Introduction
Middle East respiratory syndrome (MERS) is a highly fatal respiratory tract disease in humans that was first detected in 2012 in the Kingdom of Saudi Arabia (KSA) [Reference Zaki1]. After its first detection, MERS-coronavirus (MERS-CoV) was being reported in human patients across the Arabian Peninsula, with occasional travel-related cases in other continents. As of the end of March 2018, a total of 2189 human laboratory-confirmed cases from 27 countries have been reported to the World Health Organisation (WHO), including 782 associated deaths [2]. Dromedary camels (Camelus dromedaries) have been shown to be the natural reservoir from where spill-over to humans can occur [Reference Haagmans3, Reference Reusken4]. Human-to-human infection is also reported frequently, especially in healthcare settings [Reference Hui5]. Sustained human-to-human transmission outside of hospital settings has not been shown yet [6]. Direct or indirect human contact with camels has resulted in repeated introductions of MERS-CoV into the human population [Reference Dudas7]. It has been suggested that camels may have acquired MERS-CoV from a spill-over event from a bat reservoir, but evidence for that remains inconclusive [Reference Anthony8]. Infections with MERS-CoV generally are thought to be mild or inapparent in camels [Reference Chu9], and are therefore of low economical or animal welfare significance.
This systematic review was done to compile and analyse all published data on MERS-CoV in the global camel population to provide an overview of current knowledge on the distribution, spread and risk factors of MERS-CoV infections in dromedary camels as a basis for the design of intervention and control measures to prevent human infections.
Material and methods
On 2 May 2018, a literature search on PubMed was performed, using the terms ‘middle east respiratory syndrome coronavirus’ and ‘MERS-CoV’. Using the term ‘MERS’ did not result in any additional articles that fit the scope of this review. Only articles published in English were included. Two reviewers individually selected all original research articles containing laboratory evidence of MERS-CoV infections in dromedary camels in the field. Articles that were mentioned in Food and Agriculture Organization (FAO) updates [10] or in the references of included publications, but did not appear in the PubMed search were added. Subsequently, abstracts, follow-up studies of MERS-CoV-positive camels and genome studies without prevalence data were excluded from the analysis. Data on variables such as year of sampling, country, region, age, sex and animal origin were extracted and analysed. For each variable, the number of positive camels, total number of camels tested and the median percentage positivity was calculated. Data from experimental infection studies were not included in this analysis, but they were included in the review to provide additional information and context to the field studies. Additional information on the distribution and trade of dromedary camels was collected from references in the publications on MERS-CoV in camels and extracted from official FAO and World Organisation for Animal Health (OIE) databases [11, 12]. The additional literature on camel trade was collected in a less systematic way from PubMed.
Results
Literature search
The literature search resulted in a total of 53 papers (Fig. 1). Forty-three research papers described the results of cross-sectional studies in dromedary camel populations, six papers described outbreak investigations, including an analysis of camel samples, and four papers described longitudinal studies. In total, 33 papers describe camel studies in the Middle East, 13 studies investigated camels from Africa and the remaining seven surveys were from Spain, Australia, Japan, Bangladesh and Pakistan (Table 1).
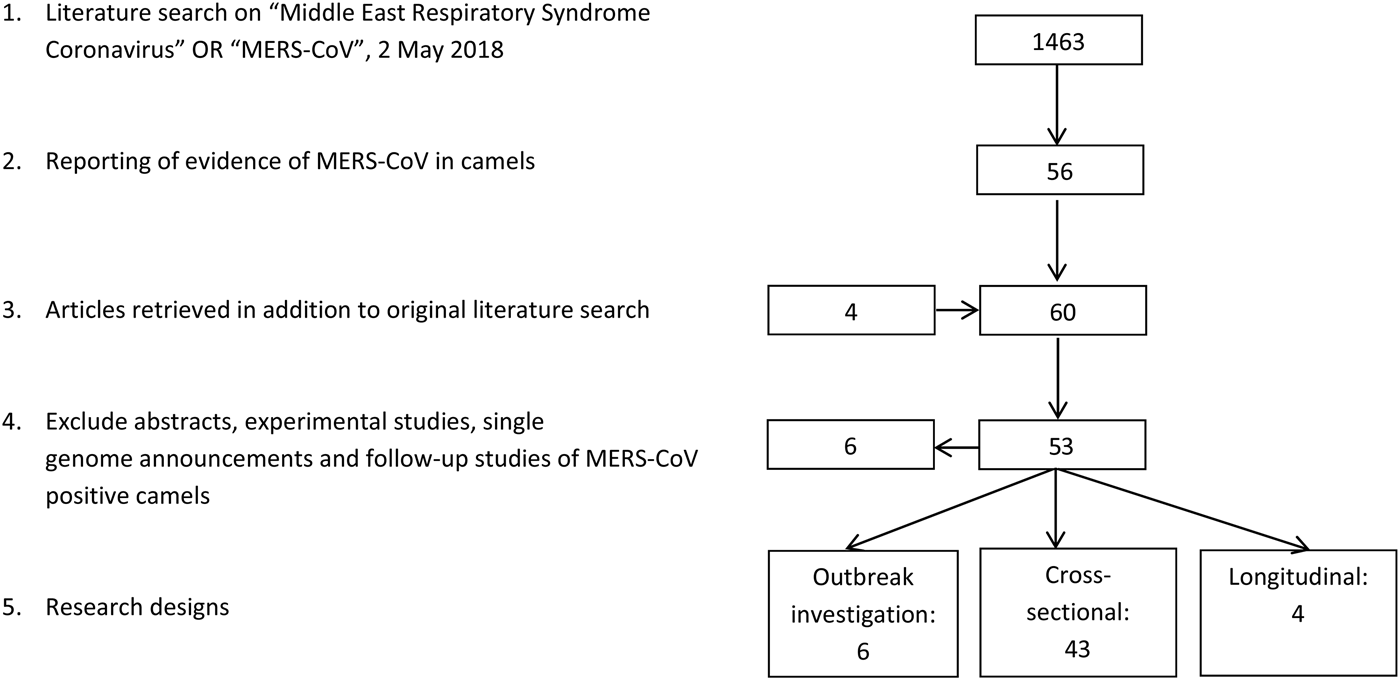
Fig. 1. Results literature search.
Table 1. Summary table of included papers
Distribution and trade of camels
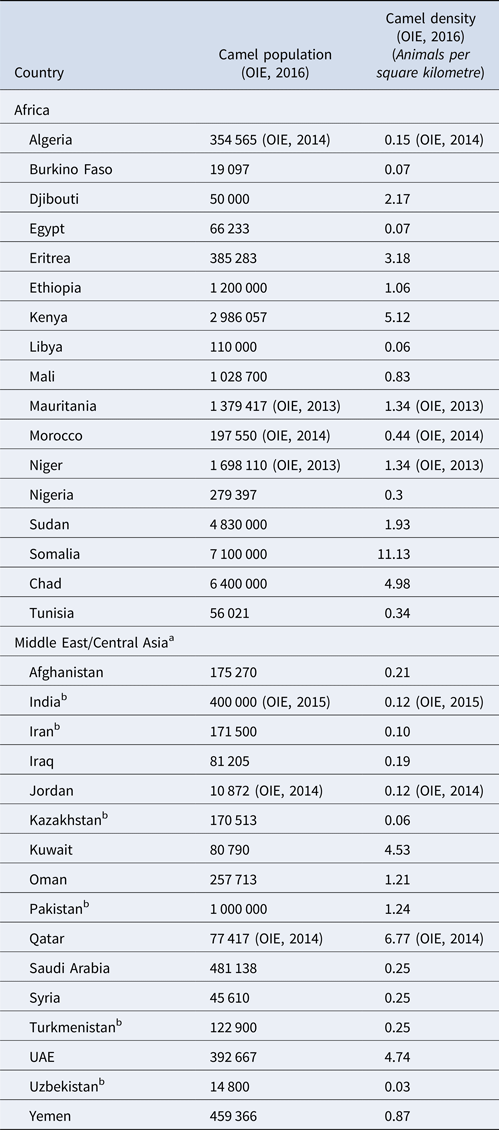
Most recent FAO statistics estimate the world population of camel to be around 29 million [11], of which approximately 95% are dromedary camels [Reference Faye13]. However, it is believed that the true population size is even larger due to inaccurate statistics and feral camels, such as the feral dromedary camel population in Australia that is estimated to be around 1 million [Reference Saalfeld and Edwards14]. Over 80% of the camel population lives in Africa. The main camel countries are Chad (6 400 000), Ethiopia (1 200 000), Kenya (2 986 057), Mali (1 028 700), Mauritania (1 379 417), Niger (1 698 110), Sudan (4 830 000), Somalia (7 100 000) and Pakistan (1 000 000) [12] (Table 2).
Table 2. Camel population and density
a Excluding China and Mongolia because the large majority of camel population are Bactrian camels.
b Camel population exists of both dromedary and Bactrian camels[Reference Ming66].
A large number of camels are being transported from the Horn of Africa to the Middle East each year. These are mainly meat camels coming from the east of Africa going to Egypt, Libya and the Gulf states, and Sudanese camels that are being imported into the Middle East to participate in camel racing competitions [Reference Younan, Bornstein and Gluecks15]. For example, the FAO reported that Somalia exported 77 000 camels in 2014 [16]. The largest camel market in Africa is the Birqash market near Cairo (Egypt), where camels from Sudan and Ethiopia are most common, but trade routes include animals from Chad, Somalia, Eritrea and Kenya [Reference Ali17]. Imported camels are usually quarantined for 2–3 days at the border before they are allowed to enter Egypt [Reference Ali17]. Most Somali and Sudanese camels that are exported to the KSA are shipped from the ports of Berbera and Bosaso in North Somalia to the KSA ports of Jizan and Jeddah [Reference Younan, Bornstein and Gluecks15].
Clinical and pathological features of MERS-CoV infections in dromedary camels
In general, only minor clinical signs of disease have been observed in animals infected with MERS-CoV and most MERS-CoV infections do not appear to cause any symptoms [Reference Chu9]. Disease symptoms that have been described after experimental and field infections are coughing and sneezing, respiratory discharge, fever and loss of appetite [Reference Adney18–Reference Khalafalla20]. Although MERS-CoV RNA can be detected in several organs after experimental infection, in studies of natural infectious virus it has only been detected in the tissues of the upper and lower respiratory tract and regional lymph nodes of the respiratory system in part of the infected camels. Histologically, a mild-to-moderate inflammation and necrosis could also be seen on the upper and lower respiratory tract. No viral antigen or lesions were detected in the alveoli. Histopathological examination showed that the nasal respiratory epithelium is the principal site of MERS-CoV replication in camels [Reference Adney18, Reference Haagmans21].
Virus shedding and antibody response
In one study investigating experimental infection of camels, MERS-CoV shedding started 1–2 days post-infection (dpi). In that study, infectious virus could be detected until 7 dpi, and viral RNA until 35 dpi in nasal swab samples and, in lower amounts, in oral swab samples [Reference Adney18]. No infectious virus or viral RNA was detected in faecal or urine samples [Reference Adney18]. Viral RNA detection in nasal, but also rectal swabs of camels after experimental infection until day 14, has been confirmed in a recent vaccine study [Reference Haagmans21].
In the field surveys included in this review, MERS-CoV RNA has been described in rectal swab samples, although other field studies report negative results [Reference Haagmans3, Reference Sabir22–Reference Hemida24] and when viral RNA can be detected, the positivity rate of rectal swabs is lower compared with nasal swab samples [Reference Hemida19, Reference Woo25–Reference Ali27]. Oral swabs are usually negative or show a lower positivity rate even when nasal swabs test positive for MERS-CoV RNA [Reference Haagmans3, Reference Hemida19, Reference Farag26]. Some studies have reported MERS-CoV RNA in milk samples [Reference Ali27, Reference Reusken28]. Longitudinal studies of camel herds show that PCR results of nasal swabs can remain positive after 2 weeks [Reference Ali27, Reference Muhairi29]. When an interval of sampling of 1 or 2 months was maintained, nasal swabs become negative for viral RNA in the next sampling round [Reference Hemida24, Reference Meyer30].
MERS-CoV infections have also been detected in camels with MERS-CoV antibodies, both in calves with maternal antibodies as well as older camels that had already acquired antibodies from a previous infection. However, virus replication and thus the virus load is generally lower in infected seropositive animals compared with seronegative camels [Reference Hemida19, Reference Haagmans21, Reference van Doremalen23, Reference Hemida24, Reference Meyer30, Reference Alagaili31].
Little is known about the longevity of antibody titres after infection from longitudinal studies. A study following camels on a closed farm found that neutralizing antibodies remained consistent during a year [Reference Meyer30], while other studies found that antibody titres rapidly drop by 1–4-fold within a period often as short as 2 weeks [Reference Hemida24, Reference Ali27].
Worldwide distribution of MERS-CoV in dromedary camels
The first evidence of MERS-CoV in camels described so far is the detection of antibodies to MERS-CoV in camel sera from Somalia and Sudan from 1983 of which 81% tested positive [Reference Muller32]. Additional serological evidence of the widespread presence of MERS-CoV infection in camels, included in this review, has been found in 18 additional countries: Bangladesh, Burkina Faso, Egypt, Ethiopia, Iraq, Israel, Jordan, Kenya, KSA, Mali, Morocco, Nigeria, Oman, Pakistan, Qatar, Spain, Tunisia and the UAE (Fig. 2). In addition, Promed mail reported that virus-positive camels had been found in Kuwait and Iran, the latter reportedly in imported animals (Archive number 20140612.2534919 and 20141029.2912385). In 11 countries, serological findings were complemented with the finding of viral RNA in dromedary camels: Burkina Faso, Egypt, Ethiopia, Iraq, Jordan, KSA, Morocco, Nigeria, Oman, Qatar and the UAE. Investigations of MERS-CoV circulation amongst dromedary camels in Australia, Japan, Kazakhstan, USA and Canada did not find any proof of MERS-CoV circulation. All countries where MERS-CoV circulates in the camel population, with the exception of Spain (Canary Islands), Pakistan and Bangladesh, are located in the Middle East or Africa [Reference Reusken4, Reference Gutierrez33]. One out of 17 camels that had MERS-CoV antibodies in Bangladesh was born in Bangladesh, 16 others were imported from India [Reference Islam34]. However, there have not been any additional reports of MERS-CoV in camels in India. There is no record of foreign origin of the seropositive camels from Pakistan [Reference Saqib35]. Moreover, in previous studies there had already been evidence of seropositive camels that originate from Pakistan [Reference Meyer37, Reference Crameri58].
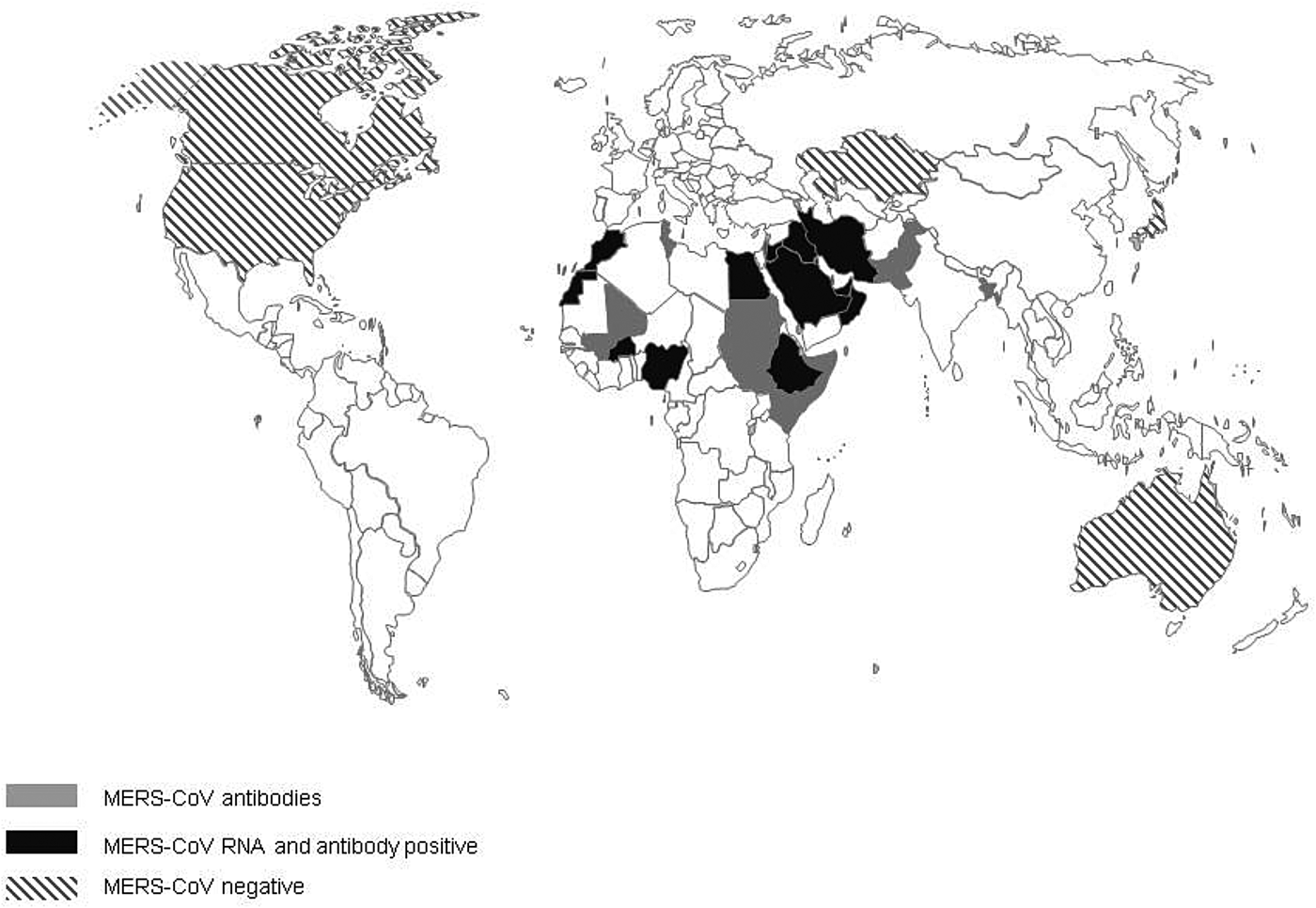
Fig. 2. Virological and serological evidence for MERS CoV in dromedary camels.
When combining serology data from all papers included in this review, the overall median seroprevalence of camels in Africa is 81% (6106/8526; range 28–98%), compared with a median seroprevalence of 93% (3230/3846; range 53–100%) in camels from the Middle East. Based on viral shedding studies from African countries, the median rate of viral shedding was 5% (1108/6318; range 1–15%), compared with 12% in camels from the Middle East (1191/14902; range 0–100%).
Risk factors of MERS-CoV in dromedary camels
Age
The seroprevalence of MERS-CoV antibodies increases with age in camels, while the fraction of camels that test positive for MERS-CoV RNA in their nasal swabs decreases with age [Reference Ali17, Reference Alagaili31, Reference Corman36, Reference Kasem38, Reference Miguel39]. When all serological results of papers that included sufficient age information is combined, the median seroprevalence of camels aged under 2 years is 52% (992/1972; range 0–100%), while the age groups 2–5 years (702/924; range 30–100%) and over 5 years old (1226/1370; range 0–100%) had a combined median seroprevalence of 97%. In the virological studies reporting age breakdown, the median rate of nasal shedding in 0–2 years old camels was 34% (718/2612; range 0–100%) of cases, compared with 2% (91/1142; range 0–100%) in camels older than 2 years.
Sex
Some individual studies show a significantly higher seroprevalence in female camels compared with males [Reference Ali27, Reference Miguel39], while others show the opposite [Reference Kasem38] or do not find any significant difference [Reference Ali17, Reference Saqib35]. Similar disagreeing results are published for the presence of MERS-CoV RNA in male vs. female camels [Reference Ali17, Reference Ali27, Reference Kasem38, Reference Miguel39].
In the studies in this review where sex of camels was recorded, a total of 4810 serum samples from female camels and 3458 samples from male camels were collected and analysed for MERS-CoV antibodies, compared with 2007 vs. 2505 nasal swabs for viral RNA testing. Approximately three times more female camels were sampled at farms, while male camels were in the majority in studies that looked at MERS-CoV prevalence of camels at slaughterhouses, live animal markets and quarantine areas. The overall median seroprevalence of male and female camels in our review is 50% and 67%, respectively (range 0–100%; excluding results from Israel and Kazakhstan). The median percentage of presence of viral RNA is 18% in nasal swabs of male camels (range 0–21%) compared with 9% in female camels (range 0–100%), in our review.
Sampling location and herd characteristics
In several studies, camels that were sampled at animal markets or quarantine facilities were seropositive more often than camels at farms [Reference Ali17, Reference Sabir22, Reference Ali27, Reference Islam34]. Combining serological laboratory results of camels in our review with sufficient background information with regard to the sampling location does not result in the same pattern, with a median seroprevalence of 84% (5632/8115; range 0–100%; excluding Australia and Spain) in camels from farms and 80% (943/1005; range 28–98%) in the camel population sampled at markets and quarantine facilities. Studies in Egypt found a significantly higher PCR positivity rate in camels sampled in abattoirs or quarantine facilities, but these results could not be confirmed by other papers in this review [Reference Ali17, Reference Ali27].
When comparing differences in seroprevalence or virus RNA-positive rate in nomadic vs. sedentary camel herds, some authors did not find a statistical difference between the two herd management types [Reference Miguel39, Reference Deem40], while others found some evidence of higher seroprevalences in nomadic herds [Reference Ali27, Reference Corman36]. One study in Kenya looked at the differences between herds with different levels of isolation, and did not find significant differences in MERS-CoV antibody levels [Reference Deem40].
Animal origin
Most studies that compared local camels with imported camels suggested that imported camels are seropositive for MERS-CoV more often [Reference Chu9, Reference Ali17, Reference Ali27, Reference Islam34, Reference Yusof41], although not all differences were significant.
Two studies in Egypt found a significantly higher RNA positivity rate in imported camels from East Africa compared with domestically bred camels [Reference Ali17, Reference Ali27], while another study executed in the KSA found a significantly higher number of MERS-CoV RNA-positive results amongst local camels vs. camels from Sudan and Somalia [Reference Sabir22].
Seasonal variation in MERS-CoV circulation in the camel population
Although MERS-CoV was detected almost year-round in camels, some studies show a relatively higher seroprevalence and viral detection during the cooler winter months [Reference Ali17, Reference Khalafalla20, Reference Ali27, Reference Kasem38].
MERS-CoV in non-dromedary animals
MERS-CoV antibodies have been detected in llamas and alpacas in Israel and in alpacas in Qatar [Reference Reusken42, Reference David43]. To date, no MERS-CoV antibodies or viral RNA have been detected in Bactrian camels [Reference Reusken4, Reference Meyer37, Reference Chan44–Reference Shirato47] (Table 1 and Table 3). Swine, goats and horses that were included in the field surveys in our review all tested negative for MERS-CoV RNA and antibodies [Reference Reusken4, Reference Ali17, Reference Alagaili31, Reference Perera48–Reference Meyer52]. MERS-CoV antibodies were detected in two studies in sheep in Egypt and Qatar, although in very low numbers [Reference Ali17, Reference Reusken51]. However, most surveys that investigated sheep did not find evidence of MERS-CoV infection or exposure [Reference Reusken4, Reference van Doremalen23, Reference Muhairi29, Reference Alagaili31, Reference Islam34, Reference Perera48–Reference Reusken51, Reference Falzarano53].
Table 3. MERS-CoV in non-dromedary animals in the field
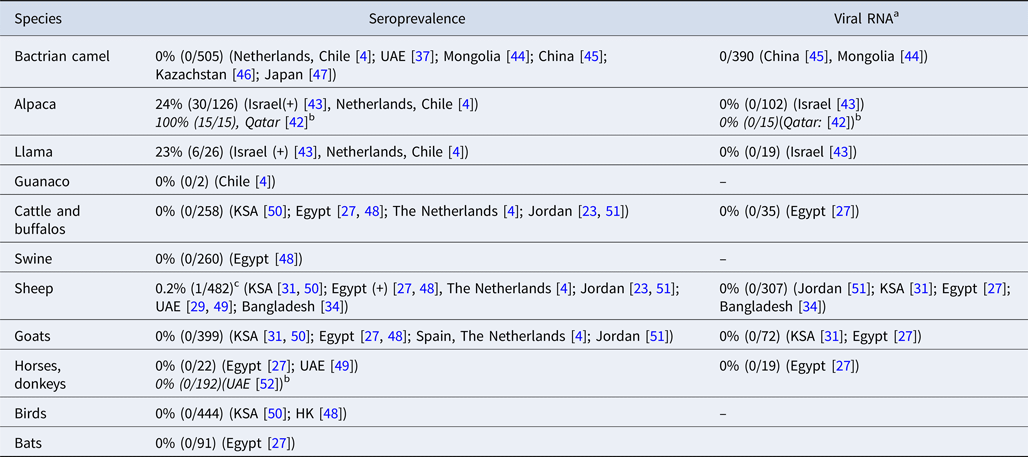
a MERS-CoV RNA in nasal swabs.
b Articles that were not included in the original literature search, because no camels were investigated in these studies.
c Six additional sera from sheep in Qatar tested positive by protein microarray (pMA), but could not be confirmed by NT.
Discussion
The publications in this review show that the MERS-CoV mainly circulates in dromedary camel populations in the Middle East and part of Africa, and has been infecting dromedary camels in Africa for more than three decades. Antibodies have also been found in Arabic camel sera from the early 90s [Reference Alagaili31, Reference Muller32]. However, MERS-CoV was discovered until 2012, after the first human cases appeared [Reference Zaki1], which is probably due to the minor clinical symptoms of MERS-CoV infections in camels [Reference Adney18]. Most camel surveys were conducted in the Middle East and some northern and eastern African countries, but significant data gaps currently still exist in the north and west of Africa, in countries that have camel populations of 100 000 to more than a million animals, such as Algeria, Libya, Mauritania and Niger. Even less is known about the central Asian region. Some evidence of MERS-CoV circulation in camels of Pakistan and Bangladesh was recently published, but data is lacking from Afghanistan and India. Knowledge on the presence of MERS-CoV in the animal reservoir is a crucial first step to assess whether MERS-CoV could be a relevant public health threat in these regions.
MERS-CoV infections are mainly detected in calves and young camels [Reference Meyer30, Reference Alagaili31]. The research included in this review shows that the IgG positivity rate increases gradually in dromedary camels of increasing age while the MERS-CoV RNA detection rate decreases. Maternal IgG antibodies in camels are acquired through the intake of colostrum during the first 24 h post-parturition. After 24 h, antibody levels in the dam's milk decrease rapidly [Reference Kamber54]. One study showed that maternal antibodies in calves peak at 7 days post-parturition and decline in the following 6 months. After 5–6 months, over half of the calves did not have maternal neutralizing antibodies in their serum any longer [Reference Meyer30]. However, in other field studies, the titre of MERS-CoV-specific antibodies is still low at 1 month of age and increases with age in dromedary calves [Reference Ali27, Reference Wernery55]. A lower or undetectable antibody levels in young camels is likely to explain the higher MERS-CoV RNA detection rate. In adult camels, a much higher MERS-CoV seroprevalence can be found, which is probably due to a long-lasting immune response against a MERS-CoV infection or multiple re-infections with MERS-CoV. Immunity is not sterilizing, as MERS-CoV infection and shedding have also been shown in adult camels that have MERS-CoV antibodies [Reference Hemida19, Reference Haagmans21, Reference van Doremalen23, Reference Hemida24, Reference Meyer30, Reference Alagaili31].
Several articles have analysed seroprevalence and virus shedding data in relation to factors, other than age, that may explain differences in seroprevalence and MERS-CoV RNA-positive rate in camels, such as sex, sampling location, herd characteristics and animal origin. Our review shows that there is considerable heterogeneity in results. In addition, comparison between studies is difficult given the lack of standardisation of study designs. A key factor to consider when comparing studies is the difference in distribution of male and female camels amongst different disciplines of camel husbandry. Females are mainly used for milking and reproduction. As a result, they often stay at farms. Male camels, especially of young age (<1 year old), are the predominant sex in slaughterhouses and amongst camels used for transport [Reference Miguel39, Reference Faye56]. This also influences the risk profile of acquiring a MERS-CoV infection. Female camels are in closer contact with calves, who are more susceptible to infection and shed virus in higher quantities compared with older camels [Reference Meyer30]. On the other hand, meat and transport camels (predominantly male) travel more, leading to increased contact with other camels and camel herds, and therefore a higher chance of exposure to MERS-CoV. Some papers in this review suggest that there is a generally lower infection rate of domestically bred camels and camels on farms compared with imported camels and camels on animal markets or in quarantine facilities. This may be explained by the same increased contact rate and mixing of camel herds, leading to an increased chance of MERS-CoV exposure and spread.
The increase in MERS-CoV circulation in winter and spring can have multiple explanations. Firstly, the winter is the calving season [10], which leads to a larger proportion of young animals that usually have a higher number of MERS-CoV infections and virus excretion. Moreover, in winter season, there is a major increase of camel and human movements due to camel racing competitions, camel breeding, trading and movements to grazing grounds, which increases the chance of virus spread. Additionally, cooler temperatures may facilitate coronavirus survival in the environment [Reference van Doremalen, Bushmaker and Munster57].
In experimental studies, llama's and alpaca's are shown to be susceptible to infection with MERS-CoV [Reference Crameri58, Reference Vergara-Alert59], which was confirmed by two papers in our review, describing serologically positive llamas and alpacas in Israel and alpacas with MERS-CoV neutralizing antibodies in Qatar [Reference Reusken42, Reference David43]. In experimental settings, animal-to-animal transmission has been shown for alpacas, making them a possible risk population for human infections [Reference Crameri58]. Two studies in our review also found anti-MERS-CoV antibodies in sheep [Reference Ali17, Reference Reusken51] but experimental inoculation of sheep did not result in MERS-CoV replication or antibody development [Reference Vergara-Alert59, Reference Adney60]. However, the DPP4 receptor, the entry receptor for MERS-CoV, is present in sheep tissues, making it possible for the virus to bind to the sheep respiratory tract which may explain the finding of MERS-CoV antibodies [Reference van Doremalen61]. Pigs also express the DPP4 receptor in their respiratory tract, and viral replication in experimental settings has been shown for pigs, but no antibodies or MERS-CoV RNA have been found in pigs during field surveys [Reference Perera48, Reference Vergara-Alert59]. This may be explained by the limited viral shedding in pigs and the absence of animal-to-animal transmission [Reference Vergara-Alert62, Reference de Wit63].
We show that dromedary camels are present in large parts of the African and Asian continent, and that MERS infections in dromedary camels are widespread. However, human infections due to spill-over from the dromedary camel reservoir have not been reported in Africa [10]. Several explanations for the difference in human cases between the Arabian Peninsula and Africa have been suggested, such as differences in cultural habits, camel husbandry, prevalence of comorbidities, under detection or genetic factors in the local population [Reference Liljander64]. Moreover, West African viruses were found to be phylogenetically and phenotypically distinct from the MERS-CoV viruses that caused human disease in the Middle East [Reference Chu65].
Increased knowledge on the animal reservoir of MERS-CoV needs to be combined with research on MERS prevalence and risk factors in humans to assess the true public health risk. Moreover, the absence of human disease, combined with the mild symptoms in camels, caused by MERS, will likely have a negative effect on the willingness to implement interventions and the cost-effectiveness of possible interventions in some areas.
Conclusion
Since the discovery of MERS-CoV in 2012, the dromedary camel has been identified as the animal reservoir of human infections with the MERS-CoV. However, the exact route of human primary infections is still unknown. Moreover, the scale of the spread and prevalence of MERS-CoV in the camel reservoir is not fully known yet since there is still a lack of MERS-CoV prevalence data in some countries that harbour a very significant proportion of the world camel population. However, knowledge of the animal reservoir of MERS-CoV is essential to develop intervention and control measures to prevent human infections. Prospective studies that include representative sampling of camels of different age groups and sex, within the different husbandry practices, are needed to fully understand the patterns of MERS-CoV circulation. Such studies are important as they may give more information on critical control points for interventions to reduce the circulation of MERS-CoV and/or exposure of humans.
Author ORCIDs
R. S. Sikkema, 0000-0001-7331-6274
Financial support
This study was financially supported by the European Commission's H2020 programme under contract number 643476 (http://www.compare-europe.eu/).
Conflict of interest
None.







