Introduction
Women with gestational diabetes mellitus (GDM) are at an increased risk of developing type-2 diabetes mellitus (T2DM) later in life, compared to women without GDM, with approximately 50% developing diabetes within 10 years [Reference Bellamy, Casas, Hingorani and Williams1]. Asian Indians stand at higher risk for earlier conversion to diabetes, compared to Caucasians. Studies from India, including our own recent data have found high conversion rates to T2DM within 5 years of delivery [Reference Gupta, Kapoor, Desai, Praveen, Joshi, Rozati, Bhatla, Prabhakaran, Reddy, Patel and Tandon2–Reference Mahalakshmi, Bhavadharini, Kumar, Anjana, Shah, Bridgette, Choudhury, Henderson, Desborough, Mohan and Ranjani5].
The development of T2DM can be prevented or delayed by lifestyle intervention and/or medical treatment in women with previous GDM [Reference Guo, Chen, Whittemore and Whitaker6–Reference Peacock, Bogossian, McIntyre and Wilkinson8]. The aim of this study was to investigate the feasibility of lifestyle intervention in women with GDM after delivery, and provide preliminary evidence of effectiveness in Indian women. It is important to note that this pilot study is not powered to determine the effects of intervention on clinical outcomes; but the results will substantially aid in estimating the required sample size, for a subsequent appropriately designed controlled study in South Asia, the information of which has been lacking so far in this region. This is important, as both prevalence of GDM and its conversion to T2DM are high in South Asia [Reference Guo, Chen, Whittemore and Whitaker6–Reference Peacock, Bogossian, McIntyre and Wilkinson8].
Methods
This is a single-arm pilot pre- and post-intervention study of a 6-month group lifestyle programme delivered at the hospital. The study was carried out at two large centres in India (All India Institute of Medical Sciences, New Delhi and MHRT-Hospital and Research Trust, Hyderabad) from 2011 to 2013. Women with a history of GDM underwent postpartum screening for glycaemic status with 75 g oral glucose tolerance test [9]. Individuals were classified as being normoglycaemic, having prediabetes or diabetes as per American Diabetes Association criteria [9]. The results for the diagnostic phase of this study, showing high rates of dysglycaemia and diabetes post-partum, have been published earlier [Reference Gupta, Kapoor, Desai, Praveen, Joshi, Rozati, Bhatla, Prabhakaran, Reddy, Patel and Tandon2]. These were considered as pre-intervention samples. Post intervention samples were obtained at end of intervention (6 months). The study was approved by ethics committees at both centres and written informed consent was obtained from all patients. The study has been performed in accordance with the ethical standards laid down in an appropriate version of the Declaration of Helsinki (as revised in Brazil 2013).
Women with normoglycaemia or prediabetes at post-partum testing (mean 18.7 months following delivery) were eligible and invited for intervention phase of the study. The intervention was based on the Greater Green Triangle (GGT) Diabetes Prevention Project which was designed for prevention of type-2 diabetes by lifestyle intervention in an Australian primary healthcare setting [Reference Laatikainen, Dunbar, Chapman, Kilkkinen, Vartiainen, Heistaro, Philpot, Absetz, Bunker, Neil, Reddy, Best and Janus10]. It was contextualised to the target population (i.e. recent mothers) in consultation with local health professionals and advisors. The components of the programme were designed using the Health Action Process Approach (HAPA) model and self-regulation theory, and intervention was delivered in six sessions. Facilitators along with theme-appropriate experts conducted these sessions.
Intervention details
One manual for the participant and two supporting manuals for facilitators on content and conduct of the sessions were prepared. Participant manuals were translated in three local languages – Hindi, Urdu and Telugu. Facilitators along with theme-appropriate experts conducted these sessions. Sessions one through five were held every 2 weeks, and session 6 was held at end of intervention (6 months).
Women recruited in the study followed the manual which outlined structured activities session-by-session. These activities were designed using adult learning principles and were suitable for women with low health literacy. The programme comprised of six 2-h sessions, with 10–15 participants in each group and focused on physical activity, nutrition, eating behaviours, motivation and barriers to lifestyle change. Activities in sessions one and two focused on risk perception, self-efficacy and outcome formation, while sessions three and four developed individual goals and plans for physical activity, diet and eating behaviour (including demonstration of fat, sugar, and fibre content in common foods and alternatives). Session five had problem-solving activities that addressed motivation, lapses, barriers and other factors that affect attainment of individual goals. Session six focused on strategies for maintenance of behaviour change post-intervention. Participants were guided to set action plans to achieve desired changes in small steps, implying that participants who completed the programme gained knowledge through interactive learning and hands-on activities. They set individual goals and action plans, and worked out solutions to common barriers through facilitated group discussion and problem-solving. The underlying application of risk perception, outcome expectation, self-efficacy, and goal setting in the programme was adapted for cultural factors.
Participants completed a structured interview to provide demographic data. The following measurements were also taken at baseline and at the end of the intervention period [6 months]: weight, height, waist circumference, body mass index (BMI), and blood pressure (BP).
Measurement details
Weight was measured with a portable weighing scale with a capacity of 125 kg and sensitivity of 0.5 kg was used. It was placed on a level surface and checked for zero-balance before every measurement. The subjects were weighed barefooted with minimum clothing, facing straight ahead and body weight distributed evenly on both the feet to obtain accurate results. The participant was weighed in kilograms to the nearest 0.1 kg (100 g). Height was measured with a wall mounted Holtain's stadiometer (Holtain Ltd, Crymych, UK) with the measurement corrected to the nearest 1 mm. BMI was calculated from measurements of height and weight as per formula (BMI = weight in kg/height in metres square). Waist circumference was measured at the end of normal expiration at the midpoint between the iliac crest and the lower edge of ribs in the mid-axillary line with the patient standing erect with abdomen relaxed, feet 25–30 cm apart, weight evenly distributed, arms loosely at their side. Measurement is corrected to the nearest 0.1 cm (WHO method of waist circumference measurement). BP was measured in the right upper limb in sitting position with a mercury sphygmomanometer after 5 min rest with an appropriate size cuff. BP was measured three times and the mean was taken. Systolic BP was taken as the point at which the Korotkoff sound appeared and diastolic BP at the point of disappearance of Korotkoff sounds.
Statistical analysis
Statistical analysis was carried out using Stata 11.0 (College Station, Texas, USA). Data are presented as mean ± s.d. or median (inter-quartile range) as appropriate for continuous variables. Normality of the variable distribution was established using skewness and kurtosis normality test before using parametric tests. The baseline characteristics for women who participated in the intervention to those who did not were compared using χ2 test (categorical variables), Student's t test (continuous and normally distributed variables) or Wilcoxon rank sum test (continuous but not normally distributed variables). Difference between paired observations (pre- and post-intervention) was assessed using paired t test for normally distributed variables and Wilcoxon sign rank test for variables not distributed normally. Significance levels were set at p < 0.05.
Results
Participant characteristics
Out of 103 women who were invited, 56 (54.4%) consented to participate in the study. The mean age and BMI (±s.d.) of the participants were 30.8 (±5.1) years and 20.7 (±3.4) kg/m2 respectively. Women who participated in the group intervention were older, were screened earlier postpartum and were more often prescribed insulin during pregnancy compared to the women who did not (Table 1).
Table 1. Baseline characteristics of invited women, and comparison among those who participated and who did not participate in lifestyle intervention programme
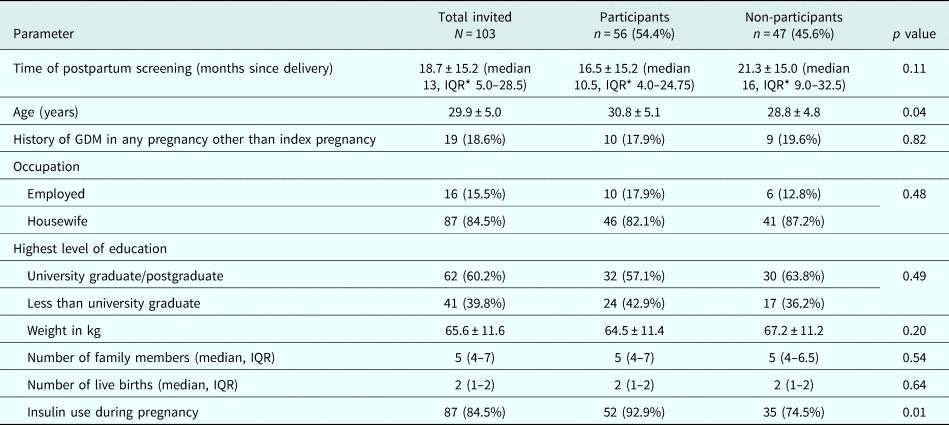
* Inter Quartile Range
Session participant rates (feasibility)
Out of 56 participants, 30 (54%) attended all six sessions, 11 (20%) attended five, four (7%) each attended four and three sessions respectively and the remaining seven (13%) attended only two sessions. In all, 80% of individuals attended four or more sessions.
Post-intervention changes
Change in glucose categories
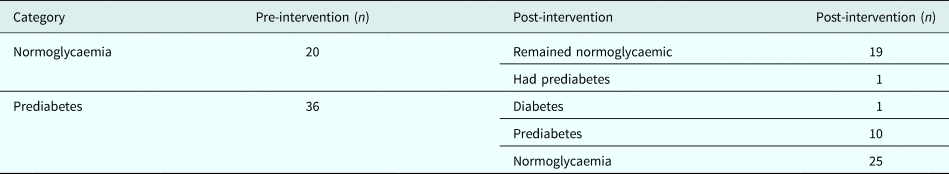
Out of 56 women, 20 (36%) were normoglycaemic and 36 (64%) had prediabetes at baseline. At the end of intervention, one normoglycaemic person progressed to prediabetes and one prediabetic progressed to diabetes, while 25 out of 36 prediabetes (69.4%) reverted to normoglycaemia (Table 2). The differences between those who reverted to normoglycaemia (n = 25) and those who did not (n = 11) was not compared due to small numbers.
Table 2. Glycaemic categories pre- and post-intervention
Absolute changes in metabolic parameters
Compared with pre-intervention measurements, there was significant reduction in mean weight (1.8 kg), BMI (0.6 kg/m2), waist circumference (2 cm), systolic BP (3.6 mmHg), fasting plasma glucose (0.3 mmol/L), 2 h post-glucose load plasma glucose (0.9 mmol/L), and triglycerides (0.15 mmol/L). Changes in total cholesterol, low-density lipoprotein-cholesterol, high-density lipoprotein-cholesterol and diastolic BP were non-significant (Table 3).
Table 3. Change in metabolic parameters after lifestyle intervention
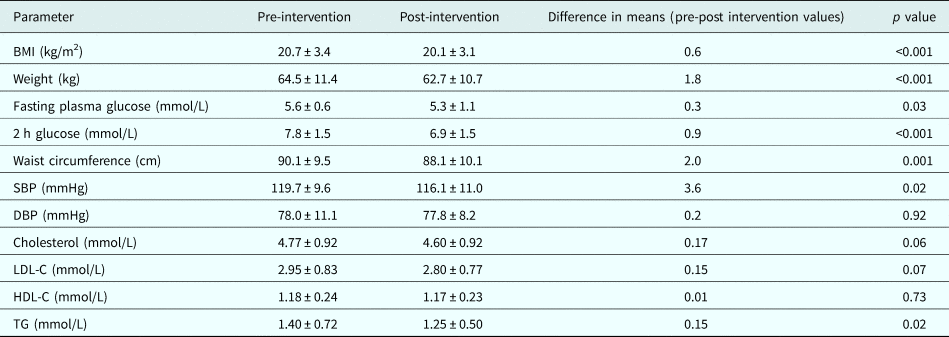
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL-C, low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol; TG, Triglyceride.
Discussion
The current study provides some evidence on the feasibility and potential effectiveness of a lifestyle intervention among women with prior GDM in the Indian context. Data are currently available from the USA, Australia and China, but are lacking from the South Asian region [Reference Guo, Chen, Whittemore and Whitaker6–Reference Peacock, Bogossian, McIntyre and Wilkinson8], where the incidence of GDM is high, as are the risks of subsequent development of T2DM. Socio-cultural and ethnic differences require the generation of local evidence [Reference Gupta, Kapoor, Desai, Praveen, Joshi, Rozati, Bhatla, Prabhakaran, Reddy, Patel and Tandon2–Reference Mahalakshmi, Bhavadharini, Kumar, Anjana, Shah, Bridgette, Choudhury, Henderson, Desborough, Mohan and Ranjani5].
There were favourable changes in metabolic parameters post-intervention in our study, with nearly 70% of prediabetic women reverting to normoglycaemia. There was significant improvement in all indices of metabolic health (weight, waist circumference, glycaemic measures, BPs and lipid profile). There was significant though modest weight loss of 1.8 kg. In the absence of a control group it is not possible to ascribe such changes to the intervention, as opposed to regression to the mean. However, the results are similar to a controlled study from Southeast Asia (Malaysia) in which 77 Asian women were randomised to a low glycaemic index dietary intervention or to usual care for a period of 6 months. At the end of study, the intervention group lost a mean of 1.3 kg in weight, 0.6 kg/m2 in BMI and 2.7 cm in waist circumference. However, the intervention implemented in the Malaysian study was close to the time of delivery (at mean of 6 months postpartum), which is relatively a difficult period for women to adopt lifestyle modification, as compared to median of 11 months post-partum in our study [Reference Shyam, Arshad, Abdul Ghani, Wahab, Safii, Nisak, Chinna and Kamaruddin11].
The fact that 70% of prediabetic women reverting to normoglycaemia may be due to reason that the women were enrolled close to last childbirth, and they may be in earlier phase of prediabetes, from which reversal to normoglycaemia is easy. Since the metabolic indices were closer to the threshold for normal, smaller changes through less intensive interventions allowed them to cross this threshold [Reference Gupta, Kapoor, Desai, Praveen, Joshi, Rozati, Bhatla, Prabhakaran, Reddy, Patel and Tandon2].
The session attendance and retention rates were encouraging in this lifestyle intervention programme. The acceptable participation rates reported in the study suggest that a lifestyle intervention programme is feasible among urban Indian women, with 80% attending more than 60% of the total sessions (i.e. ≥4 sessions), and nearly 50% having attended all six sessions. This increases the likelihood of effectiveness of this programme. The meta-analysis by Ali et al. on effectiveness of lifestyle interventions for prevention of diabetes in real world settings has shown that every additional lifestyle session attended, results in incremental weight loss of 0.26 percentage points [Reference Ali, Echouffo-Tcheugui and Williamson12]. There are some ongoing trials that are recruiting women close to delivery in different parts of the world [Reference Shih, Davis-Lameloise, Janus, Wildey, Versace, Hagger, Asproloupos, Reilly, Phillips, Ackland, Skinner, Oats, Carter, Best and Dunbar13–Reference Chasan-Taber, Marcus, Rosal, Tucker, Hartman, Pekow, Braun, Moore Simas, Solomon, Manson and Markenson17], that will provide further insights about implementation of lifestyle programmes for the prevention of type-2 diabetes, among women with prior GDM. With experience from the present study, we are now implementing a large-scale multi-national trial in South Asian countries (India, Bangladesh, Sri Lanka) on lifestyle intervention in women [LIVING study], beginning around 6 months postpartum with a median follow-up of 24 months [Reference Gupta, Kapoor, Josyula, Praveen, Naheed, Desai, Pathmeswaran, Silva, Lombard, Shamsul, Prabhakaran, Teede, Billot, Bhatla, Joshi, Zoungas, Jan, Patel and Tandon18].
The current study has some limitations. Foremost, this was an uncontrolled observational study, and therefore reliable conclusions in relation to effectiveness cannot be made. The study duration was short and women were in postpartum period varying from 2 to 55 months. However, with practically no data previously reported on this important high risk group of women from South Asia, this study is a useful addition to literature, especially as it was conducted in a population with high rates of GDM and post-partum conversion to diabetes. The current study found that lifestyle intervention is feasible and may improve metabolic parameters among Indian women with history of GDM.
Funding
This project is supported by the BRIDGES grant (ST09-016) from the International Diabetes Federation. BRIDGES, an International Diabetes Federation project is supported by an educational grant from Lilly Diabetes. There is no role of the funding agency in designing the study; in collection, analysis and interpretation of data; in the writing of the report and in the decision to submit the article for publication.
Author ORCIDs
Yashdeep Gupta http://orcid.org/0000-0002-4345-717X
Conflict of interest
None.
Authors contribution
Deksha Kapoor and Yashdeep Gupta contributed to acquisition, analysis and interpretation of data and drafting of manuscript. Nikhil Tandon, Anushka Patel and Ankush Desai contributed to conception and design of the work, interpretation of data, and revised the manuscript critically for important intellectual content. All other authors helped in interpretation of data and in revising the manuscript critically for important intellectual content. All authors approved the final submitted manuscript.
Strobe statement
This manuscript has been prepared in accordance to strobe guidelines.
We confirm that the manuscript has been read and approved by all the authors, that the requirements for authorship as stated earlier in this document have been met, and that each author believes that the manuscript represents honest work, if that information is not provided in another form.







