale di Scienze Naturali, Turin (MRSN); Natural History Museum, London (NHMUK); Natural History Museum Vienna (NHMW); Natural History Museum, Basel, Switzerland (NMBA); Paleontological Research Institute, Ithaca, USA (PRI); Academician F.N. Chernyshev Central Geological Research Museum (TsNIGR Museum); University of California Museum of Paleontology (UCMP); United States National Museum (USNM).
Systematic paleontology
Family Mactridae Lamarck, Reference Lamarck1809
Diagnosis
Shell rounded to sub-trigonal, transversely elongate, equivalve, usually subequilateral, with periostracum present, glossy; externally smooth or concentrically sculptured; valves mostly closed or slightly gaping posteriorly; pallial line with sinus normally well developed; with prominent and mostly prosogyrate umbos; left valve with an inverted V-shaped cardinal tooth (2a and 2b) fitting below the two cardinal teeth in the right valve (3a and 3b), which tend to be united dorsally. Anterior and posterior lateral teeth in left valve (AII and PII) fit into respective sockets in right valve, which are limited by, in some cases, two anterior (AI and AIII) and two posterior (PI and PIII) lateral teeth. A delicate accessory dental lamella often is present (4b). External ligament placed on a nymph, absent in some groups. Siphons usually fused to their tips; mantle margins are generally smooth (sometimes papillate) and united by cuticular junctions; ctenidia are generally smooth and homorhabdic, with inner and outer demibranchs; the outer usually with a supra-axial extension; palps large, long, narrow, partly united; foot large, compressed, heeled; heart has paired auricles and a ventricle that is traversed by the rectum. Mactridae contains the subfamilies Mactrinae, Lutrariinae, Darininae and Tanysiphoninae (Carter et al., Reference Carter, Campbell, Altaba, Anderson and Araujo2011).
Aktschagylia Starobogatov, Reference Starobogatov1970
Figure 1.1–1.12
Type species
Mactra subcaspia Andrussow, Reference Andrussow1902a (p. 66), by original designation. Type material: not examined, probably lost; Andrussow's (Reference Andrussow1902a, Reference Andrussow1905) described mactrids are not listed in the catalogues of the Chernishov Museum or Saint Petersburg University (S. Popov, personal communication, 2021). The original illustrations are herein reproduced. Type locality: not stated in the original description. Eastern European localities such as Utva, Rostosh, Chiri-Yurt, and Grozny are mentioned as sampled sites with specimens of Mactra subcaspia.

Figure 1. (1–12) Aktschagylia subcaspia (Andrussow, Reference Andrussow1902a), original illustrations. (13) Aliomactra compressa Stephenson, Reference Stephenson1952, USNM 105517, holotype. (14–16) Allomactra grateloupi Cossmann and Peyrot, Reference Cossmann and Peyrot1909, original illustrations. (17–22) Andrussella acutecarinata (Andrussow, Reference Andrussow1902a), original illustrations. Scale bars (1–13) 1 cm; (14–22) 2 cm.
Diagnosis
Shell trigonal to oval, anterior and posterior ends rounded, exterior surface with traces of concentric ornamentation; umbo prosogyrate; hinge plate with two cardinal teeth in the right valve (3a and 3b), inverted V-shaped cardinal tooth (2a-2b) present in left valve; posterior laterals longer than anterior ones; lateral teeth close to the cardinals; pallial line with a very small sinus.
Occurrence
Uppermost Miocene–Lower Pleistocene. Caspian region, East Europe, northwestern Kazakhstan, Azerbaijan, western Turkmenistan, Ukraine, Georgia, Taman Peninsula, Russia.
Remarks
Starobogatov (Reference Starobogatov1970) proposed Aktschagylia as a replacement name for Mactra of Ali-Zade, Reference Ali-Zade1967 (non Mactra Linnaeus, Reference Linnaeus1767). Nevesskaja et al. (Reference Nevesskaja, Popov, Goncharova, Guzhov, Janin, Plubotko, Biakov and Gavrilova2013) mentioned Aktschagylia as a valid genus. The Akschagylian mollusk faunas are characterized by the presence of endemic mactrid and cardiid bivalve species with eight species assigned to the genus Aktschagylia (Danukalova, Reference Danukalova1996).
Aliomactra Stephenson, Reference Stephenson1952
Figure 1.13
Type species
Aliomactra compressa Stephenson, Reference Stephenson1952 (p. 125), by original designation. Type material: USNM 105517, holotype; paratypes: USNM 105518a, b, 2 specimens; USNM 105519, 2 specimens; USNM 105520, 1 specimen. Type locality: Templeton Member on an east-west road near head of a small branch, 3 miles northeast of Sherman Junction, Grayson County, Texas, USA.
Diagnosis
Shell outline like in Mactra but more compressed, large, length up to 55 mm; anterior end sharply rounded, posterior end rounded; dorso-posterior area straight and longer than the slightly concave dorso-anterior area; umbos posteriorly oriented; external ligament, short, placed in a narrow groove; internal ligament placed on a spoon-shaped, right hinge with thin unfused cardinal (3a and 3b) and long anterior lateral teeth (AI and AIII), left hinge with fragile, inverted V-shaped cardinal tooth (2a-2b) and one long anterior lateral tooth (AII); posterior lateral shorter than anterior; long; pallial sinus wide and deep.
Occurrence
Cenomanian, Upper Cretaceous of North America.
Remarks
Aliomactra is monospecific. Stephenson (Reference Stephenson1952) highlighted that Aliomactra differs from Priscomactra by having a more ventrally projected chondrophore and longer anterior lateral teeth, and from Cymbophora by the presence of a groove where the external ligament is seated, by having longer lateral teeth, and by lacking strong transverse striations on the sides of lateral teeth. No additional records of this genus were found in the literature.
Allomactra Tomlin, Reference Tomlin1931
Figure 1.14–1.16
Type species
Mactra grateloupi Cossmann and Peyrot, Reference Cossmann and Peyrot1909 (p. 242), by typification of replaced name. Type material: not examined, original illustration is reproduced. Type locality: Burdigalian deposits of Dax, France.
Diagnosis
Shell trigonal, flattened, large, length up to 40 mm; ends rounded, the anterior end more pronounced, lunule and escutcheon similar to Eomactra; exterior surface smooth with commarginal and fine undulations; hinge plate with fused, inverted V-shaped cardinal tooth (2a-2b) in the left valve, thin, posteriorly flanked by an accessory lamella (4b); posterior lateral teeth (PI, PII, and PIII) more closely positioned near the cardinals than the anterior ones (AI, AII, and AIII); internal surface covered with fine granulations; chondrophore moderately projected; pallial sinus U-shaped, long, and broad.
Occurrence
Burdigalian, Lower Miocene of Europe (Cossmann and Peyrot, Reference Cossmann and Peyrot1909) and India (Lyngdoh et al., Reference Lyngdoh, Tiwari and Kachhara1999; Jauhri et al., Reference Jauhri, Tiwari and Lyngdoh2004), and Middle Miocene of Iraq (Al-Abbasi, Reference Al-Abbasi2011).
Remarks
Allomactra is a replacement name for Heteromactra Cossmann and Peyrot, Reference Cossmann and Peyrot1909 (p. 242) (non Lamy, Reference Lamy1906, p. 45, Cyamiidae).
Andrussella Korobkov, Reference Korobkov1954
Figure 1.17–1.22
Type species
Mactra acutecarinata Andrussow, Reference Andrussow1902a (p. 75), by original designation. Type material: not examined, probably lost. The original illustrations are herein reproduced. Type locality: Aktschagyl is mentioned in the original description, additional specimens collected at Kögnja-Arap, Azerbaijan.
Diagnosis
Shell oval to trapezoid, moderately elongated, with a posterior area defined by a sharp keel; anterior end rounded; exterior surface smooth; umbos prosogyrate; hinge plate shortened, two cardinal teeth (3a and 3b) in the right valve, unfused, two anterior (AI and AIII) and two posterior (PI and PIII) lateral teeth equal in length; left valve with the inverted V-shaped lateral tooth (2a-2b) and two lateral teeth, one anterior (AII) and one posterior (PII), closer to the cardinals, pallial sinus unknown.
Occurrence
Upper Pliocene–Lower Pleistocene of Eastern Europe to western Asia, reported from Georgia, Azerbaijan, Turkmenistan, and Russia from the Northern Caspian region.
Remarks
Andrussella was described as a subgenus of Avimactra (Korobkov, Reference Korobkov1954). Later, Keen (Reference Keen, Cox, Newell, Boyd, Branson, Casey and Chavan1969a) treated it as a subgenus of Mactra. Recently, Nevesskaja et al. (Reference Nevesskaja, Popov, Goncharova, Guzhov, Janin, Plubotko, Biakov and Gavrilova2013) raised Andrussella to genus level. Tscheltzov (Reference Tscheltzov1964) analyzed and illustrated the morphological convergence between Akchagylian mactrids and cardiids. Korobkov (Reference Korobkov1954) only included Mactra acutecarinata Andrussow within Andrussella. Recently, Nevesskaja et al. (Reference Nevesskaja, Popov, Goncharova, Guzhov, Janin, Plubotko, Biakov and Gavrilova2013) reported this species from Akschagylian deposits exposed at Krasnovodsk Peninsula.
Avimactra Andrussow, Reference Andrussow1905
Figure 2.1–2.4
Type species
Mactra (Avimactra) aviculoides Andrussow, Reference Andrussow1905 (p. 393), by monotypy. Type material: not examined, probably lost (see type material section of Aktschagylia). The original illustrations are herein reproduced. Type locality: limestone rocks of Shorsulu, Azerbaijan.

Figure 2. (1–4) Avimactra aviculoides (Andrussow, Reference Andrussow1905), original illustrations. (5–8) Barymactra burdigalensis (Mayer-Eymar, Reference Mayer-Eymar1864), NMBA 3030, syntypes. (9–17) Caspimactra naphtalanica Ali-Zade and Kabakova in Ali-Zade, Reference Ali-Zade1969, original illustrations. Scale bars (1–4, 9–17) 2 cm; (5–8) 4 cm.
Diagnosis
Shell trigonal, dorsal margin long and straight, ventral margin short and acute; ends rounded; exterior surface smooth with a radial fold from umbo to ventral edge that divides the exterior surface in two halves; umbos centrally placed; hinge plate with moderately thick cardinal teeth in the right valve (3a and 3b), lateral teeth not observed; chondrophore large, slightly posteriorly oriented; pallial sinus unknown.
Occurrence
Oligocene–Pliocene of Eastern Europe and western Asia.
Remarks
Several species have been mentioned within Avimactra or Aktschagylia by different authors (Tscheltzov, Reference Tscheltzov1967; Nevesskaja et al., Reference Nevesskaja, Popov, Goncharova, Guzhov, Janin, Plubotko, Biakov and Gavrilova2013; among others). Both genera are currently considered as valid (Nevesskaja et al., Reference Nevesskaja, Popov, Goncharova, Guzhov, Janin, Plubotko, Biakov and Gavrilova2013). Tscheltzov (Reference Tscheltzov1967) described seven species included within Avimactra from Akschagylian deposits. A full revision of Eastern European Neogene mactrids is needed to confirm the number of valid fossil species.
Barymactra Cossmann and Peyrot, Reference Cossmann and Peyrot1909
Figure 2.5–2.8
Type species
Mactra burdigalensis Mayer-Eymar, Reference Mayer-Eymar1864 (p. 351), by original designation. Type material: NMBA 3030, two syntypes. Type locality: Aquitanian deposits of Leognan, Bordeaux, France.
Diagnosis
Shell large, thick, trigonal, length up to 90 mm; anterior and posterior ends rounded; dorso-posterior area convex, dorso-anterior area concave; exterior surface smooth, with commarginal growth lines; umbos prosogyrate; hinge plate shortened, curved, right valve with two cardinal teeth (3a and 3b), and two short anterior (AI and AIII) and two short posterior (PI and PIII) lateral teeth, the ventral ones stronger; left valve with a thin, inverted V-shaped cardinal tooth (2a-2b), the 2a curved upwards, and one short anterior (AII) and one elongated posterior (PII) lateral tooth; chondrophore spoon-like; pallial sinus short, triangular, V-shaped, slightly descending.
Occurrence
Eocene–Miocene of Europe and tropical West Africa.
Remarks
Currently, the fossil type species Mactra burdigalensis Mayer-Eymar, Reference Mayer-Eymar1864, and the living Mactra rostrata Spengler, Reference Spengler1802, have been placed within Barymactra (Huber, Reference Huber2010; Cosel and Gofas, Reference Cosel and Gofas2019; MolluscaBase, Reference MolluscaBase2022).
Caspimactra Ali-Zade and Kabakova in Ali-Zade, Reference Ali-Zade1969
Figure 2.9–2.17
Type species
Caspimactra naphtalanica Ali-Zade and Kabakova in Ali-Zade, Reference Ali-Zade1969 (p. 4, 65–66), by original designation. Type material: not examined, deposited at the Museum of Geosciences, Lomonosov Moscow State University, according to the original authors. The original illustrations were analyzed. Type locality: Naftalan, Azerbaijan.
Diagnosis
Shell oval, elongate, medium size, length up to 50 mm; ends rounded, the anterior one positioned higher than the posterior one; exterior surface smooth; umbos prosogyrate; hinge plate shortened; right hinge with two weak cardinals (3a and 3b), V-shaped left cardinal tooth (2a-2b) present; lateral right dorsal teeth (AI and PI) reduced or almost absent; left lateral teeth small (AII and PII); chondrophore trigonal, posteriorly curved; pallial line with a short and round sinus.
Occurrence
Upper Pliocene–Lower Pleistocene of Asia, Azerbaijan, Turkmenistan.
Remarks
Caspimactra was described as a subgenus of Lutraria (Ali-Zade, Reference Ali-Zade1969). Recently, Nevesskaja et al. (Reference Nevesskaja, Paramonova and Babak1997, Reference Nevesskaja, Popov, Goncharova, Guzhov, Janin, Plubotko, Biakov and Gavrilova2013) accepted it at the genus level. Caspimactra includes the species Caspimactra naphtalanica Ali-Zade and Kabakova in Ali-Zade, Reference Ali-Zade1969, and Caspimactra andrussovi Ali-Zade and Kabakova in Ali-Zade, Reference Ali-Zade1969, which are commonly reported from deposits exposed at Naftalan, Azerbaijan (Nevesskaja et al., Reference Nevesskaja, Paramonova and Babak1997).
Chersonimactra Paramonova, Reference Paramonova and Nevesskaja1978
Figure 3.1–3.6
Type species
Mactra bulgarica Toula, Reference Toula1892 (p. 435), by original designation. Type material: NHMW 1998/0001/0005, 4 syntypes. Type locality: ‘Seitenschlucht in Balžik,’ German for ‘side gorge/ in Balchik, Bulgaria.

Figure 3. (1–6) Chersonimactra bulgarica (Toula, Reference Toula1892), NHMW 1998/0001/0005, 4 syntypes. (7–9) Spisula (Crepispisula) amekiensis Eames, Reference Eames1957: (7, 8) holotype (NHMUK L. 48224), (9) paratype (NHMUK L. 48219). (10–18) Cryptomactra pesanseris (Mayer-Eymar, 1857), lot NMBA 1053 from Sevastopol. (19–23) Cymbophora ashburnerii (Gabb, Reference Gabb1864): (19, 20) ANSP 4441b and (21, 22) ANSP 4441a, paralectotypes; (23) ANSP, lectotype 4441. Scale bars (1–18) 1 cm; (19–23) 2 cm.
Diagnosis
Shell trigonal, thick, inflated; exterior surface smooth, with concentric irregular growth lines; umbo very inflated and prosogyrate; hinge plate shortened; inverted V-shaped cardinal tooth (2a-2b) asymmetric, the posterior one shorter; lateral teeth strong and short; pallial sinus very shallow.
Occurrence
Upper Sarmatian deposits (upper Middle Miocene) of Eastern Paratethys, Eastern Europe, and western Asia.
Remarks
Currently, Chersonimactra is considered a subgenus of Mactra (Nevesskaja et al. (Reference Nevesskaja, Popov, Goncharova, Guzhov, Janin, Plubotko, Biakov and Gavrilova2013). A further revision of Chersonimactra and Sarmatimactra is needed. Dobrogimactra Ionesi, Reference Ionesi1986, is an objective junior synonym with the same type species.
Crepispisula Eames, Reference Eames1957
Figure 3.7–3.9
Type species
Spisula (Crepispisula) amekiensis Eames, Reference Eames1957 (p. 65) (new name for Mactra semisulcata Newton, Reference Newton1922, non Lamarck, Reference Lamarck1805), by original designation. Type material: NHMUK L. 48224, holotype. Type locality: Eocene deposits of Ameki, District of Omobialla, Nigeria.
Diagnosis
Shell medium size, thin, sub-triangular, strongly inflated, shell outline similar to Scissodesma; anteriorly and posteriorly sub-carinated; exterior surface with concentric sculpture; umbo prosogyrate; hinge plate with short but prominent inverted V-shaped cardinal tooth (2a-2b); posterior laterals (PI, PII, and PIII) longer than anterior ones (AI, AII, and AIII); chondrophore narrow and triangular, flanked anteriorly by a thin lamella (4b); pallial sinus narrow, deep.
Occurrence
Eocene of western Africa.
Remarks
Crepispisula was described as a subgenus of Spisula (Eames, Reference Eames1957). Currently, it is considered a subgenus of Scissodesma (Cosel and Gofas, Reference Cosel and Gofas2019). Crepispisula is similar to Scissodesma in shell outline, but differs in the concentric ornament pattern, less-marked posterior carina, smaller ligament slit, and the lateral teeth not crenulated. Crepispisula amekiensis is a new name of Eames (Reference Eames1957) for the misidentified Mactra semisulcata of Newton (Reference Newton1922) (non Lamarck, Reference Lamarck1805). Lamarck's species, from the Eocene of the Paris Basin, shows a different outline, being less triangular, less antero-ventrally angulated, and having weaker ornament.
Cryptomactra Andrussow, Reference Andrussow1902
Figure 3.10–3.18
Type species
Lucina pesanseris Mayer-Eymar, Reference Mayer-Eymar1857 (p. 57), by monotypy. Type material: not examined. The lot collected by Mayer-Eymar NMBA 1053 from Crimea, Sevastopol, Middle Sarmatian deposits was examined. Type locality: Cape Ak-Bouroun, Volhynie, Ukraine.
Diagnosis
Shell small but thick, trapezoidal in shape; ends rounded and low; exterior surface with one or two strong radial folds extending from the beaks to the ventral edge, making ventral margin sinuous; umbos markedly prosogyrate, inflated; hinge plate with two cardinal teeth in each valve; one or two anterior teeth (AI and AIII) and one posterior lateral tooth (PI) in the right valve and single ones in the left valve (AII and PII) (Nevesskaja et al., Reference Nevesskaja, Popov, Goncharova, Guzhov, Janin, Plubotko, Biakov and Gavrilova2013); pallial sinus not observed.
Occurrence
Middle Sarmatian deposits (upper Middle Miocene) of Eastern Paratethys, Eastern Europe, and western Asia.
Remarks
Cryptomactra pesanseris has been recorded from the Taman Trough, eastern part of the Kerch Peninsula, Taman Peninsula, and Ciscaucasia, Russia, defined by Cryptomactra beds (Kolesnikov, Reference Kolesnikov and Arkhangel'skij1940; Rostovtseva, Reference Rostovtseva2009). The basin includes a molluscan community of Cryptomactra pesanseris (Kolesnikov, Reference Kolesnikov and Arkhangel'skij1940) accompanied by both benthic and planktonic diatom species. Cryptomactra is currently considered a valid genus (Nevesskaja et al., Reference Nevesskaja, Popov, Goncharova, Guzhov, Janin, Plubotko, Biakov and Gavrilova2013).
Cymbophora Gabb, Reference Gabb1869
Figure 3.19–3.23
Type species
Mactra ashburnerii Gabb, Reference Gabb1864 (p. 153), by monotypy. Type material: ANSP 4441, lectotype (Saul, Reference Saul1974); paralectotypes: ANSP 4441a, 4441b (Saul, Reference Saul1974). The lots labeled as paratypes from UCMP 14925, 14926, and 14927 also were examined. Type locality: Cretaceous deposits of Texas Flat, Butte County, California, USA.
Diagnosis
Shell trigonal, moderately elongated; anterior end rounded, posterior end rounded to sub-truncate, dorso-posterior area convex; antero-ventral margin nearly straight; exterior surface with commarginal ornamentation; selenis, understood here as the area defined by a groove on the anterior slope of the valve, is poorly defined; umbos moderately prominent; hinge plate with thin cardinal teeth (3a and 3b; 2a-2b), flanked by a thin cardinal lamella (4b), lateral teeth distant from the umbos and elongate, with fine granulations on the upper part of their dorsal surfaces; tensilifer as long as the resilifer, extended behind the umbo; pallial sinus not observed.
Occurrence
Upper Cretaceous of eastern and western North America, Europe, Japan.
Remarks
Cymbophora is distinguished from other genera by the elevated margins of the chondrophore and the open ligament groove (Saul, Reference Saul1974). Twenty-three species have been included in Cymbophora from Cretaceous deposits of North America, eastern Russia, and Japan (Gabb, Reference Gabb1864; Nagao and Otatume, Reference Nagao and Otatume1938; Stephenson, Reference Stephenson1941, Reference Stephenson1952; Saul, Reference Saul1974; among others). Additional records of Cymbophora from Algeria and Chile are dubious and must be revisited.
Darcinia Clark in Clark and Durham, Reference Clark and Durham1946
Figure 4.1–4.3
Type species
Darcinia colombiana Clark in Clark and Durham, Reference Clark and Durham1946 (p. 74), by original designation. Type material: UCMP 34873 holotype, a right valve. Type locality: Bolivar, Colombia.
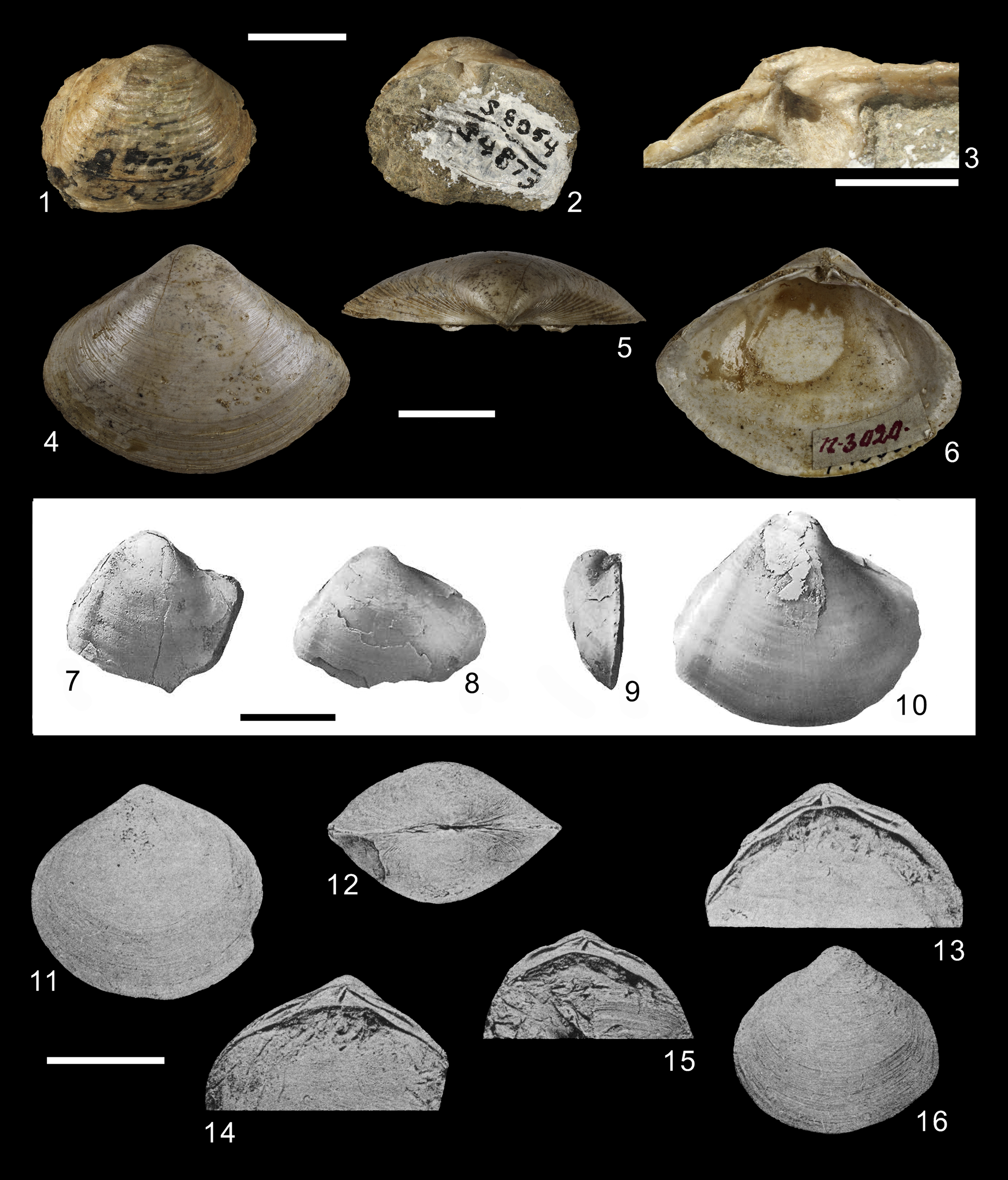
Figure 4. (1–3) Darcinia colombiana Clark and Durham, Reference Clark and Durham1946, UCMP 34873, holotype. (4–6) Eomactra basteroti (Mayer-Eymar, 1853), NMBA 3020, probable syntype. (7–10) Eopapyrina darienensis (Dall, Reference Dall1898), original illustrations of Dall, Reference Dall1898: (7) USNM 112271, syntype; (8, 9) USNM 647513, hypotype; (10) USNM 647512, hypotype. (11–16) Geltena subequilatera: (11, 12) USNM 103761, holotype, original illustration; (13–16) USNM 103762A, 103762B, 103762C, and 103762D, respectively, paratypes. Scale bars (1, 2, 4–16) 1 cm; (3) 5 mm.
Diagnosis
Shell thick, sub-circular, sub-truncate, posteriorly gaping; dorso-posterior area defined by a ridge; exterior surface with concentric growth lines; umbos prosogyrate; hinge plate with two heavy cardinal teeth (3a and 3b), well-developed anterior laterals (AI, AII, and AIII) and nearly obsolete posterior laterals; chondrophore deep, spoon-shaped, ventrally projected; pallial sinus unknown.
Occurrence
Eocene of western South America.
Remarks
Darcinia was described from Eocene deposits exposed at Bolivar Department, Colombia. The authors mentioned that this genus shares morphological characters with Darina and Eastonia. It is currently placed within the subfamily Darininae (Carter et al., Reference Carter, Campbell, Altaba, Anderson and Araujo2011).
Eomactra Cossmann in Cossmann and Peyrot, Reference Cossmann and Peyrot1909
Figure 4.4–4.6
Type species
Mactra basteroti Mayer-Eymar, 1853 (p. 80), by original designation. Type material: not examined; the lot NMBA 3020, with 31 specimens collected by Mayer-Eymar in Bordeaux, France was examined and reproduced. Type locality: Belpberg, Switzerland.
Diagnosis
Shell trigonal, large to medium in size, length up to 35 mm; anterior end rounded, posterior end pointed; exterior surface smooth except for concentric ribs on lunule and escutcheon; hinge plate narrow; left valve with one anterior (AII) tooth and one posterior (PII) lateral tooth, elongated and thin, equidistant to the cardinal teeth; inverted V-shaped cardinal tooth (2a-2b) strong; chondrophore small, not ventrally projected; pallial sinus wide and short; ventral margin with undulations on the inner side.
Occurrence
Eocene–Pliocene of Europe.
Remarks
Currently, Eomactra is considered a subgenus of Mactra (Nevesskaja et al., Reference Nevesskaja, Popov, Goncharova, Guzhov, Janin, Plubotko, Biakov and Gavrilova2013). Eomactra is mainly recorded from Eocene deposits of Europe (d’Orbigny, Reference d'Orbigny1850–1852; Mayer-Eymar, Reference Mayer-Eymar1853; Cossmann and Peyrot, Reference Cossmann and Peyrot1909; among others). Non-European records of this genus were mentioned by Garvie (Reference Garvie2013) and Tiwari and Kachhara (Reference Tiwari and Kachhara2003). However, shell comparisons suggest a different generic placement. Currently four species are included.
Eopapyrina Woodring, Reference Woodring1982
Figure 4.7–4.10
Type species
Mactra (Mactrella) darienensis Dall, Reference Dall1898 (p. 895), by original designation. Type material: USNM 112271, syntype; USNM 647512, 647513, and 647514 are hypotypes illustrated by Woodring (Reference Woodring1982). Type locality: Eocene Gatun beds, corresponding to the Claibornian, at Vamos-vamos Station on the line of the Panama Canal, Isthmus of Darien. Eopapyrina darienensis was also noted in the Búcaro Formation, Tonosí area, southwestern Panama.
Diagnosis
Shell trigonal, thin, moderately elongated, inequilateral, medium size, outline similar to Mactrellona; anterior end more extended than posterior; slightly gaping at posterior end; dorso-posterior slope narrow, defined by a carina; exterior surface with concentric growth lines; umbos inflated, strongly convex; hinge plate unknown; pallial sinus unknown.
Occurrence
Eocene to Miocene of North and Central America.
Remarks
Although its hinge plate is unknown, Woodring (Reference Woodring1982) placed Eopapyrina within Mactridae. This genus is monospecific. No additional records of this genus were found in the literature.
Geltena Stephenson in Vokes, Reference Vokes1946
Figure 4.11–4.16
Type species
Geltena subequilatera Stephenson in Vokes, Reference Vokes1946 (p. 201), by original designation. Type material: USNM 103761, holotype (length 20 mm, height 17.8 mm, thickness 11.8 mm); paratypes: USNM 103762A, 1 specimen; 103762B, 1 specimen; 103762C, 1 specimen; 103762D, 1 specimen; 105522, 15 specimens. Type locality: Lewisville Member on Johnson Creek, 1 mile east of Arlington, Tarrant County, Texas, USA.
Diagnosis
Shell sub-circular to broadly sub-ovate; moderately to strongly inflated; anterior and posterior ends rounded, postero-dorsal area with concentric ornamentation; exterior surface smooth with concentric growth lines, lunule long and broad, defined by a weakly impressed line; external ligament placed on a heavy nymph; hinge plate with two cardinal teeth in each valve, the left one inverted V-shaped (2a-2b); anterior lateral (AI, AII, and AIII) and posterior lateral (PI, PII, and PIII) teeth well developed with deep grooves; chondrophore very small; pallial sinus shallow.
Occurrence
Upper Cretaceous–Miocene of North America, South America, and Europe.
Remarks
Geltena is a valid genus commonly reported in North American deposits (Saul, Reference Saul1973; Nevesskaja et al., Reference Nevesskaja, Popov, Goncharova, Guzhov, Janin, Plubotko, Biakov and Gavrilova2013). Additional records were mentioned from Cenomanian–Turonian deposits of Sergipe Basin, Brazil (Ayoub-Hannaa et al., Reference Ayoub-Hannaa, Bengtson, Fürsich and Andrade2019) and the Lower Cretaceous of England (Woods, Reference Woods1907). Currently, eight valid species are included within Geltena.
Ionesimactra Signorelli nom. nov.
Figure 5.1–5.7
Type species
Mactra caspia Eichwald, Reference Eichwald1840 [1841, 1842] (p. 260), by typification of replaced name. Type material: not examined, Eichwald's collections mainly housed at Saint Petersburg University, but not listed at the university collections. Illustrations of Eichwald (Reference Eichwald1840 [1841, 1842]) (Fig. 5.1) and Macarovici (Reference Macarovici1940) (Fig. 5.2–5.7) are reproduced. Type locality: calcareous deposits exposed at Tükkaragani, Russia.

Figure 5. (1–7) Ionesimactra caspia (Eichwald, Reference Eichwald1840 [1841, 1842]) n. comb.: (1) original illustration; (2–7) illustrations from Macarovici (Reference Macarovici1940). (8–14) Kirghizella pisum (Andrussow, Reference Andrussow1902a), original illustrations. (15–20) Leptomactra delumbis (Conrad, Reference Conrad1832), ANSP 30532, syntypes. Scale bar (8–20) 2 cm.
Other species
In the original description, Ionesi (Reference Ionesi1986) included the species M. caspia Eichwald, Reference Eichwald1840 [1841, 1842]; M. timida Zhizhchenko, Reference Zhizhchenko1934; M. sinzovi Pavlov, Reference Pavlov1925; M. intermedia Macarovici, Reference Macarovici1935; M. supernaviculata Macarovici, Reference Macarovici1935; and M. rostrata Macarovici, Reference Macarovici1935.
Diagnosis
Shell small to medium size, trigonal to oval, thin, moderately elongated; anterior and posterior ends rounded; exterior surface smooth with concentric growth lines; umbos small but pointed; hinge plate strong in comparison with valve size, inverted V-shaped cardinal tooth (2a-2b) thin, with both branches almost completely fused, lateral teeth short and well developed, except AIII and PIII which are slightly smaller; pallial sinus shallow.
Occurrence
Upper Sarmatian deposits (upper Middle Miocene) of Eastern Paratethys, Eastern Europe, and western Asia.
Etymology
Honoring Bica Ionesi for her contribution on the Sarmatian Mactridae.
Remarks
Ionesimactra nom. nov. is a replacement name for Caspimactra Ionesi, Reference Ionesi1986 (non Caspimactra Ali-Zade and Kabakova in Ali-Zade, Reference Ali-Zade1969). Ionesimactra caspia n. comb. is similar to Chersonimactra and Sarmatimactra, but differs by having more reduced cardinal teeth and a thinner shell. Additional studies are needed to confirm its taxonomic position.
Kirghizella Andrussow, Reference Andrussow1902
Figure 5.8–5.14
Type species
Mactra pisum Andrussow, Reference Andrussow1902a (p. 131), by subsequent designation (Nevesskaja et al., Reference Nevesskaja, Popov, Goncharova, Guzhov, Janin, Plubotko, Biakov and Gavrilova2013). Type material: not examined, probably lost. The mactrids described by Andrussow (Reference Andrussow1902a, Reference Andrussow1905) are not listed in the catalogues of Chernishov Museum or Saint Petersburg University (S. Popov, personal communication, 2021).Type locality: Akschagyl, Kögnja-Arap, Azerbaijan is mentioned in the original description.
Diagnosis
Shell small, trigonal to sub-oval, moderately to strongly inflated, length up to 10 mm; anterior and posterior ends rounded, the anterior more pointed; exterior surface smooth; umbos prosogyrate; hinge plate with two cardinal teeth (3a and 3b) on the right valve, and one fused cardinal (2a-2b) on the left, obliquely backwards oriented; one anterior lateral tooth and one posterior lateral tooth (AII and PII) in the left valve, the anterior is shorter than the posterior; mantle line without sinus.
Occurrence
Upper Middle Pliocene–Lower Pleistocene of Eastern Europe, Asia, Russia, Azerbaijan.
Remarks
Nevesskaja et al. (Reference Nevesskaja, Paramonova and Babak1997, Reference Nevesskaja, Popov, Goncharova, Guzhov, Janin, Plubotko, Biakov and Gavrilova2013) included two species within Kirghizella: K. pisum (Andrussow, Reference Andrussow1902a) and K. modiolopsis (Tscheltzov, Reference Tscheltzov1967), the latter originally placed within Avimactra.
Leptomactra Ward, Reference Ward1992
Figure 5.15–5.20
Type species
Mactra delumbis Conrad, Reference Conrad1832 (p. 26), by original designation. Type material: ANSP 30532, syntypes. Type locality: James River, near Smithfield, Virginia, USA.
Diagnosis
Shell large to medium size, thin, fragile, ovate to trigonal, moderately compressed, anterior end rounded, posterior end slightly sulcate, lunule not defined but indicated by a depression, dorso-posterior area convex, demarked by a low ridge; dorso-anterior area concave, elongated, with thin and concentric lines; umbos prosogyrate, moderately inflated; right valve with two thin cardinal teeth (3a and 3b), unfused, 3a larger than 3b; left valve with an inverted V-shaped cardinal tooth (2a-2b); lateral teeth (AII and PII) thin, elongated, and not-striated; chondrophore trigonal, deep, posteriorly inclined; adductor muscle scars distinct, subovate, subequal; pallial sinus poorly defined, deep.
Occurrence
Middle Miocene to Upper Pliocene, Maryland to Florida, USA.
Remarks
Leptomactra was described at a genus level status (Ward, Reference Ward1992). However, shell outline of Leptomactra is similar to Mactromeris and Simomactra. A conclusive opinion about the taxonomic status of this genus needs additional analysis. The species included within Leptomactra historically had been placed in the subgenus Hemimactra (Dall, Reference Dall1898). However, the subgenus Hemimactra is characterized by a more oval shell outline, umbos less inflated, striated lateral teeth, and more distinct pallial line. Leptomactra currently includes six species.
Mactrodesma Conrad, Reference Conrad1869
Figure 6.1–6.8
Type species
Mactra ponderosa Conrad, Reference Conrad1830 (p. 228) (non Eichwald, Reference Eichwald1830, p. 207; non Philippi, Reference Philippi1844, p. 165), (= Mactra subponderosa d’Orbigny, Reference d'Orbigny1852, by monotypy). Type material: ANSP 30497, lectotype, designated by Ward (Reference Ward1992), and six paralectotypes. Type locality: St. Marys River, Maryland, USA.

Figure 6. (1–8) Mactrodesma ponderosa (Conrad, Reference Conrad1830): (1, 2) ANSP 30497, lectotype; (3–8) paralectotypes. (9–11) Mactrona mula (Marwick, Reference Marwick1948): (9) GS3528, R11/f7014, TM1280 holotype; (10) GS4348, S22/f6473; (11) GS4265, S22/f6455, all taken from Beu (Reference Beu2004). (12–19) Miorangia johnsoni (Dall, Reference Dall1892), USNM MO 107033, syntypes. Scale bars (1–11) 2 cm; (12–19) 1 cm.
Diagnosis
Shell sub-trigonal to sub-circular, ends rounded, dorso-anterior area straight, dorso posterior area convex; exterior surface smooth with concentric growth lines; umbos prosogyrate, not inflated; right valve with two unfused cardinal teeth (3a and 3b) and two anterior lateral (AI and AIII) and two short posterior lateral (PI and PIII) teeth in the; left valve with profoundly elevated inverted V-shaped cardinal tooth (2a-2b) and one anterior lateral tooth (AII) and one posterior lateral tooth (PII), short but thick; chondrophore large, ventrally projected; adductor muscle scars large; pallial sinus narrower and deeper than in Mactra.
Occurrence
Miocene of eastern North America.
Remarks
Mactrodesma was described with a genus level status (Ward, Reference Ward1992). It was mainly reported in Miocene deposits exposed at Chesapeake Bay in eastern North America (Gardner, Reference Gardner1943). The type species originally was named Mactra ponderosa and later renamed M. subponderosa by d’Orbigny (Reference d'Orbigny1852) to correct the homonymy of Conrad (Reference Conrad1830).
Mactrona Marwick, Reference Marwick1952
Figure 6.9–6.11
Type species
Mactra (Mactrula) mula Marwick, Reference Marwick1948 (p. 22), by typification of replaced name. Type material: TM1280, holotype. Illustration of Beu (Reference Beu2004) is herein reproduced. Type locality: Waipipian deposits (Upper Pliocene), GS3528, R11/f7014, Otahuhu well, Auckland, New Zealand.
Diagnosis
Shell large to medium size, strongly trigonal, thick and solid; anterior end rounded; posterior end pointed; exterior surface smooth with weak growth ridges; umbos narrow, inflated, prosogyrate; right valve with two small cardinal teeth (3a and 3b) and two anterior (AI and AIII) and two posterior (PI and PIII) lateral teeth; left valve with a small inverted V-shaped cardinal tooth (2a-2b) flanked by a very small lamella (4b), and one anterior lateral tooth (AII) and one posterior lateral tooth (PII), both pustulose, in the left valve; adductor scars oval, relatively small, near valve margins; pallial sinus small, semicircular.
Occurrence
Pliocene to Lower Pleistocene of New Zealand.
Remarks
Mactrona, a replacement name for Mactrula Marwick, Reference Marwick1948 (preoccupied by Mactrula Risso, Reference Risso1826), groups species with Spisula-type ligament and pustulose lateral teeth. Beu (Reference Beu2004) synonymized Mactrona with Pseudocardium and pointed out that this is one of the few taxa distributed in New Zealand and western North American localities. However, Marshall and Spencer (Reference Marshall and Spencer2013), based on biogeographical distribution, rejected this synonymy (see Pseudocardium remarks). In addition to biogeographical differences, the hinge morphology of Pseudocardium has longer anterior lateral teeth than Mactrona. Additional analysis is needed to make a conclusive decision.
Miorangia Dall, Reference Dall1894
Figure 6.12–6.19
Type species
Gnathodon johnsoni Dall, Reference Dall1892 (p. 165), by monotypy. Type material: USNM MO 107033, syntypes. Type locality: Chesapeake Miocene of the Pascagoula clays, at Shell Bluff, Pascagoula River, Greene County, Mississippi, USA.
Diagnosis
Shell small, ovate-triangular to sub-mytiliform, extremely inequilateral; exterior surface smooth or with irregular and concentric growth lines; umbo prosogyrate, prominent, compressed; hinge plate asymmetrical; right valve with two small and thin cardinal teeth (3a and 3b), two short anterior (AI and AIII) and two long posterior (PI and PIII) lateral teeth, the posterior ones arched; left valve with inverted V-shaped cardinal tooth (2a-2b), and one anterior lateral tooth (AII) and one posterior lateral tooth (PII) fitting in the right; lateral teeth with pustules; chondrophore narrow, oblique; muscular impressions small, distinct; pallial sinus obsolete.
Occurrence
Miocene of eastern North America.
Remarks
Miorangia was described by Dall (Reference Dall1894) as a subgenus of Gnathodon (= Rangia Desmoulins). Currently, it is considered a subgenus of Rangia Desmoulins, Reference Desmoulins1832, and includes only the type species. Miorangia has been recorded from different Middle to Upper Miocene coastal Mississippi deposits and drill holes (Gardner, Reference Gardner1940; Campbell and Otvos, Reference Campbell and Otvos1992).
Mulinoides Olsson, Reference Olsson1944
Figure 7.1–7.5
Type species
Mulinoides chilca Olsson, Reference Olsson1944 (p. 217), by original designation. Type material: PRI 4854, holotype; paratypes: PRI 4855, 4856. Type locality: Baculites Zone, Maastrichtian Stage, Upper Cretaceous, Piura Department, Peru.

Figure 7. (1–5) Mulinoides chilca Olsson, Reference Olsson1944: (1) PRI 4854, holotype; (2, 3) PRI 4855, paratype, (4, 5) PRI 4856, paratype. (6, 7) Nelltia stenzeli Stephenson, Reference Stephenson1952, USNM 105431, holotype. (8–10) Nymphactra jonasseni Stilwell and Zinsmeister, Reference Stilwell and Zinsmeister1992: (8, 10) PRI 452, holotype (ex USNM 441633), (9) PRI 1077, paratype (ex USNM 441634). (11) Ovamactra cyma Woodring, Reference Woodring1982, USNM 135235, holotype. (12–14) Tellina mathewsonii Gabb, Reference Gabb1864: (12) UCMP 10077, paralectotype, (13) UCMP 10072, paralectotype, (14) UCMP 10078, paralectotype. Scale bars (1–10, 12–14) 2 cm; (11) 1 cm.
Diagnosis
Shell sub-circular, thick, solid, similar to Cymbophora; ends rounded, postero-dorsal area well marked, set off by ridge; lunule present; exterior surface with concentric growth lines; umbos wide and convex; hinge plate known only in parts, with lateral teeth strong, chondrophore moderately developed, mactroid in form; pallial sinus unknown.
Occurrence
Upper Cretaceous of western South America.
Remarks
Mulinoides resembles Cymbophora, but differs by having rounded ends, stronger shell, postero-dorsal area more defined and broad lunular area (Olsson, Reference Olsson1944). Currently three species are included within this genus (DeVries, Reference DeVries2019).
Nelltia Stephenson, Reference Stephenson1952
Figure 7.6, 7.7
Type species
Nelltia stenzeli Stephenson, Reference Stephenson1952 (p. 113), by original designation. Type material: USNM, 105431, holotype; USNM 10432, 2 paratypes; USNM 105433, 1 paratype; USNM 105434, 1 paratype; USNM 105435a, b, 2 paratypes; USNM 105436, 1 paratype; USNM 105437, 1 paratype; USNM 105438, 35 paratypes. Type locality: near Chicago, Rock Island and Pacific Railroad, 0.9–1 mile west of the Dallas County line, in Tarrant County, Texas, USA.
Diagnosis
Shell large, sub-elliptical, compressed; anterior and posterior ends rounded; exterior surface smooth; umbos prosogyrate, small, not prominent; external ligament opisthodetic; right hinge with two cardinal teeth (3a and 3b), one anterior lateral tooth (AI) aligned with the anterior cardinal tooth (3a), and one posterior lateral tooth (PI) elongated; left hinge with thick-limbed, inverted V-shaped cardinal tooth (2a-2b), posterior laterals weak; pallial sinus moderately deep and rounded.
Occurrence
Aptian–Turonian, Lower to Upper Cretaceous of North America.
Remarks
Although different authors have placed Nelltia in Tellinidae (Stephenson, Reference Stephenson1952; Keen, Reference Keen, Cox, Newell, Boyd, Branson, Casey and Chavan1969b), hinge morphology undoubtedly places it within Mactridae (Saul, Reference Saul1973).
Nymphactra Stilwell and Zinsmeister, Reference Stilwell and Zinsmeister1992
Figure 7.8–7.10
Type species
Nymphactra jonasseni Stilwell and Zinsmeister, Reference Stilwell and Zinsmeister1992 (p. 76), by original designation. Type material: PRI 452, holotype (ex USNM 441633), dimensions: length 68.5 mm, height 52.5 mm, width 17.5 mm; paratype: PRI 1077 (ex USNM 441634). Type locality: Middle–Upper Eocene deposits of Seymour Island, Antarctica.
Diagnosis
Shell large, thin, sub-oval to sub-circular, sub-equilateral; anterior and posterior ends rounded; exterior surface with concentric lines; umbos small; nymph well developed; hinge plate narrow, left valve with strong inverted V-shaped cardinal tooth (2a-2b), one anterior lateral tooth (AII) and one posterior lateral tooth (PII), thick and elongated; right hinge not examined; trigonal and small chondrophore, not ventrally projected; pallial sinus shallow, V-shaped.
Occurrence
Middle–Upper Eocene deposits of Seymour Island, Antarctic Peninsula.
Remarks
Nymphactra is monospecific. It was proposed for mactrids with well-developed nymphs and narrow hinge plate (Stilwell and Zinsmeister, Reference Stilwell and Zinsmeister1992). Beu (Reference Beu2009) suggested that Nymphactra belongs to Cardiidae based in the lack of lateral teeth. However, the types of N. jonasseni, herein examined, show well-developed lateral teeth and an inverted V-shaped left cardinal tooth, which confirm its taxonomic position within Mactridae.
Ovamactra Woodring, Reference Woodring1982
Figure 7.11
Type species
Ovamactra cyma Woodring, Reference Woodring1982 (p. 651−652), by original designation. Type material: USNM 135235, holotype. Type locality: Eocene rocks exposed at Canal Zone off Palenquilla Point, submerged by Gatun Lake, Panama.
Diagnosis
Shell large, thin, ovate, elongated, sub-equilateral, moderately inflated; anterior end broadly rounded, posterior end rounded but more acute; exterior surface with concentric undulations, more visible near the umbo, posterior slope narrow; umbos not inflated; hinge unknown; pallial sinus unknown.
Occurrence
Eocene of Central America.
Remarks
Ovamactra is monospecific. Its taxonomic placement is uncertain because its hinge is unknown. Woodring (Reference Woodring1982) mentioned that ornamentation suggests affinities with Harvella.
Petromactra Saul, Reference Saul1973
Figure 7.12–7.14
Type species
Tellina mathewsonii Gabb, Reference Gabb1864 (p. 158), by original designation. Type material: ANSP 4373, lectotype designated by Saul (Reference Saul1973); paralectotypes: ANSP 4373a; UCMP 10072– 10080. Type locality: Martinez, Contra Costa County, California, USA.
Diagnosis
Shell outline similar to Willimactra, distinguished by morphology and placement of the ligament, which is deeply sunken, mostly internal, exposed only near the umbo; resilium separated from the ligament by a strong flange.
Occurrence
Campanian–Maastrichtian, Upper Cretaceous of Baja California, British Columbia.
Remarks
Petromactra was described Saul (Reference Saul1973) as a subgenus of Willimactra. It differs from Willimactra by having a deeply sunken ligament that is only exposed externally near the umbo (Saul, Reference Saul1973). Shell morphology suggests that this subgenus might have evolved from Willimactra s. s. (Saul, Reference Saul1973). Recent lineages, such as Lutraria, may have evolved from Petromactra where the chondrophore is enlarged, the lateral teeth are close to the cardinals, and the shape is tellinoid (Saul, Reference Saul1973). Petromactra includes two species.
Planimactra Ionesi, Reference Ionesi1986
Figure 8.1, 8.2
Type species
Mactra alata Macarovici, Reference Macarovici1940 (p. 217) (non Spengler, Reference Spengler1802, p. 99) (= Mactra plana Belokrys, Reference Belokrys1964 (p. 1843, pl. 2 figs. 1–9), by original designation. Type material: Mactra alata not examined, type measurements mentioned in the original description: 9.5 mm length, 7.5 mm height, 1.5 mm width; Mactra plana holotype, Ukrainian Academy of Sciences, N° 1647/27-11, River Yuzhnyy Bug, Konstantinovka; Kherson horizon (upper Sarmatian). Type locality: Tighina (currently Bender), Moldavia.
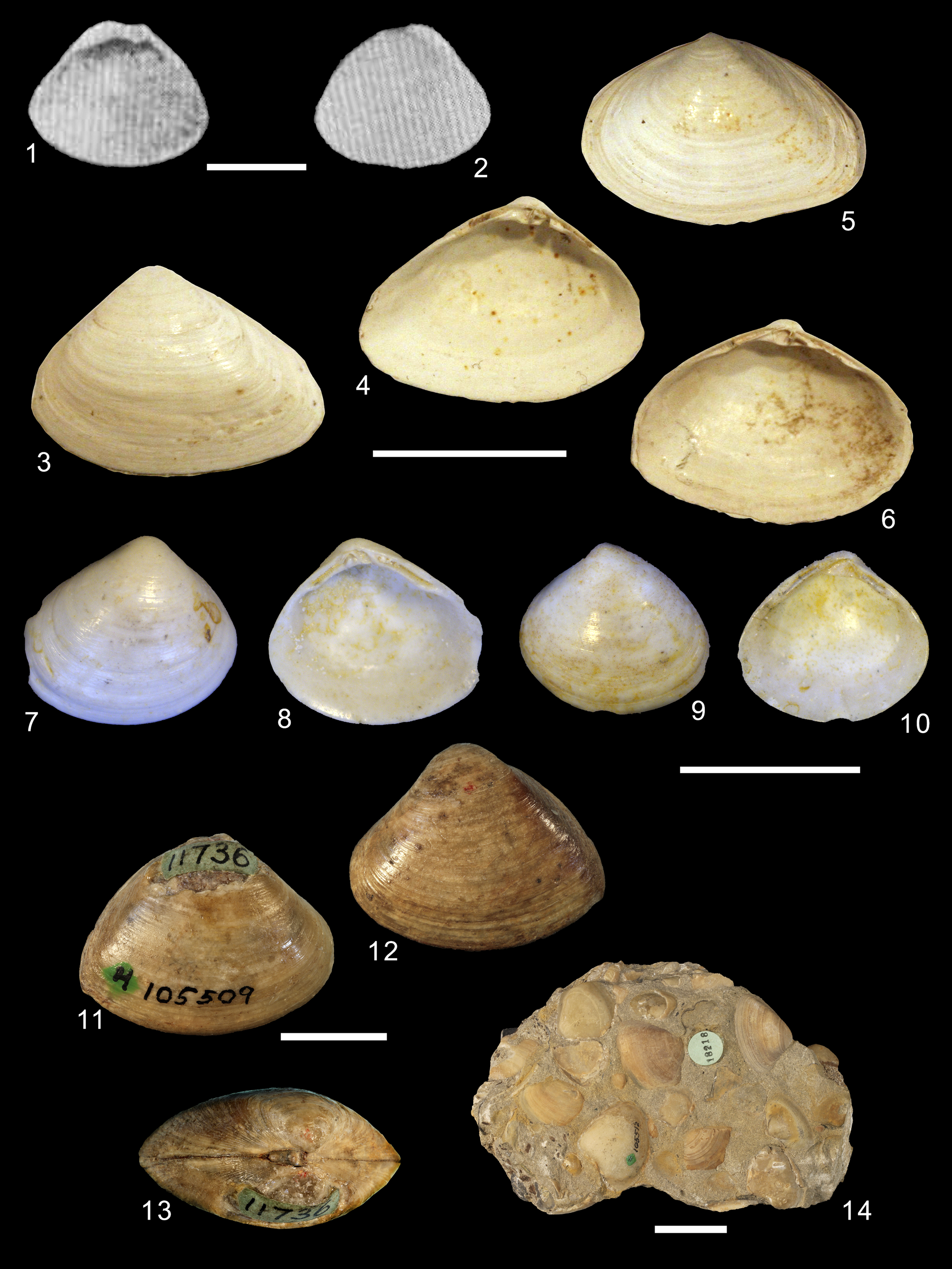
Figure 8. (1, 2) Planimactra alata (Macarovici, Reference Macarovici1940), original illustration. (3–6) Mactra fragilis var. buglovensis Laskarew, Reference Laskarew1903, syntypes, TsNIGR Museum, col. 2430, (3, 4) specimen 77/2430, (5, 6) specimen 79/2430 (Podolimactra eichwaldi [Laskarew, Reference Laskarew1914] is a replacement name). (7–10) Praerangia minuscula Cossmann, Reference Cossmann1908: (7, 8) IRSNB 02954, (9, 10) IRSNB 2955. (11–14) Priscomactra cymba Stephenson, Reference Stephenson1952: (11–13) USNM 105509, holotype; (14) USNM 105512, paratype. Scale bars (1–6, 11–13) 1 cm; (7–10) 3 mm; (14) 2 cm.
Diagnosis
Shell small, oval to trigonal, compressed, thin, length up to 30 mm; anterior end rounded, posterior end with a circular keel, postero-dorsal area flat to slightly convex; external ligament short; exterior surface with very fine growth lines; umbos small and pointed, flattened; hinge plate similar to Caspimactra, but narrower, cardinal tooth 3b longer than 3a, lateral teeth well developed, but relatively short; pallial sinus straight or slightly concave.
Occurrence
Upper Sarmatian deposits (upper Middle Miocene) of Eastern Paratethys, Eastern Europe.
Remarks
Planimactra includes the single species Mactra alata Macarovici, Reference Macarovici1940 (non Spengler, Reference Spengler1802 [=Mactra plana Belokrys, Reference Belokrys1964]).
Podolimactra Ionesi Reference Ionesi1986
Figure 8.3–8.6
Type species
Mactra eichwaldi Laskarew, Reference Laskarew1914 (p. 433) (replacement name for Mactra fragilis Laskarew, Reference Laskarew1903, p. 84, preoccupied), by original designation. Type material: Mactra fragilis var. buglovensis Laskarew, Reference Laskarew1903, TsNIGR Museum, col. 2430, specimens 77/2430–86/2430, 10 syntypes. Type locality: Pliska, Bulgaria mentioned by Laskarew (Reference Laskarew1903, p. 84).
Diagnosis
Shell oval-triangular to oval-elongated outline, thin, fragile, medium size; anterior and posterior ends rounded; exterior surface smooth, dorso-posterior area weakly defined by a ridge; umbos prosogyrate, not inflated; hinge plate narrow, mactroid, with thin cardinal and lateral teeth, AIII and PIII smaller and thinner than AI and PI; pallial sinus shallow.
Occurrence
Lower to middle Sarmatian deposits, upper Middle Miocene of Paratethys, Eastern and Southern Europe and western Asia.
Remarks
Podolimactra is widely distributed from lower to middle Sarmatian deposits. The type species, P. eichwaldi, has been included within Sarmatimactra by some authors (Nevesskaja et al., Reference Nevesskaja, Goncharova, Paramonova, Popov, Babak, Bagdasarian and Voronina1993). However, hinge plate morphology, shell thickness, and pallial sinus depth easily distinguish both genera. Besides the type species, Podolimactra currently includes twelve species (Ionesi and Tabara, Reference Ionesi and Tabara2004).
Praerangia Cossmann, Reference Cossmann1908
Figure 8.7–8.10
Type species
Praerangia minuscula Cossmann, Reference Cossmann1908 (p. 35), by original designation. Type material: not examined; addition material examined: IRSNB 02954 and IRSNB 02955, collected at Mons, Puit Coppée, Danian of Belgium. Type locality: Mons (F. Obourg), Calcaires de Mons, Danian of Belgium.
Diagnosis
Shell trigonal to subcircular, cyreniform, small, thin, fragile; anterior and posterior ends rounded; exterior surface smooth with irregular and concentric growth lines; umbos prosogyrate, external ligament small, positioned behind the beaks; hinge plate curved, left valve with inverted V-shaped cardinal tooth (2a-2b) thin, anterior lateral tooth (AII) shorter than posterior one (PII); right valve with two cardinal teeth (3a and 3b), two anterior (AI and AIII), and two posterior (PI and PIII) lateral teeth, with the inner surfaces serrated; chondrophore trigonal, not ventrally projected; pallial sinus shallow.
Occurrence
Danian, Paleocene of Europe.
Remarks
Keen (Reference Keen, Cox, Newell, Boyd, Branson, Casey and Chavan1969a) considered Praerangia to be a subgenus of Rangia. However, hinge, pallial sinus depth, and ligament placement of the type species, herein examined, reject this taxonomic position. Praerangia shows posterior lateral teeth longer than the anterior ones, but considerably shorter than posterior lateral teeth observed in Rangia. The anterior lateral teeth also differ significantly. In Praerangia, they are short and straight, whereas in Rangia they are curved downward. Finally, the ligament is exclusively internal in Rangia, whereas in Praerangia, there is a small nymph, very close to the beaks, that suggests the presence of a rudimentary external ligament. At the moment, Praerangia is monospecific.
Priscomactra Stephenson, Reference Stephenson1952
Figure 8.11–8.14
Type species
Priscomactra cymba Stephenson, Reference Stephenson1952 (p. 124), by original designation. Type material: USNM 105509, holotype; paratypes: USNM 105510a–c, 3 specimens; USNM 105511, 28 specimens; USNM 105512, 24 specimens; USNM 105513, 2 specimens. Type locality: Lewisville Member near the Chicago, Rock Island and Pacific Railroad, 0.9 mile west of the Dallas County line, in Tarrant County, Texas, USA.
Diagnosis
Shell trigonal, thick, medium size, outline similar to Cymbophora, Spisula, or Mactra; anterior and posterior ends rounded; exterior surface smooth; umbos prosogyrate and inflated, external ligament placed in a deep groove, separated from the internal ligament by a thick ridge; hinge plate with strong and trigonal cardinal tooth (2a-2b) in the left valve, not evidently bifid as in other genera; lateral teeth well developed with internal surfaces striated; chondrophore elevated, slightly concave; pallial sinus not observed.
Occurrence
Upper Cretaceous of North America.
Remarks
Priscomactra currently includes two species (Stephenson, Reference Stephenson1952).
Pseudocardium Gabb, Reference Gabb1866
Figure 9.1–9.3
Type species
Cardium gabbii Rémond, Reference Rémond1863 (p. 13) (= Mulinia densata Conrad, Reference Conrad1857, p. 313), by monotypy. Type material: not examined; Rémond (Reference Rémond1863, p. 13) mentioned that the specimens “…are in the collection of Mr. W. M. Gabb and my own…”. One of Gabb's (Reference Gabb1866) figured specimens (MCZ 15047) of Pseudocardium is from Sierra Bonita, below Tres Pinos (Stewart, Reference Stewart1930). The illustration of Gabb (Reference Gabb1866) is reproduced. Type locality: vicinity of Kirker's Pass, Contra Costa County, California, USA.

Figure 9. (1–3) Pseudocardium gabbii (Rémond, Reference Rémond1863), illustration of Gabb (Reference Gabb1866). (4–7) Pseudomactra poroschini Steklov, Reference Steklov1960, original illustration. (8–15) Pseudoxyperas proaspersa Sacco, Reference Sacco, Bellardi and Sacco1901: (8, 9) MRSN BS 144.04.001, (10, 11) MRSN BS 144.04.002; (12, 13) MRSN BS 144.04.003; (14, 15) MRSN BS 144.04.004, syntypes. (16–19) Mactra elongata Quoy and Gaimard, 1835, MNHN-IM-2000-35987, syntypes. Scale bars (4–7) 5 mm; (8–19) 2 cm.
Diagnosis
Shell ventricose, thick, heavy, rounded trigonal, variable in shape, higher than long; ends rounded; exterior surface sculpted by irregular growth lines; umbos strongly curved; ligament exclusively internal; hinge plate with two large and prominent lateral teeth in each valve, AI and PI in the right valve, and AII and PII in the left one; pallial sinus short, rounded.
Occurrence
Miocene–Pliocene of western North America.
Remarks
Pseudocardium-group mactroid species are characterized by a ventricose and thick shell. Beu (Reference Beu2004) pointed out that Mactra mula (type species of Mactrona, replacement name for Mactrula Marwick, preoccupied) (Fig. 6.9–6.11) is not morphologically distinguishable from Pseudocardium gabbii, and concluded that Mactrona is a junior synonym of Pseudocardium. This conclusion placed Pseudocardium along the southeastern Pacific, which expanded the distribution of this genus from Pliocene deposits of New Zealand to western North American localities. However, Marshall and Spencer (Reference Marshall and Spencer2013) rejected the synonymy between Mactrona and Pseudocardium due to biogeographical reasons. Some differences in hinge morphology between both taxa were observed (see Mactrona remarks). If the genus Pseudocardium is restricted to western North America exclusively, then 10 fossil species are currently included.
Pseudomactra Steklov, Reference Steklov1960
Figure 9.4–9.7
Type species
Pseudomactra poroschini Steklov, Reference Steklov1960 (p. 89), by original designation. Type material: not examined. According the original description, the holotype is deposited in the Russian Academy of Sciences of Moscow under the number 1367. Measures of the type series (from Steklov, Reference Steklov1960): length 7–14 mm, height 5.5–9 mm, width 2–6 mm. Type locality: Upper Sarmatian deposits mentioned in the original description.
Diagnosis
Shell equivalve, trigonal, outline irregular, ventral margin arched, small and thin; anterior end elongated and narrowly rounded, posterior end broadly rounded; exterior surface with concentric undulations, central area with more or less clear depression; gaped at both ends, more opened at posterior end; umbos small, pointed; hinge plate large in relation to shell size, wide; right valve with two short anterior lateral teeth (AI and AIII), two cardinal teeth (3a and 3b), and two posterior short lateral teeth (PI and PIII), of which the lower one almost merges with the nymph; left valve, with a one short anterior lateral tooth (AII) placed near the anterior cardinal lamellar tooth, and one posterior lateral tooth (PII) almost merged with the lower part of the nymph; chondrophore large, ventrally projected beyond the edge of the hinge plate, anteriorly oriented; adductor muscle scars strongly depressed; pallial sinus not observed.
Occurrence
Upper Sarmatian deposits, upper Middle Miocene of Eastern Europe.
Remarks
Pseudomactra poroschini was reported from shallow-water upper Sarmatian deposits (Iljina et al., Reference Iljina, Nevesskaja and Paramonova1976; Nevesskaja et al., Reference Nevesskaja, Goncharova, Paramonova, Popov, Babak, Bagdasarian and Voronina1993; among others). The late Sarmatian bivalve fauna shows significant differences from the middle Sarmatian fauna, when Chersonimactra and Pseudomactra arose from Sarmatimactra forms (Belokrys, Reference Belokrys1964).
Pseudoxyperas Sacco, Reference Sacco, Bellardi and Sacco1901
Figure 9.8–9.15
Type species
Pseudoxyperas proaspersa Sacco, Reference Sacco, Bellardi and Sacco1901 (p. 27), by original designation. Type material: MRSN BS. 144.04.001, 144.04.002, 144.04.003, BS. 144.04.004, syntypes. Type locality: “Elveziano: Colli torinesi, Baldissero” understood as Turin hills, Baldissero, Italy.
Diagnosis
Shell elliptical, elongated, moderately thick, slightly inflated, large to medium size, length up to 100 mm; anterior and posterior ends rounded but pointed; exterior surface with commarginal cords to smooth growth lines; yellowish to light brown periostracum, with dark spots over the exterior surface in some species; umbos not inflated; hinge plate shortened and narrow, right valve with two fragile cardinal teeth (3a and 3b) and anterior and posterior lateral teeth positioned close to the cardinals; left valve with thin, inverted V-shaped cardinal tooth (2a-2b), and one anterior lateral tooth (AII) and one posterior lateral tooth (PII), both elongated and thin; pallial sinus very deep.
Occurrence
Pliocene of Italy and Lower Eocene–Lower Miocene of New Zealand. Living species of the Indo-Pacific region from India to Japan, New Zealand, and tropical West Africa.
Remarks
The synonymy of Pseudoxyperas, Longimactra Finlay, Reference Finlay1928, and Oxyperas is still open. Keen (Reference Keen, Cox, Newell, Boyd, Branson, Casey and Chavan1969a) mentioned that P. proaspersa has transversely striated lateral teeth, as in Spisula, rather than the smooth lateral teeth of Oxyperas elongatum (Quoy and Gaimard, Reference Quoy and Gaimard1835) (Fig. 9.16–9.19). Beu (Reference Beu2006) suggested that the similarity among genera might reflect homoplasy rather than a close phylogenetic relationship. Examination and comparison of type material of the type species revealed an evident shell shape variability. Oxyperas lentiginosum (Gould, Reference Gould1852) (= Mactra triangularis Lamarck, Reference Lamarck1818, type of Oxyperas), Oxyperas bernardi (Pilsbry, Reference Pilsbry1904). and Oxyperas coppingeri (Smith, Reference Smith1884) show trigonal shells with the anterior and posterior ends pointed, and the exterior surface with conspicuous wrinkles. On the other hand, Oxyperas elongatum (Quoy and Gaimard, Reference Quoy and Gaimard1835) (type of Longimactra, Fig. 9.16–9.19), Oxyperas aspersum (Sowerby I, Reference Sowerby I1825), and Oxyperas egenum (Reeve, Reference Reeve and Reeve1854) show a more convex dorso-posterior area, anteriorly inclined hinge plate, and smoother exterior surface. Finally, Oxyperas bellianum (Oliver, Reference Oliver1915) and Oxyperas transversum (Reeve, Reference Reeve and Reeve1854) show intermediate forms. Oxyperas proaspersum (Sacco, Reference Sacco, Bellardi and Sacco1901), which is the type species of Pseudoxyperas, is similar to trigonal species and was mentioned as living in tropical West Africa (Cosel and Gofas, Reference Cosel and Gofas2019). Future genetic work will confirm or reject the synonymy of all related genera.
Pteroluter Saul, Reference Saul1973
Figure 10.1–10.4
Type species
Pteroluter othnius Saul, Reference Saul1973 (p. 16), by original designation. Type material: LACMIP 10753-1, specimen N° 10109, holotype; paratype: LACMIP 10753-2, specimen N° 10110. Type locality: Melton Place, Little Cow Creek, Shasta County, California, USA.

Figure 10. (1–4) Pteroluter othnius Saul, Reference Saul1973: (1, 2) LACMIP 10753-1, specimen N° 10109, holotype; (3, 4) LACMIP 10753-2, specimen N° 10110, paratype. (5–8) Spisula (Ruellia) bernayi (Cossmann, Reference Cossmann1886), MNHN.F.J07108, 2 specimens, illustrated by Cossmann, 1904, collected from Bartonien deposits of Le Ruel, France (Credit: Recolnat ANR-11-INBS-0004, Peter Massicard 2015). (9, 10) Rugosoxyperas asperaeformis Nomura and Zinbo, Reference Nomura and Zinbo1934, Museum of Tohoku University N° 50396, holotype. (11, 12) Sarmatimactra vitaliana (d'Orbigny, 1844), MNHN.F.R54699, syntype. (13–16) Mactra crassa Hutton, Reference Hutton1884: (13, 14) CMC M.831, holotype, taken from Beu (Reference Beu2004); (15) CMC M.832, paratype, left valve; (16) GS12857, V20/f136, right valve. Scale bars (1–12) 1 cm; (13–16) 2 cm.
Diagnosis
Shell thin, moderately elongated, medium size, tellinoid shape as in Macoma; ends rounded, but posteriorly gaped; exterior surface smooth with concentric growth lines; umbos posteriorly oriented, not prominent, ligament short, placed in an alate nymph; hinge plate with two cardinal teeth (3a and 3b) in the right valve, fragile, lamellar; left valve with inverted V-shaped cardinal tooth (2a-2b) flanked posteriorly by a lamella (4b); no posterior lateral teeth developed; anterior lateral teeth lamellar; chondrophore trigonal; pallial sinus unknown.
Occurrence
Known from Turonian deposits, Upper Cretaceous of western North America.
Remarks
Saul (Reference Saul1973) suggested that species included within Pteroluter are similar to those belonging to Aliomactra. Both genera show elliptical and compressed shells, with smooth surface and alate nymph, but can be distinguished by lacking posterior lateral teeth and shorter anterior lateral teeth in Pteroluter. Currently Pteroluter is monospecific.
Ruellia Cossmann, Reference Cossmann1913
Figure 10.5–10.8
Type species
Mactra bernayi Cossmann, Reference Cossmann1886 (p. 63), by original designation. Type material: not examined; two specimens collected from Bartonian deposits of Le Ruel, France (MNHN.F.J07108) are herein reproduced. Type locality: Le Ruel, France, Bernay collection.
Diagnosis
Shell medium size, elongated, dorso-posterior area convex, dorso-anterior area slightly concave; ventral margin rounded; anterior end widely rounded, posterior end more narrowly rounded; exterior surface smooth with a posterior dorsal carina well observed; umbos moderately inflated; hinge plate narrow, shortened, right valve with two cardinal teeth (3a and 3b), the 3a horizontally oriented, and two short anterior (AI and AIII) and two elongated posterior (PI and PIII) lateral teeth; left valve with inverted V-shaped cardinal tooth, thin, the 2a flanking the trigonal chondrophore, one anterior lateral tooth (AII), and one posterior lateral tooth (PII); pallial sinus U-shaped, deep, ~50% of shell length.
Occurrence
Bartonian deposits of Le Ruel, Eocene of France.
Remarks
Ruellia was described as a subgenus of Mactra (Cossmann, Reference Cossmann1913). Recently, Ruellia has been considered a subgenus of Spisula (Le Renard and Pacaud, Reference Le Renard and Pacaud1995). Currently, this genus includes only the type species, Spisula (Ruellia) bernayi.
Rugosoxyperas Habe, Reference Habe1977
Figure 10.9, 10.10
Type species
Mactra (Spisula) asperaeformis Nomura and Zinbo, Reference Nomura and Zinbo1934 (p. 156), by original designation. Type material: the authors reported only a single left valve deposited at Museum of Tohoku University N° 50396, holotype, type measurements: 32 mm length, 18.5 mm height, 5.5 mm width. Type locality: Pleistocene deposits of the Plateaux area near Kamikatetu, Kikai-zima, Kagoshima Prefecture, Japan.
Diagnosis
Shell elliptical, elongated, compressed, moderately fragile, similar to Oxyperas; anterior and posterior ends rounded; exterior surface with two recognized zones, the anterior zone with concentric wrinkles and grooves near the ventral edge, and the posterior zone defined by scales or granules, stronger on the posterior end; hinge plate wide, left valve with inverted V-shaped cardinal tooth (2a-2b), asymmetrical, with 2a larger that 2b, and very short anterior lateral tooth (AII) and elongated posterior lateral tooth (PII); right valve with two anterior and two posterior lateral teeth; pallial sinus deep, rounded, almost 60% of shell length.
Occurrence
Pleistocene of Japan.
Remarks
Rugosoxyperas was described by Habe (Reference Habe1977) as a subgenus of Oxyperas Mörch, Reference Mörch1853. The subgenus is currently monospecific. Oxyperas (Rugosoxyperas) asperaeformis can be distinguished from the Indo-Pacific Oxyperas (Oxyperas) aspersum (Sowerby I, Reference Sowerby I1825) by having a different posterior sculpture. Additional records of Rugosoxyperas were not found.
Sarmatimactra Korobkov, Reference Korobkov1954
Figure 10.11, 10.12
Type species
Mactra vitaliana d’Orbigny, Reference d'Orbigny1844 (p. 479), by original designation. Type material: MNHN.F.R54699, syntype. Type locality: Doutchina, Bessarabian County, Moldavia, collected by Xavier Hommaire de Hell.
Diagnosis
Shell sub-trigonal to sub-circular, thick, length up to 50 mm; anterior end rounded, posterior end keel-like, weakly defined; exterior surface smooth with concentric growth lines; umbos wide but moderately inflated; hinge plate strong with thick cardinal teeth (2a-2b, 3a and 3b), and strong, short lateral teeth in each valve; chondrophore large, ventrally projected; pallial sinus very shallow; pallial line deeply incised.
Occurrence
Miocene of East Europe.
Remarks
Currently, Sarmatimactra is considered a subgenus of Mactra (Nevesskaja et al. (Reference Nevesskaja, Popov, Goncharova, Guzhov, Janin, Plubotko, Biakov and Gavrilova2013). Ionesi and Tabara (Reference Ionesi and Tabara2004) studied the mollusk fauna of the Scheia Formation, Romania, identifying ~40 taxa including M. (Sarmatimactra) vitaliana. The type species examined herein revealed that Sarmatimactra differs from Eomactra in lacking a defined lunule and escutcheon, and by the fusion of the two left cardinal teeth at half of their height.
Spisulona Marwick, Reference Marwick1948
Figure 10.13–10.16
Type species
Mactra crassitesta Finlay, Reference Finlay1927 (p. 531) (replacement name for Mactra crassa Hutton, Reference Hutton1884; non Turton, Reference Turton1822), by original designation. Although designation of the type species is not affected, the replacement name is unnecessary, therefore the original combination of Hutton is Hemimactra crassa (i.e., no homonym was generated). Type material: Hemimactra crassa, holotype CMC M.831, with 1 paratype, M.832, illustration of Beu (Reference Beu2004) is herein reproduced. Type locality: Wanganui, Nukumaruan, New Zealand.
Diagnosis
Shell medium size, length up to 60 mm, sub-trigonal, thick and solid, moderately inflated, almost equilateral; antero-dorsal margin straight but short, postero-dorsal margin slightly longer; ventral margin strongly convex; exterior surface smooth with concentric growth lines from umbo to ventral edge; umbos not inflated; hinge plate thick, curved; right valve with two anterior (AI and AIII) and two posterior (PI and PIII) lateral teeth, long, thick, transversely striated, and one anterior lateral tooth (AII) and one posterior lateral tooth (PII) in the left valve; cardinal teeth small; chondrophore small, spoon-shaped; adductor muscle scars oval, similar in size and shape; deeply impressed; pallial sinus small, rounded.
Occurrence
Lower Pliocene of the Southern Pacific.
Remarks
Spisula was described by Marwick (Reference Marwick1948) as a subgenus of Spisula. Beu (Reference Beu2004) regarded Spisulona and Notospisula Iredale, Reference Iredale1930, as junior synonyms of Spisula. After examination of the type species of Spisula and Notospisula, their taxonomic placement is not definitive. Spisulona differs from Spisula by having a more trigonal shell outline, an arched and compressed hinge plate, an anterior and posterior dorsal margin straight and downward inclined, and a less-developed pallial sinus. In addition, Notospisula differs from Spisula by having a more trigonal shell with a more pointed dorso-posterior area and by having a shallower pallial sinus. Spisula, Spisulona, and Notospisula are herein considered three valid entities. Spisulona currently includes three species.
Stereomactra Stewart, Reference Stewart1930
Figure 11.1–11.6
Type species
Schizodesma abscissa Gabb, Reference Gabb1866 (p. 20), by original designation. Type material: ANSP 4548, holotype (Stewart, Reference Stewart1930, p. 210–211) (Richards, Reference Richards1968, p. 29). Type locality: Miocene deposits south of Martinez, Contra Costa County, California, USA.

Figure 11. (1–6) Stereomactra abscissa (Gabb, Reference Gabb1866), ANSP 4548, holotype. (7, 8) Stiphromactra welwitschi Böhm, Reference Böhm1929, original illustrations. (9, 10) Tenuimactra hodkinsoni Garvie, Reference Garvie1996: (9) PRI 33074, holotype; (10) PRI 30443, paratype. (11, 12) Thyasira sanctiandreae Maury, Reference Maury1925, PRI 985, holotype. Scale bars (1–6) 4 cm; (9–12) 1 cm.
Diagnosis
Shell trigonal, large, heavy, resembling Scissodesma, but with longer lateral teeth, anterior and posterior ends rounded; dorso-posterior area carinated; external surface smooth with irregular concentric growth lines; umbos prosogyrate, inflated; hinge plate strong, with heavy anterior lateral teeth (AI, AII, and AIII); chondrophore wide and deep; pallial sinus not observed.
Occurrence
Upper Miocene–Pliocene of western North America.
Remarks
Stewart (Reference Stewart1930) described Stereomactra as a subgenus of Spisula, but based on lateral teeth morphology, he suggested that it fits better as subgenus of Mactromeris. Stereomactra has smooth lateral teeth without transversal striae in contrast to the lateral teeth observed in Spisula and Hemimactra. Moore (Reference Moore2003) considered Stereomactra as a valid genus.
Stiphromactra Böhm, Reference Böhm1929
Figure 11.7, 11.8
Type species
Mactra (Stiphromactra) welwitschi Böhm, Reference Böhm1929 (p. 452), by monotypy. Type material: not examined; it is not in the fossil bivalve collections of the BGR. The original illustration is herein reproduced. Type locality: Eocene deposits near Mossámedes, Angola.
Diagnosis
Shell medium size; sub trigonal, inflated; anterior and posterior ends rounded; lunule poorly defined, broad, triangular and slightly concave; exterior surface smooth; outer ligament placed in a nymph over the edge of shell surface; umbos strongly curved; hinge plate wide with very small cardinal teeth, right valve with very small, unfused cardinal teeth (3a and 3b), and two anterior (AI and AIII) and two posterior (PI and PIII) lateral teeth, elongated and lamellar; left valve with inverted V-shaped cardinal tooth (2a-2b) flanking the antero-ventral corner of the trigonal chondrophore; pallial sinus unknown.
Occurrence
Eocene of western Africa.
Remarks
The taxonomic placement of Stiphromactra is not definitive because the type species is missing and cannot be revised. The types possibly were destroyed during World War II. The original illustration suggests a mactroid hinge, but additional specimens must be examined to confirm the taxonomic position. Here, a neotype is needed. Only Stiphromactra welwitschi is included within this genus.
Tenuimactra Garvie, Reference Garvie1996
Figure 11.9, 11.10
Type species
Tenuimactra hodgkinsoni Garvie, Reference Garvie1996 (p. 35), by original designation. Type material: PRI 33074, holotype, a right valve; paratypes: PRI 30443–30446. Type locality: locality 4, bluff on Ridge Creek, south of Missouri, Kansas, and Texas, USA, railroad trestle and county road bridge; bluff on Ridge Creek.
Diagnosis
Shell small, ovate-trigonal, inflated; anterior ends rounded; exterior surface with concentric sculpture; umbos not inflated; hinge plate with a large, inverted V-shaped cardinal tooth (2a-2b) in the left valve, anterior lateral teeth absent, posterior lateral teeth obsolete, chondrophore small, escutcheon defined by an impressed line, pallial sinus not observed.
Occurrence
Eocene of North America.
Remarks
Garvie (Reference Garvie1996) pointed out that outline of Tenuimactra is similar to venerids, but a hinge plate with an inverted V-shaped cardinal tooth places it in Mactridae. In addition, the antero-dorsal margin and the small chondrophore differentiate this genus from other North American fossil mactrids. Tenuimactra hodkinsoni is the only member of this genus.
Trinitasia Maury, Reference Maury1928
Figure 11.11, 11.12
Type species
Thyasira sanctiandreae Maury, Reference Maury1925 (p. 318), by original designation. Type material: PRI 985, holotype. Type locality: station N° 316, at Manzanilla, Trinidad y Tobago.
Diagnosis
Shell trigonal to subcircular, inequilateral, strong, length up to 57 mm; dorsal margin concave in front of umbos, anterior end rounded; escutcheon not defined, ventral margin convex; exterior surface smooth; umbos prosogyrate, inflated; right valve with two cardinal teeth (3a and 3b) unfused and fragile, two anterior lateral teeth (AI and AIII) elongated and similar in size and shape, and two posterior lateral teeth (PI and PIII), the ventral one larger and elongated; left valve with two short lateral teeth (AII and PII), with one cusp, inverted V-shaped cardinal tooth (2a and 2b) flanked by an accessory lamella (4b); trigonal chondrophore, ventrally projected; pallial sinus V-shaped, deep, to ~50% of shell length.
Occurrence
Miocene of Trinidad y Tobago, Venezuela, and Colombia. Also recorded as living along the Gulf and Atlantic coasts of Central and South America.
Remarks
After the advice of Dall, the type species of Trinitasia was originally placed in Thyasira by Maury (Reference Maury1925, p. 318). Later, Maury (Reference Maury1928) recognized it as belonging to the new genus Trinitasia. Subsequent authors had placed Trinitasia in Lucinidae (Chavan, Reference Chavan, Cox and Moore1969; Vokes, Reference Vokes1980). However, Woodring (Reference Woodring1982), based on internal shell characters, confirmed it as belonging to Mactridae.
Willimactra Saul, Reference Saul1973
Figure 12.1–12.3
Type species
Willimactra popenoei Saul, Reference Saul1973 (p. 18), by original designation. Type material: LACMIP 23633-2, holotype, specimen 10115; paratypes: LACMIP 10847-1, specimen 10116; LACMIP 23295-1, specimen 10120; LACMIP 23298-15, specimen 10119; LACMIP 23298-16, specimen 11154; LACMIP 23625-1, specimen 10117; LACMIP 23625-2, specimen 10118. Type locality: Chico Creek, Butte County, California, USA.

Figure 12. (1–3) Willimactra popenoei Saul, Reference Saul1973: (1) LACMIP 23633-2, holotype, specimen 10115; (2, 3) LACMIP 23298-15, specimen 10119, paratype. (4–7) Zenatiopsis angustata Tate, Reference Tate1879: (4, 5) NHMUK PI L. 6613 syntype; (6, 7) NHMUK PI L. 9830, syntype. (8–10) Zenatia (Zenatraria) vellai Beu, Reference Beu1966, taken from Beu (Reference Beu2006), holotype, TM3841, GS1164 re-collection, R22/f6348, right valve. Scale bars (1–3) 1 cm; (4–10) 2 cm.
Diagnosis
Shell medium to large size, elongate, compressed, tellinoid shape; ends rounded; exterior surface smooth with concentric ornamentation; umbo prosogyrate, moderately inflated; gaped posteriorly; hinge plate mactroid, posterior left cardinals (2b) flanked by a thin accessory lamella (4b), chondrophore posteriorly oriented; pallial sinus deep, horizontally oriented; nymph is exclusively external in Willimactra s. s.
Occurrence
Turonian–Maastrichtian, Upper Cretaceous of North America.
Remarks
Saul (Reference Saul1973) described Willimactra and included Petromactra as a subgenus. She pointed out that Willimactra is similar to Aliomactra and Pteroluter in shell outline. However, it can be distinguished from the former by the presence of two posterior lateral teeth in the right valve, and from the latter by having posterior lateral teeth (Saul, Reference Saul1973). Pteroluter and Aliomactra have an opisthogyrate umbo, which was not observed in Willimactra.
Zenatiopsis Tate, Reference Tate1879
Figure 12.4–12.7
Type species
Zenatiopsis angustata Tate, Reference Tate1879 (p. 129), by original designation. Type material: NHMUK PI L. 6613 syntype; NHMUK PI L. 9830, syntype. Type locality: Muddy Creek, Victoria, Australia.
Diagnosis
Shell equivalve, inequilateral, elongated; outline narrower than Zenatia; both ends rounded, gaped; exterior surface smooth, with concentric growth lines; umbo anterior, not inflated, internally supported by a thick umbonal rib; hinge plate with strong cardinal teeth in each valve, left cardinals (2a-2b) fused, inverted V-shaped, without lateral teeth; chondrophore postero-obliquely oriented, well developed; anterior adductor scar close-up under the hinge line as in Zenatia; pallial sinus deep, horizontal.
Occurrence
Miocene–Quaternary deposits of New Zealand.
Remarks
A synopsis on the genus Zenatiopsis was carried out by Gill and Darragh (Reference Gill and Darragh1963) who described new fossil taxa from New Zealand. Currently, Zenatiopsis is considered a valid genus (Beu, Reference Beu2006).
Zenatraria Beu, Reference Beu1966
Figure 12.8–12.10
Type species
Zenatia (Zenatraria) vellai Beu, Reference Beu1966, by original designation. Type material: holotype articulated pair TM3841; paratype consisting of articulated valves from Mangatuatau Stream, inland Manawatu, Nukumaruan (GS2773, T23/f6485) and Hautawa Shellbed, Lower Nukumaruan, Hautawa Road, north of Hunterville, Rangitikei Valley (GS3096, T22/f8492). Illustration of Beu (Reference Beu2006) is herein reproduced. Type locality: Nukumaru Brown Sand (Nukumaruan), Nukumaru Beach, 50 m east of Nukumaru fault plane, coast of Wanganui, New Zealand.
Diagnosis
Shell large, strongly inflated, dorsal area flattened behind the umbos, moderately gaped at both ends; posterior end rounded, anterior end ventrally truncated; exterior surface smooth with concentric growth lines; umbos more prominent than in Zenatia s. s.; hinge plate with posterior arm of the inverted V-shaped cardinal tooth (2b) flanking the chondrophore, which is trigonal and ventro-posteriorly inclined, no lateral teeth; anterior adductor scar oval, posterior adductor scar circular; pallial sinus depth, ~60% of shell length, broad, U-shaped.
Occurrence
Upper Pliocene–Lower Pleistocene of New Zealand.
Remarks
Beu (Reference Beu1966) described Zenatraria as a subgenus of Zenatia. However, Beu (Reference Beu2006) recently regarded Zenatraria as junior synonym of Psammophila Brown, Reference Brown1827, and concluded that the morphology of the chondrophore, pedal retractor, and hinge plate is more similar to Zenatia species than to Lutraria species, referring Zenatia to the subfamily Lutrariinae.
Discussion
This catalogue listed the mactrid genera based exclusively on fossil type species. The evolution of hinge structure in mactrids has been discussed by Saul (Reference Saul1973). The oldest records of mactrid genera with poorly developed resilifer are from the Aptian, Albian, and Cenomanian stages in the Cretaceous of North America. Currently, only eight genera have been described from Cretaceous deposits (Vokes, Reference Vokes1946; Stephenson, Reference Stephenson1952; Saul, Reference Saul1973).
A different scenario is observed during the Paleogene and Neogene. several taxa were described from Eocene deposits in North, Central, and northern South America (Cossmann and Peyrot, Reference Cossmann and Peyrot1909; Clark and Durham, Reference Clark and Durham1946; Garvie, Reference Garvie1996; among others). In Europe, the family Mactridae has a large number of taxa during the Miocene. Burdigalian deposits from northern Italy and France include the genera Allomactra and Pseudoxyperas from the Tethys. In the Paratethys, the number of species increased considerably. Sarmatian and Akschagylian Mactridae have almost 100 names. However, after a few revisions, the number of valid species has been restricted to a few dozen (Sidorova, Reference Sidorova1959a, Reference Sidorovab, Reference Sidorova1960a, Reference Sidorovab; Belokrys, Reference Belokrys1964). In several cases, the number of valid taxa is far from resolved or the type material of type species are lost or destroyed.
A full revision of Paratethys mactrids is needed to understand the evolution of not only Akschagylian species (Pliocene), but also Sarmatian (Middle–Upper Miocene) taxa. This complete revision, currently in progress, will determine if designation of neotypes is necessary. Neogene mactrids include genera, based on fossil type species, that include both living and species in some cases.
Fossil genera described for southern South America, Asia, or Africa are scarce in the literature. Currently a full revision of fossil Argentinean Mactridae is in progress, and descriptions of new taxa at genus level are under evaluation. This work is part of a global revision of the superfamily Mactroidea that will elucidate the evolutive history of the group, and constitutes the basis for future revisions related to fossil taxa of the family Mactridae from different regions.
Acknowledgments
I would like to acknowledge all the curators, technicians, and additional people who assisted me in the revision of type materials and additional reproduced specimens. They are W. Etter and S. Kühni (Natural History Museum Basel), M. Goodwin and D. Strauss (UCMP), L.L. Skibinski (PRI), J. Nemoto (Tohoku University Museum), J. Sessa (ANSP), J. Todd (NHMUK), A. Sokolov (TSNIGR Museum), M. Florence (USNM), A. Beu (GNS Science, Wellington, New Zealand), and A. Pistarino (MRSN, Turin). Special thanks to S. Schneider, S. Nielsen, and S. Popov for their comments on an earlier version of the manuscript. This work was partially supported by grant PICT-2019-03433 of the Agencia Nacional de Promoción Científica y Técnica. This is the contribution N° 172 of Larbim.
Declaration of competing interests
Javier H. Signorelli from CONICET declares none.















