Introduction
Recording changes in population status and identifying anthropogenic factors responsible for species declines are fundamental for evaluating potential risks to biodiversity and developing effective conservation management actions (Segan et al., Reference Segan, Bottrill, Baxter and Possingham2011; Turvey et al., Reference Turvey, Risley, Moore, Barrett, Hao and Zhao2013; Bland et al., Reference Bland, Bielby, Kearney, Orme, Watson and Collen2017). However, it can be difficult to obtain direct information on such parameters for species of conservation concern, particularly in geographical regions that have large human populations but little focus on formal scientific research or monitoring of exploitation (Turvey et al., Reference Turvey, Trung, Quyet, Nhu, Thoai and Tuan2015). One such case is the limited population research conducted on the Chinese horseshoe crab Tachypleus tridentatus and mangrove horseshoe crab Carcinoscorpius rotundicauda in China, despite China being largely responsible for driving the recent decline in horseshoe crab populations across Asia (Liao & Li, Reference Liao and Li2001; Weng et al., Reference Weng, Xie, Xiao, Huang, Li, Wang and Zhang2012; Gauvry, Reference Gauvry, Carmichael, Botton, Shin and Cheung2015). Tachypleus tridentatus occurs in the Seto Inland Sea of Japan, along the coast of China, and southwards to Borneo and Sumatra in Indonesia, whereas C. rotundicauda is commonly recorded in Indonesia, Malaysia, Singapore, the Philippines, Thailand, Cambodia, Vietnam and the Bay of Bengal in India (Sekiguchi, Reference Sekiguchi1988). Horseshoe crabs are ecologically important in coastal ecosystems, serving as both prey and predators, hosts for epibionts, and bioturbators for sediment–water nutrient exchanges (Botton et al., Reference Botton, Shuster, Keinath, Shuster, Barlow and Brockmann2003; Botton, Reference Botton, Tanacredi, Botton and Smith2009). They also have high commercial value in the manufacture of Tachypleus amoebocyte lysate, which is used in the testing of bacterial contamination in biomedical products (Walls et al., Reference Walls, Berkson and Smith2002; Gauvry, Reference Gauvry, Carmichael, Botton, Shin and Cheung2015). Despite being listed as Key Protected Aquatic Wildlife in four provinces of China, T. tridentatus populations are heavily exploited to meet the increasingly high demands for Tachypleus amoebocyte lysate production and local food consumption (Liao et al., Reference Liao, Hong and Li2001; Hong, Reference Hong2011; Gauvry, Reference Gauvry, Carmichael, Botton, Shin and Cheung2015). Other factors contributing to the depletion of horseshoe crab populations include the destruction of their spawning shores as a result of coastal reclamation, and marine pollution, as reported in Taiwan (Hsieh & Chen, Reference Hsieh, Chen, Carmichael, Botton, Shin and Cheung2015), Hong Kong (Kwan et al., Reference Kwan, Hsieh, Cheung and Shin2016) and Japan (Seino et al., Reference Seino, Uda, Tsuchiya and Tsuchiya2003). Carcinoscorpius rotundicauda populations in Hong Kong and Singapore are also decreasing, mainly as a result of the loss of mangrove and mudflat habitats (Shin et al., Reference Shin, Li, Cheung, Tanacredi, Botton and Smith2009; Cartwright-Taylor et al., Reference Cartwright-Taylor, Von Bing, Chi and Tee2011).
Tachypleus tridentatus and C. rotundicauda are currently categorized as Data Deficient on the IUCN Red List (WCMC, 1996a,b), but based on the available evidence it is likely that their populations will decline further because their protection is a low priority for local governments (Bland et al., Reference Bland, Bielby, Kearney, Orme, Watson and Collen2017). In general, public awareness of the conservation of marine invertebrates is low (Cardoso et al., Reference Cardoso, Borges, Triantis, Ferrández and Martín2011). There is an urgent need for up-to-date information on the distribution and population trends of Asian horseshoe crabs in China, and the threats they face, to protect remaining populations across their distribution range. To this end, a broad approach that facilitates rapid, inexpensive and wide-scale collection of population data must be developed. An important means of addressing information gaps regarding species conservation and management, especially in data-poor situations, is the collection of local knowledge (Segan et al., Reference Segan, Bottrill, Baxter and Possingham2011; Nash et al., Reference Nash, Wong and Turvey2016; Pan et al., Reference Pan, Wei, Cunningham, Li, Chen, Milner-Gulland and Turvey2016). This so-called Wisdom of Crowds technique is based on a mechanism that aggregates knowledge of a large sample population to generate quantitative estimates and spatial reasoning (Whitmore, Reference Whitmore2016). Although the application of this method is relatively new, the merits of the approach, including its independence, diversity of opinion, decentralization and aggregation, are recognized as of value for informing natural resource management and conservation (Surowiecki, Reference Surowiecki2004; Arlinghaus & Krause, Reference Arlinghaus and Krause2013). Previous studies have found that fishers’ perceptions about population trends matched scientific data reasonably well in some circumstances (Rochet et al., Reference Rochet, Prigent, Bertrand, Carpentier, Coppin and Delpech2008; Daw et al., Reference Daw, Robinson and Graham2011; Arlinghaus & Krause, Reference Arlinghaus and Krause2013). In the case of Asian horseshoe crabs, local community members, especially fishers, may be able to report the historical and recent status of a species as well as underlying drivers of species decline (sensu Ainsworth et al., Reference Ainsworth, Pitcher and Rotinsulu2008).
We conducted a study in three coastal cities along Beibu Gulf in south-western China to obtain data on the potential distribution of spawning/nursery grounds of T. tridentatus and C. rotundicauda, and anthropogenic threats to the populations, through community interviews. Beibu Gulf is considered to be the most critical spawning/nursery ground for Asian horseshoe crab populations, particularly T. tridentatus (Hu et al., Reference Hu, Wang, Chen, Cheung, Shin and Li2009; Li, Reference Li2010). We verified our findings on the distribution of the two species by surveys of intertidal habitats for juvenile populations. Such findings can strengthen our understanding of the extent to which local knowledge can assist in the conservation of Asian horseshoe crabs in locations where these species are under intense anthropogenic pressure.
Study area
Community interviews were conducted during 19 September–1 October 2015 in 30 fishing villages and small towns around the three coastal cities (Fangchenggang, Qinzhou and Beihai) along Beibu Gulf in Guangxi Zhuang Autonomous Region, China (Table 1). Beibu Gulf is a semi-closed gulf located in the northern South China Sea between China and Vietnam. The Gulf is 330 km wide at its widest and has a total area of 128,000 km2 (Chen et al., Reference Chen, Xu, Qiu, Lin and Jia2009). It has extensive estuarine ecosystems, with considerable freshwater input from seven major rivers (Fangcheng, Qin, Dafeng, Nanliu, Red, Beilun, Changhua; Chen et al., Reference Chen, Gu and Gao1991). The rivers carry a significant load of nutrients from the land, which are essential for phytoplankton growth, which in turn supports the rich biodiversity and productive fisheries in the northern South China Sea (Yu & Mu, Reference Yu and Mu2006).
Table 1 Villages and towns along the shores of Beibu Gulf in Guangxi Zhuang Autonomous Region, China (Fig. 1), where interview surveys were conducted to gather local knowledge about the Asian horseshoe crabs Tachypleus tridentatus and Carcinoscorpius rotundicauda, with the number of responses collected at each location.
Both T. tridentatus and C. rotundicauda are recorded frequently in mangrove-dominated estuaries with patchy seagrass beds and extensive sandy mudflats along the coastline of Beibu Gulf (Hu et al., Reference Hu, Wang, Chen, Cheung, Shin and Li2009; Chen et al., Reference Chen, Yang, Fan, Qiu, Liao and Hsieh2015). In general, horseshoe crabs require three types of coastal habitats during their entire life cycle: adults nest on open, sandy beaches near the high water mark or along the edge of mangrove forests, juveniles forage and grow within the intertidal zones of the same shores or their adjacent flats, and subadults and adults inhabit shallow subtidal areas (Chen et al., Reference Chen, Yang, Fan, Qiu, Liao and Hsieh2015; Smith et al., Reference Smith, Brockmann, Beekey, King, Millard and Zaldívar-Rae2017). During the spawning season local people encounter adult C. rotundicauda mating pairs in amplexus burrowing in the mud along the edges of mangrove forests. Mating pairs of T. tridentatus are occasionally entangled in fishing nets cast along the coastline to harvest fish when the tide recedes.
Methods
The 30 villages and towns were chosen for the interviews because sightings of horseshoe crabs on the beaches nearby had been reported in the grey literature. Respondents were intercepted at random as the survey party walked through each village. We aimed to survey people of both sexes and of various ages, to capture potentially diverse knowledge and experience of horseshoe crabs across the sampling locations. Only one person per household was surveyed, to minimize the chance of double counting the same information from various family members. We interviewed 400 people in total, and the number of respondents from each village varied (4–27 individuals), depending on people's willingness to participate in the interviews. Interview methods followed the guidelines of the American Sociological Association, including informing all respondents at the outset about the study's general aims, assuring them that the data would be anonymized, and conducting the interviews only after participants had given verbal consent. Interviews were conducted by research team members and student helpers, all of whom were native Mandarin Chinese speakers. There was also at least one team member present during each visit who could speak local dialects. All respondents were interviewed one-to-one using a standard anonymous questionnaire in simplified Chinese characters, containing descriptive and multiple-choice questions in three sections: present occurrences, population trends and threats, and fishing activities targeted at horseshoe crabs (Supplementary Material). Respondents could choose more than one answer for questions regarding the horseshoe crab species observed, perceived causes of the population decline, and fishing methods used for harvesting adult crabs (Supplementary Material). To improve the accuracy of the information collected, responses were included in the analysis only if the respondent had seen a horseshoe crab in the wild. The respondents who could not read or write in simplified Chinese were assisted by the researchers through oral communication in either Mandarin Chinese or native dialects.
As horseshoe crabs may utilize sandy mudflats as their spawning and/or nursery grounds, the potential shores indicated by the respondents were considered to be a mixture of spawning and nursery grounds. Based on the information provided by the respondents, and given budget constraints, a total of 10 potential spawning/nursery shores were chosen at random and verified by field surveys conducted at each site in March and June 2016. The sites were chosen to cover at least two potential spawning/nursery grounds from each of the three coastal cities in Beibu Gulf. In Southern China, summer is the best season to conduct juvenile population surveys, as the crabs feed actively in the intertidal habitats after prolonged hibernation in winter and early spring (Chiu & Morton, Reference Chiu and Morton2004; Hu et al., Reference Hu, Wang, Chen, Cheung, Shin and Li2009). During each field visit a team of five trained researchers walked at random and searched for juveniles within the intertidal area, 1.6–2.0 m above chart datum (i.e. the lowest astronomical tide as defined by the UK Hydrographic Office) for 120 minutes. The total monitoring area for each shore was 62,300–77,100 m2. Juvenile T. tridentatus were distinguished from C. rotundicauda by the triangular cross section of their telson, as well as the presence of three distinct immovable spines above the telson insertion and three spines growing along the central ridge on the dorsum of the opisthosomal carapace (Chen et al., Reference Chen, Yang, Fan, Qiu, Liao and Hsieh2015; Hu et al., Reference Hu, Kwan, Wang, Cheung, Shin, Carmichael, Botton, Shin and Cheung2015).
Statistical analysis was performed using SPSS v. 16.0 (SPSS, Inc., Chicago, USA), after data had been checked for normality and homogeneity of variance using the Shapiro–Wilk and Levene's tests, respectively. If these tests failed, data were logarithmically transformed. An independent samples t-test was applied to analyse the difference in the age of respondents who had or had not observed mating pairs of horseshoe crabs on shores, and who had or had not eaten adult T. tridentatus before.
Results
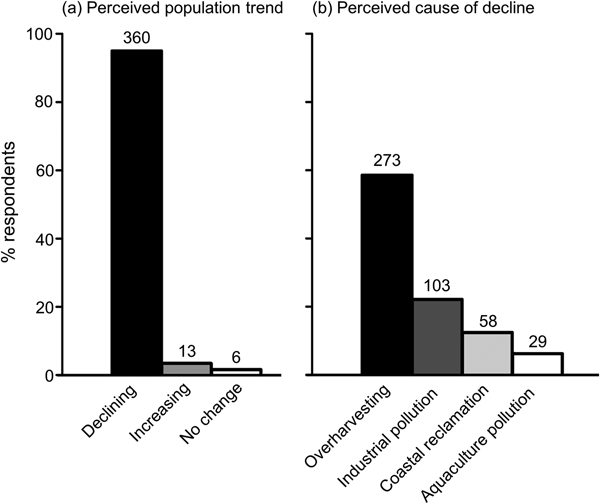
We interviewed 325 men and 75 women, with a mean age of 53.3 ± SD 16.2 years (range 15–99). According to their responses, juvenile and/or mating pairs of horseshoe crabs had been observed at a total of 45 sites (Fig. 1a). Field monitoring confirmed the presence of juvenile T. tridentatus and/or C. rotundicauda at all 10 of the sites selected for monitoring (Fig. 1b). These sites were previously believed to be spawning/nursery grounds for T. tridentatus only; however, juvenile C. rotundicauda were recorded at five of the sites, along with juvenile T. tridentatus (Fig. 1a, b). This may reflect the difficulty for non-experts of distinguishing juvenile C. rotundicauda from T. tridentatus. Only T. tridentatus were found at Zhakou, and only C. rotundicauda were found at Shankou during the two field visits (Fig. 1b). Considering our findings along with the previously published population data (Liang, Reference Liang1985; Hu et al., Reference Hu, Wang, Chen, Cheung, Shin and Li2009, Reference Hu, Kwan, Wang, Cheung, Shin, Carmichael, Botton, Shin and Cheung2015; Chen et al., Reference Chen, Yang, Fan, Qiu, Liao and Hsieh2015), there are 19 known nursery sites for juvenile Asian horseshoe crabs in Beibu Gulf (Fig. 1b). This is a conservative number, as more spawning/nursery grounds may be confirmed by field surveys. Respondents who had seen a mating pair of horseshoe crabs on the shore were significantly older (59.9 ± SD 13.7 years) than those who had not (48.0 ± SD 16.2 years; df = 329, t = 7.172, P = 0.000). Ninety-five percent of the respondents agreed that there had been a population decline among horseshoe crabs in Beibu Gulf in the previous 5 years, 3.4% stated that the population had increased, and 1.6% did not perceive any obvious change (Fig. 2a). Only one respondent could provide a specific location where there had potentially been an increase in the population.
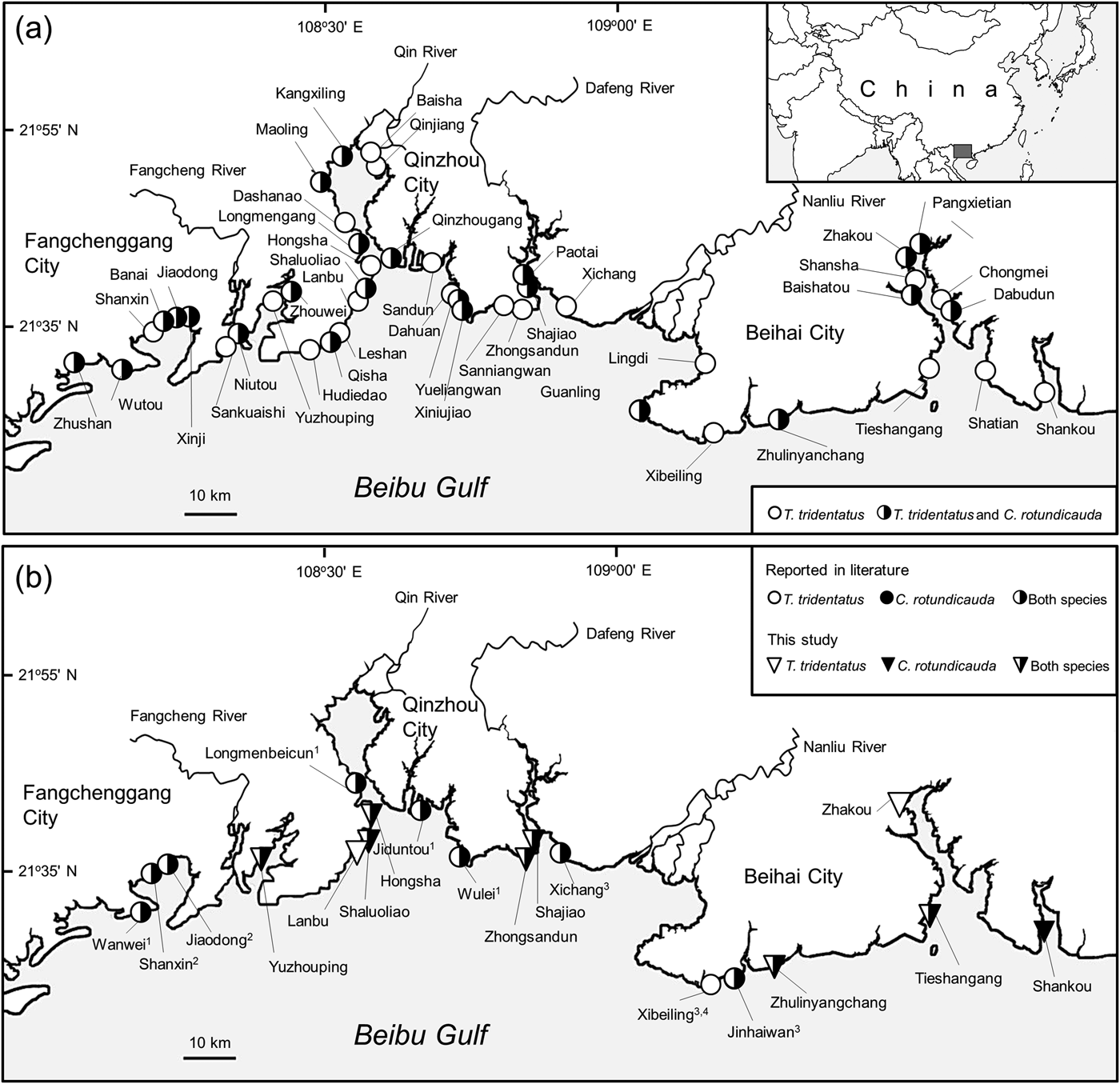
Fig. 1 (a) Locations of potential spawning/nursery grounds of the Asian horseshoe crabs Tachypleus tridentatus and Carcinoscorpius rotundicauda in Beibu Gulf, Guangxi, China, as indicated by interviews with local people in 30 villages and towns along the coast, and (b) locations of spawning/nursery grounds of T. tridentatus and C. rotundicauda reported in the literature (1, Liang, Reference Liang1985; 2, Chen et al., Reference Chen, Yang, Fan, Qiu, Liao and Hsieh2015; 3, Hu et al., Reference Hu, Kwan, Wang, Cheung, Shin, Carmichael, Botton, Shin and Cheung2015; 4, Hu et al., Reference Hu, Wang, Chen, Cheung, Shin and Li2009) and confirmed by field monitoring in this study.
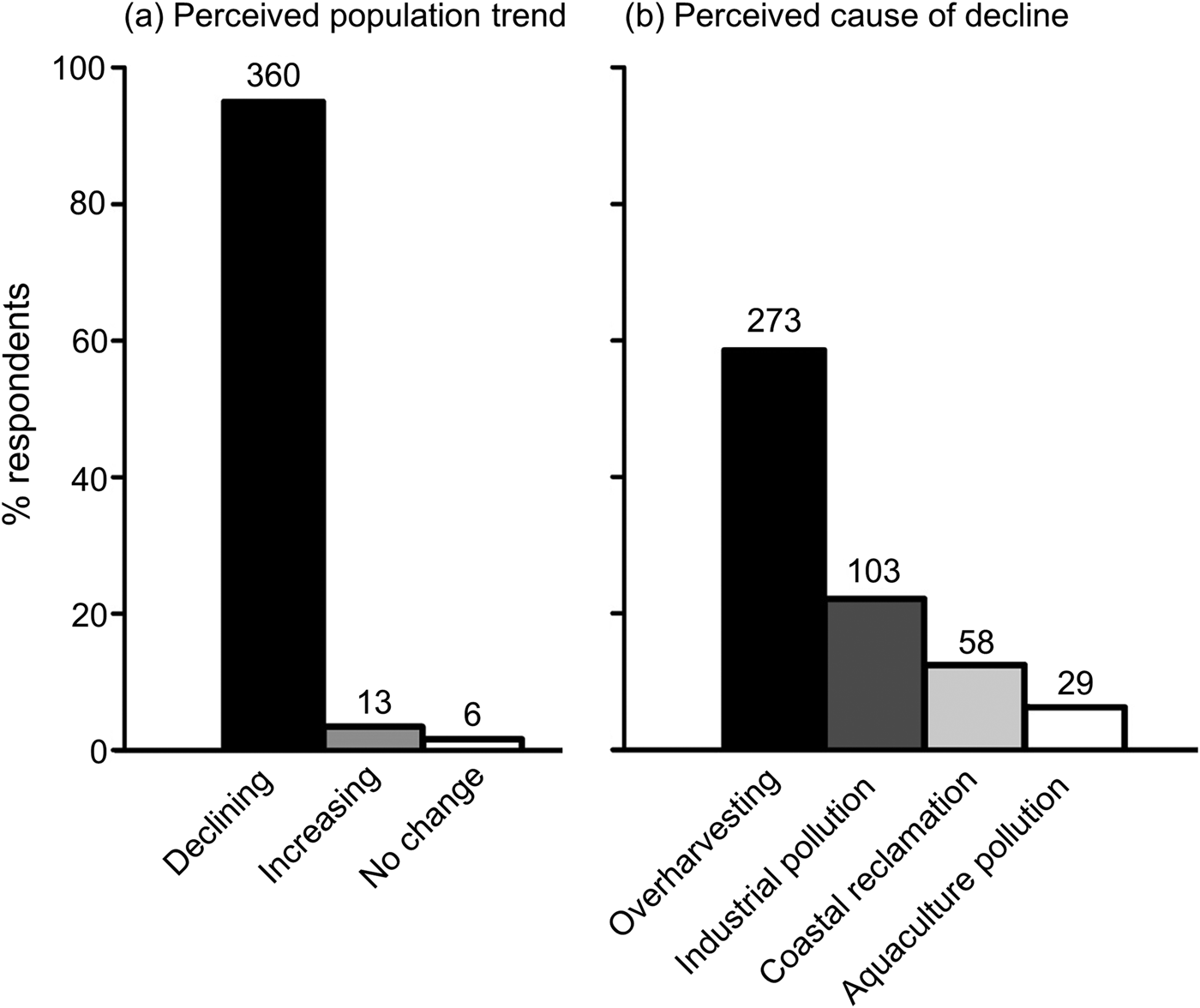
Fig. 2 (a) Respondents’ perceptions of the population trend of adult horseshoe crabs in Beibu Gulf, China (Fig. 1), and (b) perceived causes of population decline. The number of responses is indicated above the bars.
Respondents attributed the decline of horseshoe crabs to overharvesting (75.8%), followed by industrial pollution (28.6%) and coastal reclamation (16.1%; Fig. 2b). Mass harvesting of adult T. tridentatus had been witnessed by 74.1% of respondents (n = 223). The methods used to catch horseshoe crabs included bottom trawling (36.9%), gill-netting (31.1%) and purse-seine fishing (29.0%). According to the respondents’ observations, 69.9% of T. tridentatus harvested were adults or subadults (prosomal width > 10 cm), and 23.8% were 10th-instar or larger juveniles. The mean daily catch rate during 2011–2016 as estimated by the interviewees was 0–30 individuals (n = 144 respondents), which was significantly lower compared to the mean catch rate in the 1990s, which was 50–1,000 individuals per day (n = 35). Respondents reported that the consumption of adult T. tridentatus was common practice (95.2%; n = 378). The majority (96%; n = 382) considered T. tridentatus to be edible, although 71% (n = 115) had heard of food-poisoning incidents caused by other horseshoe crab species. There was no statistical difference between the age of respondents who habitually ate horseshoe crabs and those who did not (df = 385, t = −0.122, P = 0.903).
Discussion
In mainland China horseshoe crabs occur in the south of the Yangtze River Delta, along the coastline of Shanghai, Fuzhou, Xiamen and Shantou Cities, and southwards to Leizhou, Beibu Gulf and Hainan Island (Liao et al., Reference Liao, Hong and Li2001). Beibu Gulf is believed to accommodate the highest density of T. tridentatus globally (Liao & Li, Reference Liao and Li2001; Brockmann & Smith, Reference Brockmann, Smith, Tanacredi, Botton and Smith2009; Weng et al., Reference Weng, Xie, Xiao, Huang, Li, Wang and Zhang2012). According to the grey literature and other information sources, Asian horseshoe crabs were probably distributed extensively over all mudflats, beaches and shallow waters in Beibu Gulf at least until the 1990s. In the 1980s T. tridentatus production could reach 0.4–1.4 million individuals per year (Liang, Reference Liang1985; Liao & Li, Reference Liao and Li2001). Despite the species’ unusually high occurrence in Beibu Gulf, information about its abundance and distribution is limited. The first quantitative data on the daily harvest of T. tridentatus and C. rotundicauda on five shores within Beibu Gulf were reported in 1985 (3–7 pairs; Liang, Reference Liang1985). During 2008–2009, Hu et al. (Reference Hu, Kwan, Wang, Cheung, Shin, Carmichael, Botton, Shin and Cheung2015) discovered three previously unknown nursery grounds of T. tridentatus and C. rotundicauda (Fig. 1b), with a juvenile density of 0.88–3.19 individuals per 100 m2. Chen et al. (Reference Chen, Yang, Fan, Qiu, Liao and Hsieh2015) found two more new nursery grounds on the west coast of Beibu Gulf, and recorded a juvenile density of 0.02–0.25 individuals per 100 m2. By gathering local knowledge from 30 fishing communities, we identified 45 potential spawning/nursery grounds, distributed widely along the shores of Beibu Gulf, 10 of which were confirmed by surveys. This suggests that local knowledge is useful and effective in identifying potential habitats for juvenile T. tridentatus and C. rotundicauda.
Previous studies have reported declining trends in populations of Asian horseshoe crabs along the coastline of Zhejiang, Fujian, Guangdong, Guangxi and Hainan provinces (Liao & Ye, Reference Liao and Ye2000; Li & Hu, Reference Li and Hu2011; Weng et al., Reference Weng, Xie, Xiao, Huang, Li, Wang and Zhang2012). It is estimated at least a million pairs of adult T. tridentatus are exploited each year to satisfy the demand for consumption and biomedical applications (Liao & Ye, Reference Liao and Ye2000; Li & Hu, Reference Li and Hu2011), and these are mainly imported from Vietnam. Such intense harvesting pressure in addition to habitat destruction may have driven horseshoe crab populations to the edge of extinction. Our interview results indicated that the horseshoe crab population in Beibu Gulf has declined substantially: (1) nearly all interviewees reported an overall reduction in the abundance of horseshoe crabs in the previous 5 years; (2) older respondents were more likely to have seen horseshoe crab mating pairs on the shores than younger respondents; and (3) the respondents reported a mean daily catch rate of 0–30 adult T. tridentatus, which is a significant decline compared with 50–1,000 individuals per day in the 1990s. The reduction in population abundance was attributed mainly to unsustainable fishing practices. Our findings are consistent with previous reports of T. tridentatus decline (Liao & Li, Reference Liao and Li2001; Huang et al., Reference Huang, Lin, Gao, You and Lai2003; Weng et al., Reference Weng, Xie, Xiao, Huang, Li, Wang and Zhang2012), and indicated that most people in the region, regardless of their age, consumed adult T. tridentatus, despite the species being listed as Key Protected Aquatic Wildlife in Guangxi province. In general, local people avoid consuming adult C. rotundicauda and unlaid eggs, following reports of food poisoning caused by the presence of saxitoxin and tetrodotoxin (Shumway, Reference Shumway1995; Liao et al., Reference Liao, Liu and Zhou2012). However, the cultural norm of consuming horseshoe crab as a delicacy may impede efforts to motivate local communities to conserve the remaining T. tridentatus population in Beibu Gulf. Long-term and local-based conservation education programmes (e.g. raising laboratory-cultured juvenile horseshoe crabs at secondary schools, as described by Kwan et al., Reference Kwan, Cheung, Law, Cheung and Shin2017) are useful in promoting positive changes in students’ behaviour and attitudes towards horseshoe crab conservation. Such participatory educational activities, supported by public awareness programmes, could have a significant influence in deterring local communities from eating horseshoe crabs.
As the problems of increasing human disturbance and overharvesting are expected to persist, there is an immediate need for a management strategy for T. tridentatus conservation. At the 2012 IUCN World Conservation Congress, a motion for the conservation of Asian horseshoe crabs was passed unanimously, with a recommendation encouraging members to identify critical habitats and to protect them using appropriate integrated marine and coastal management approaches (IUCN, 2012). However, territory-wide distribution surveys of Asian horseshoe crabs in China would be costly and require significant effort, particularly given limited baseline data. Gathering local knowledge could be of particular use in countries or areas where conservation budgets are insufficient to support well-structured quantitative monitoring (Whitmore, Reference Whitmore2016). Follow-up confirmatory field surveys, as described here, have the potential to attract funding for further studies. Given that both T. tridentatus and C. rotundicauda are categorized as Data Deficient, the Wisdom of Crowds method could be used to collect data and facilitate assessment of the species’ conservation status. Fishing communities identified the possible extent of occurrence for both species, which are distributed widely along the shores of Beibu Gulf. However, despite their widespread occurrence, there is a perception among fishers that horseshoe crab populations are in decline, based on their direct observations of mating pairs on the shores, and the catch rate of adult T. tridentatus offshore. The causes of the decline, including unsustainable fishing, industrial pollution and coastal reclamation, may be irreversible, and the available evidence should be sufficient to urge the provincial Chinese authorities to implement management conservation actions, particularly to ensure sustainable use of horseshoe crab resources by fishers and the Tachypleus amoebocyte lysate industry. Other recommendations for local authorities include (1) reducing illegal trade of horseshoe crabs; (2) promoting establishment of protected areas for horseshoe crabs; (3) implementing effective management and restoration of breeding/nursery habitat; (4) strengthening research and conservation capacity; and (5) raising public awareness of horseshoe crab conservation. Regarding the increasing need for cheap and rapid population assessment tools, Wisdom of Crowds methods are an overlooked but potentially valuable tool for conservation, particularly in the Asia–Pacific region, where conventional field survey methods are largely unaffordable and human disturbance of horseshoe crab populations is intense.
Acknowledgements
This project was funded by the National Natural Science Foundation of China (41466003), Guangxi Natural Science Foundation (2015GXNSFDA139016), Guangxi Marine Science and Technology Fund (GXZC2015-G3-3692-GXJX), Guangxi Key Laboratory of Beibu Gulf Marine Biodiversity Conservation, Qinzhou University (2015ZA02) and Guangxi Colleges and Universities Innovation Research Team. We are grateful to the students from the Ocean College of Qinzhou University for their assistance in conducting community interviews and field surveys.
Author contributions
YL led the research project, developed the overall research framework and methods, compiled and clustered data for analysis, and prepared the article. SX, QZ, JL, ML, HF, LX, WL and XX conducted community interviews and field population surveys. HLH and CPC provided input on local context to ensure the research framework and methods were relevant. SGC conducted statistical analysis and prepared the figures. BKYK supervised the research to ensure it met scientific requirements. HLH, CPC, SGC and BKYK reviewed and edited the article, which was read and approved by all authors.
Biographical sketches
Y. Liao studies the population biology of Asian horseshoe crabs in Southern China. H. L. Hsieh and C. P. Chen work on habitat conservation and population restocking of Chinese horseshoe crabs in Taiwan and Southern China. S. Xu, Q. Zhong, J. Lei, M. Liang, H. Fang and L. Xu conduct ecological studies of Chinese white dolphins, mangroves and fishery resources along the shores of Beibu Gulf in Guangxi, China. W. Lin and X. Xiao are involved in mobilizing local community participation in horseshoe crab and migratory bird conservation. S.G. Cheung participates in research and education outreach projects on the conservation of horseshoe crabs in Hong Kong. B. K. Y. Kwan is interested in studying how ecological and social information facilitates wildlife conservation, with a particular focus on conservation of Chinese horseshoe crabs.