Dysmenorrhea is a debilitating gynecological condition that affects 50% to 90% of women, causing serious adverse physical and mental health effects and reducing quality of life (McKenna & Fogleman, Reference McKenna and Fogleman2021). It is a form of discomfort that usually accompanies ovulatory menstruation (Rogers, Reference Rogers1964), and abdominal cramp is the most common one. Dysmenorrhea is mainly categorized into primary dysmenorrhea and secondary dysmenorrhea, with the presence or absence of organic pelvic changes as the main point of differentiation. The underlying pathological mechanisms remain unclear and require further study (Iacovides et al., Reference Iacovides, Avidon and Baker2015). Epilepsy is the fourth most common neurological disorder worldwide (GBD 2015 Disease and Injury Incidence and Prevalence Collaborators, 2016; Disease and Injury Incidence and Prevalence Collaborators, 2016). The global lifetime prevalence of epilepsy is estimated to be about 7.6 cases per 1000 people (Olusanya et al., Reference Olusanya, Wright, Nair, Boo, Halpern, Kuper, Abubakar, Almasri, Arabloo, Arora, Backhaus, Berman, Breinbauer, Carr, de Vries, Del Castillo-Hegyi, Eftekhari, Gladstone and Hoekstra2020). Understanding the epidemiology of epilepsy is critical to developing healthcare policy, implementing prevention strategies, and understanding the health resource needs of these challenging patient populations (S. Wang et al., Reference Wang, Yao, Zhang, Xia, Yu, Peng, Xiang and Liu2023).Current observational studies suggest a correlation between dysmenorrhea and epilepsy (Ingudomnukul et al., Reference Ingudomnukul, Baron-Cohen, Wheelwright and Knickmeyer2007; Octaviana et al., Reference Octaviana, Sumapraja, Wiratman, Indrawati and Budikayanti2022; Pohl et al., Reference Pohl, Cassidy, Auyeung and Baron-Cohen2014). Dysmenorrhea is more prevalent among women with epilepsy (Serret-Montoya et al., Reference Serret-Montoya, Villasís-Keever, Ríos-Zúñiga, Sánchez-Vaca, Zurita-Cruz and Hernández-Cabezza2014). A case-control study with 66 participants similarly confirmed this result (Octaviana et al., Reference Octaviana, Sumapraja, Wiratman, Indrawati and Budikayanti2022). However, the direction and mechanism of the correlation between dysmenorrhea and epilepsy are unclear.
Mendelian randomization (MR) is a sophisticated epidemiological technique that assesses the causal relationship between risk factors and diseases by simulating the random allocation of participants in a randomized clinical trial using germline genetic variants as instrumental variables (IVs), also known as single-nucleotide polymorphisms (SNPs; Burgess et al., Reference Burgess, Butterworth and Thompson2013). The use of this technique has emerged as an invaluable tool for overcoming the constraints and biases inherent in observational research, enabling a more precise assessment of the causal relationship between exposures and results (Davey Smith & Hemani, Reference Davey Smith and Hemani2014). In the current investigation, we applied a bidirectional two-sample MR study employing the genomewide association study (GWAS) summary statistics data, hoping to shed light on the potential causal relationships between dysmenorrhea and epilepsy. We endeavored to ascertain more precisely whether dysmenorrhea exerts a causative influence on epilepsy, or if this causation operates in the opposite direction.
Materials and Methods
Study Design Description
A summary of this bidirectional MR design between dysmenorrhea and epilepsy is shown in Figure 1. The current MR analysis fulfills the following three basic assumptions: (1) genetic variants are strongly related to the exposure; (2) genetic variants are independent of confounding factors of the exposure-outcome; and (3) genetic variants influence outcomes solely through the selected exposure.
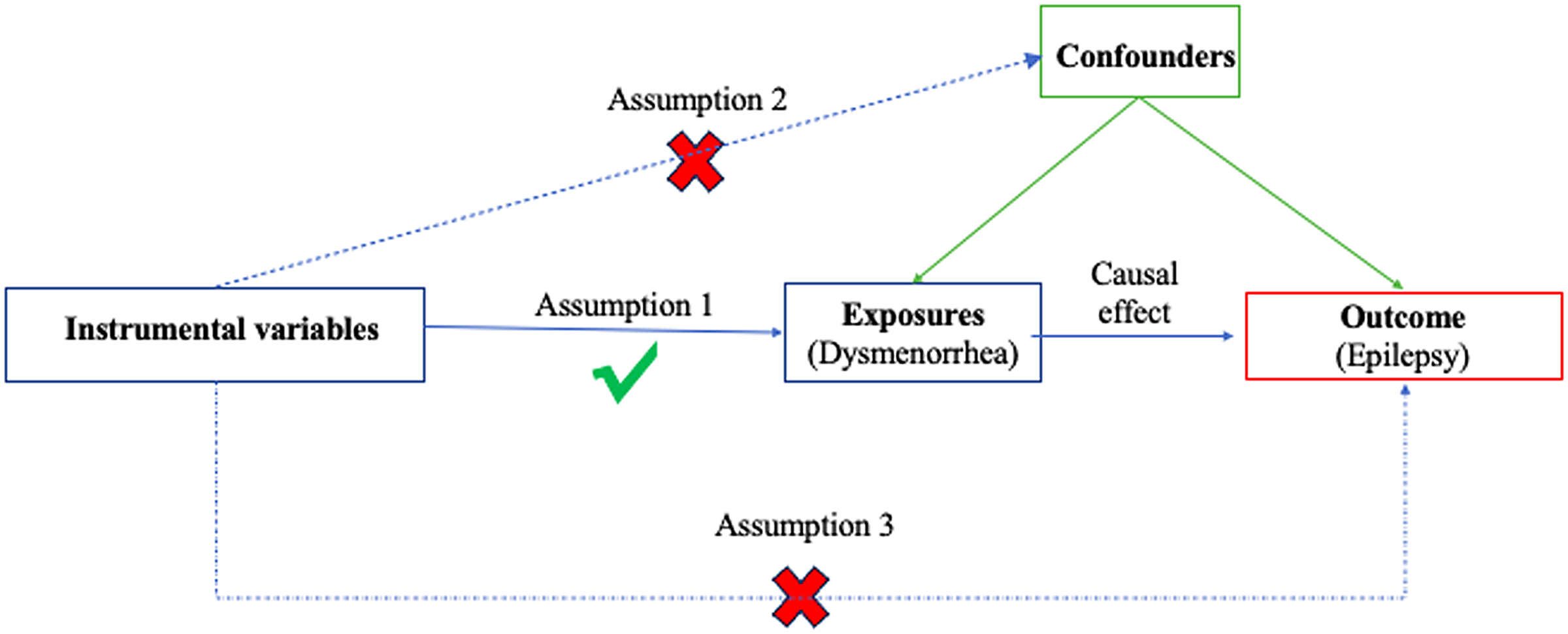
Figure 1. The overview flowchart of hypothesis and study design. This study was a two-sample Mendelian randomization analysis testing the causal effects between dysmenorrhoea and epilepsy. There were three assumptions required for a valid genetic instrument: the SNPs were significantly correlated with dysmenorrhoea, they were independent of confounders, and epilepsy was only related through dysmenorrhoea.
Utilizing summary statistics from a GWAS, we conducted two MR analyses to investigate the bidirectional relationship between dysmenorrhea and epilepsy. In contrast to the reverse MR analysis, which used epilepsy as the exposure and dysmenorrhea as the outcome, the forward MR analyses used dysmenorrhea as the exposure. The statistical analyses were conducted using the TwoSampleMR package (version 0.5.7) in the R program environment (version 4.2.3). Identification of outliers were conducted using the MRPRESSO package (version 1.0). See Figure 2 for a flow chart of the whole Mendelian randomization process.
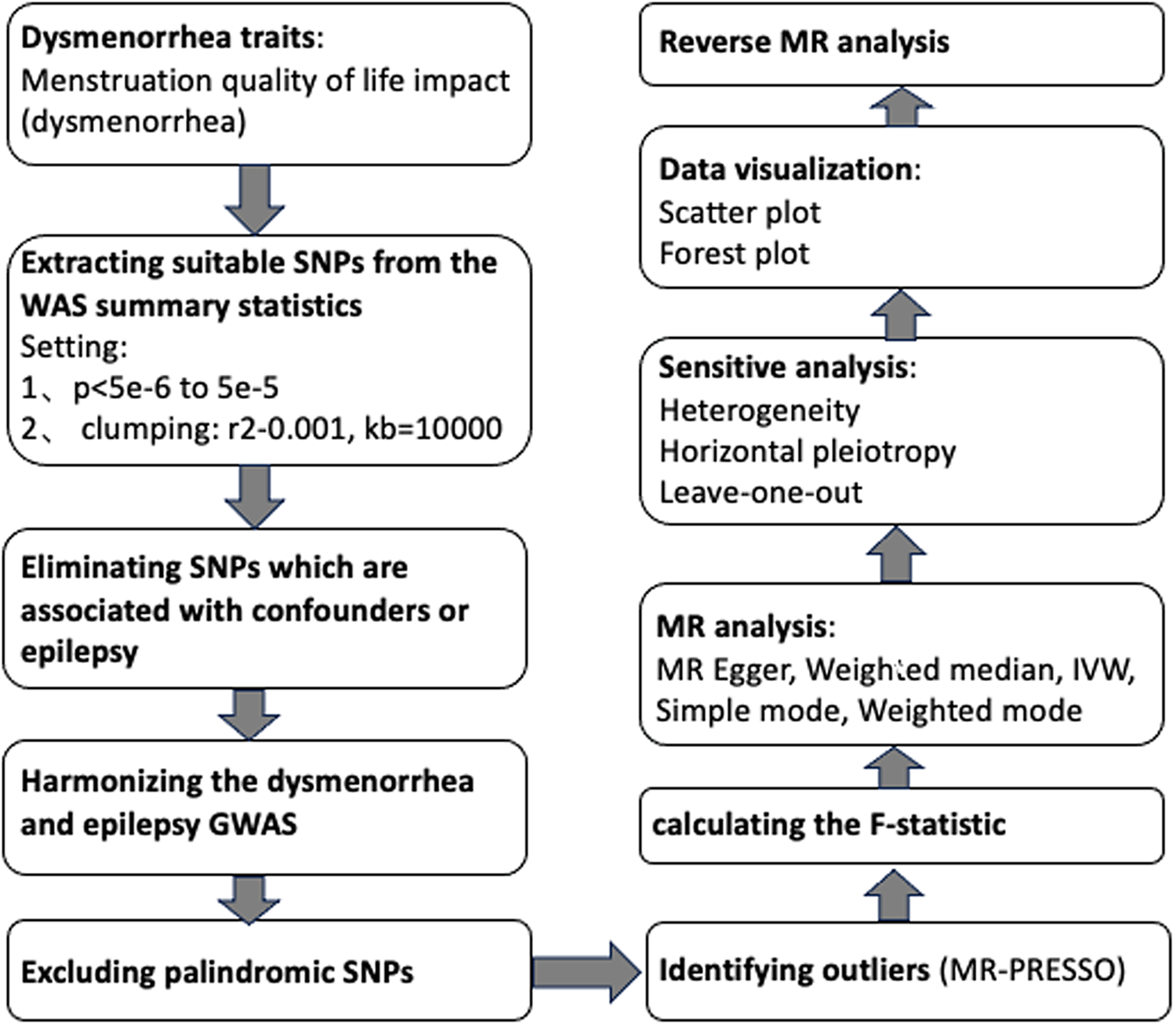
Figure 2. Mendelian randomization flow chart.
Data Source
Dysmenorrhea
We obtained the genetic instruments of menstruation quality of life impact (dysmenorrhea) to measure dysmenorrhea from GWAS aggregate statistics published by the European Bioinformatics Institute (EBI) (GWAS Catalogue, n.d.). These statistics, which included 3573 cases and 2161 controls of East Asian descent, are the most recent and comprehensive aggregated statistics on dysmenorrhea of East Asian that we know of. The diagnosis of dysmenorrhea is determined based on signs and symptoms, and other conditions causing pain are excluded.
Epilepsy
Summary statistics for epilepsy were provided by BioBank Japan (BBJ), which comprised 2143 epilepsy patients and 210,310 controls with East Asian ancestry (BBJ, n.d.). Cases were either identified using the International League Against Epilepsy (ILAE) criteria or by self-reported epilepsy history.
Selection of the Genetic Instrumental Variables
To determine the IVs for dysmenorrhea and epilepsy, some procedures were done to ensure that the first assumption was true. First, the genetic variants were extracted with association thresholds at p < 5 × 10–6, which are mostly used in MR analysis to elucidate a greater variation when few SNPs are available for exposure (Kwok & Schooling, Reference Kwok and Schooling2021). If necessary, we will make informed adjustments based on the number of IVs acquired. Second, independent variants were identified using a clumping procedure implemented in R software, in which a linkage-disequilibrium threshold of r 2 < .001 within a 10,000 kb window was set. The PhenoScanner tool (www.phenoscanner.medschl.cam.ac.uk; Kamat et al., Reference Kamat, Blackshaw, Young, Surendran, Burgess, Danesh, Butterworth and Staley2019) was used to cross-reference the IVs and exclude any SNPs associated at a genomewide significance with potential confounders or outcomes. We used p < 1e-5 as the primary threshold and p < .001 as the criterion to retrieve results for analyzing the potential relationship between dysmenorrhea and epilepsy. Next, palindromic SNPs were excluded when harmonizing the dysmenorrhea and epilepsy GWAS. The MR Pleiotropy RESidual Sum and Outlier (MR-PRESSO) test was employed to discern potential outlier SNPs for correcting potential horizontal pleiotropy (Rosoff et al., Reference Rosoff, Clarke, Adams, McIntosh, Davey Smith, Jung and Lohoff2021; Verbanck et al., Reference Verbanck, Chen, Neale and Do2018). Finally, the F statistic was calculated for each leftover SNP to measure the strength of genetic IVs, with the following formula (Shim et al., Reference Shim, Chasman, Smith, Mora, Ridker, Nickerson, Krauss and Stephens2015):
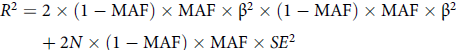 $$\eqalign{ & {R^2} = 2 \times (1 - {\rm{MAF}}) \times {\rm{MAF}} \times {\rm \beta ^2} \times (1 - {\rm{MAF}}) \times {\rm{MAF}} \times {\rm\beta ^2} \cr
& \quad \quad + 2N \times (1 - {\rm{MAF}}) \times {\rm{MAF}} \times S{E^2} \cr}$$
$$\eqalign{ & {R^2} = 2 \times (1 - {\rm{MAF}}) \times {\rm{MAF}} \times {\rm \beta ^2} \times (1 - {\rm{MAF}}) \times {\rm{MAF}} \times {\rm\beta ^2} \cr
& \quad \quad + 2N \times (1 - {\rm{MAF}}) \times {\rm{MAF}} \times S{E^2} \cr}$$
In the formula, MAF is minor allele frequency, β is the genetic effect of a given SNP on exposure factors, SE is standard error, and N is the sample size of the exposure. Considering that the genetic databases we used were from small studies, instead of simply adopting the classical criterion of F statistic >10, we used the broader criterion of F statistic >3 and combined several tests, including the MR-Egger and MR-PRESSO tests (Burgess et al., Reference Burgess and Thompson2011). The remaining SNPs were utilized for MR analysis.
MR Analysis
We used the inverse variance weighted (IVW) approach to get the main result of the MR analysis in R software. By using Wald ratios, this method integrates SNP-specific estimations and then uses a meta-analysis method to create a pooled causal influence of the exposure on the result (Bowden et al., Reference Bowden, Spiller, Del Greco, Sheehan, Thompson, Minelli and Davey Smith2018). To supplement IVW estimations, weighted median and MR-Egger regression analyses were also carried out. The confidence in the veracity of the causal evidence would be greatly increased if estimates from several MR approaches converged in a consistent way. However, if the causal effect estimates of these models are inconsistent, the results of IVW methods are considered as the main outcome. Using the formula β = ln (OR), the estimated combined effect β was converted into OR. The ORs and respective 95% CIs for the results of the MR analysis showed the effect of a unit change in exposure on the probability of the outcome. P < .05 indicated a statistically significant finding of causality.
Sensitivity Analysis
We performed numerous studies to find any underlying pleiotropy and heterogeneity that can possibly undermine the accuracy of the results. These studies comprised the Cochran’s Q test, the MR-Egger regression, and the leave-one-out analysis.
Quantified results from Cochran’s Q test for the IVW model can be used to determine whether there is underlying heterogeneity (p < .05 indicates potential heterogeneity; Greco et al., Reference Greco, Minelli, Sheehan and Thompson2015). A reliable indicator of horizontal pleiotropy was the intercept obtained from the MR-Egger regression (p < .05 confirming its presence; Bowden et al., Reference Bowden, Davey Smith and Burgess2015). Finally, a leave-one-out analysis was conducted to guard against the chance that any one SNP could have an unwarranted influence on the causal estimate.
Results
Dysmenorrhea to Epilepsy
We found a set of seven genetic instrumental variables for dysmenorrhea after a thorough procedure of LD clumping and exclusion potential confounders. All of them showed stronger robust statistical power, with F statistics ranging from 3.23 to 22.56, beyond the cut-off point of 3. Please refer to the Supplementary dataset for more information on the genetic variations. The chosen seven SNPs were then combined and harmonized with the epilepsy outcome dataset (Supplementary Table 1). We used this threshold to exclude rs17817634 and then used a very strict threshold to find confounders that were still present. Referring to the relevant literature (Cui & Tian, Reference Cui and Tian2021; Ye et al., Reference Ye, Liu, Bai, Cao, Lin, Lyu, Meng, Dai, Ye, Pan, Wang, Mao and Chen2023), we chose the former scheme.
The results (OR = 1.26; 95% CI [1.07, 1.47]; p = 4.42 × 10-3) show that a potential causal effect of dysmenorrhea on epilepsy is evident via the IVW model. However, the MR-Egger method (OR: 1.03; 95% CI [0.53, 2.00]; p = 9.38 × 10 -1) and weighted median analysis (OR: 1.17; 95% CI [0.96, 1.42]; p = 1.15 × 10-1) gained opposing results and failed to attain statistical significance (Figure 3).
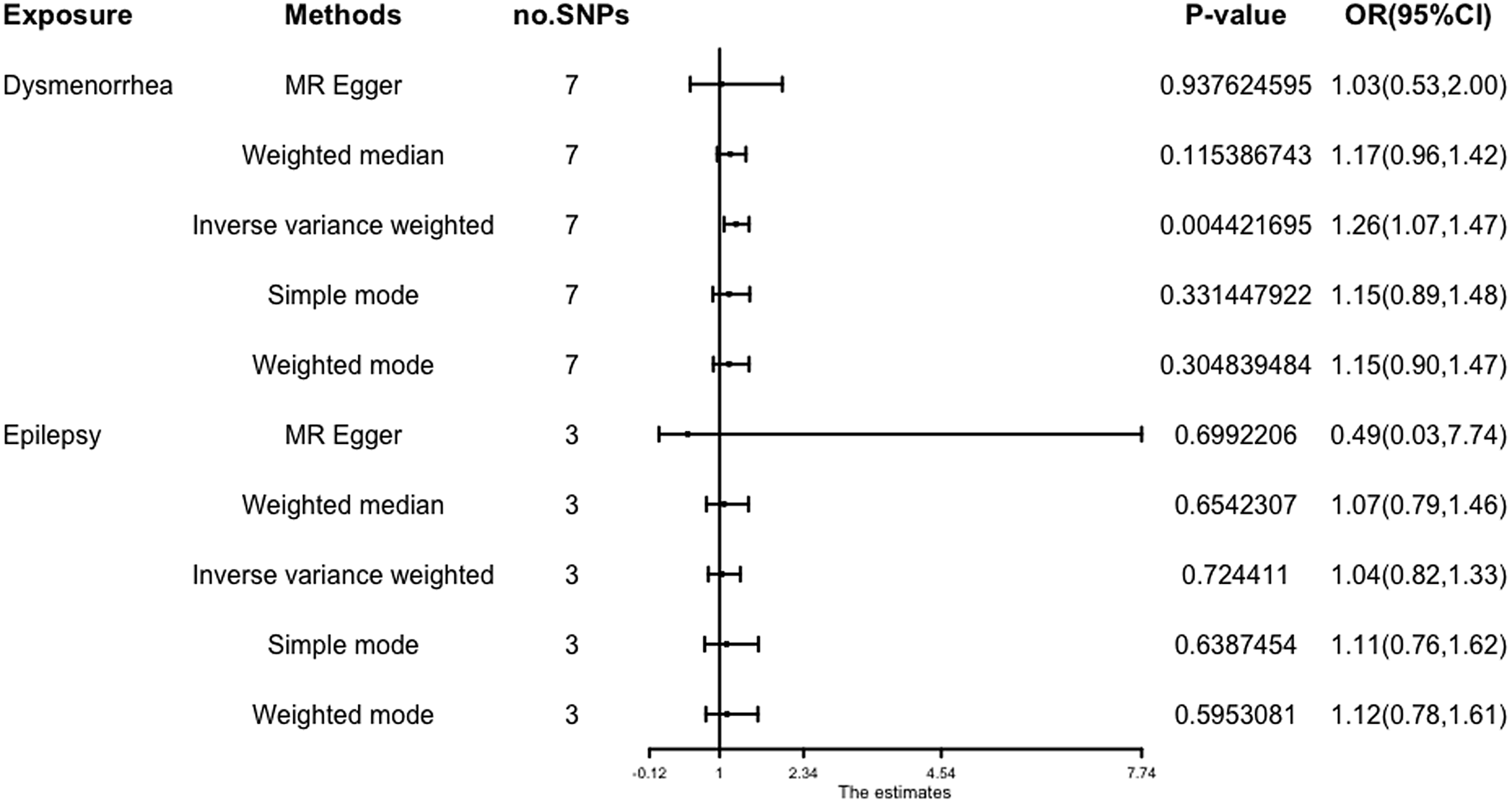
Figure 3. Forest plot of causal relationships estimated for dysmenorrhoea and epilepsy using five methods.
Cochran’s Q test was performed using different methods to assess heterogeneity, and both IVW results (p = .37) and MR Egger (p = .31) suggested that there was no heterogeneity between instrumental variables. MR-Egger regression did not reveal any indication of pleiotropic effects in our datasets (Egger intercept = 0.036; p = .57). To validate earlier findings and identify any outliers, we performed MR-PRESSO and found no outliers. The results (p < .05) showed that the effect of genetic variation on outcome estimates was significant (Supplementary Table 2). The leave-one-out analysis, which we further conducted, revealed that no single SNP severely violated the overall effect (Supplementary Table 3). The results of power calculations indicated a strong statistical power of .81.
Epilepsy to Dysmenorrhea
Referring to some recent studies (Jiang et al., Reference Jiang, Gill, Butterworth and Burgess2023; Sanna et al., Reference Sanna, van Zuydam, Mahajan, Kurilshikov, Vich Vila, Võsa, Mujagic, Masclee, Jonkers, Oosting, Joosten, Netea, Franke, Zhernakova, Fu, Wijmenga and McCarthy2019), we used p < 5×10-5 to obtain exposure-related SNPs. After implementing a meticulous process of LD clumping and removal of potential confounders, we successfully identified 25 genetic instrumental variables for epilepsy. The chosen SNPs had stronger statistical significance, as shown by their F statistics, which ranged from 4.14 to 7.28 and were higher than the usual cut-off of 3. Three SNPs were retained for further investigation (rs2002685, rs2238014, and rs269492; Supplementary Table 1).
The results obtained from the IVW model (OR: 1.04; 95% CI [0.82, 1.33]; p = .72), weighted median (OR: 1.07; 95% CI [0.79, 1.46]; p = 0.65), and MR-Egger (OR: 0.49; 95% CI [.03, 7.74]; p = .70) demonstrated a high degree of similarity, suggesting that there is limited evidence to support a significant association between an increase in epilepsy and the risk of developing dysmenorrhea (Figure 3).
When Cochran’s Q test was used to analyze heterogeneity, it did not find any significant evidence of heterogeneity (p = .31), and MR-Egger regression analysis did not find any evidence of pleiotropic effects (p = .68). A leave-one-out analysis further revealed that no single SNP had a significant impact on the overall effect (Supplementary Tables 2 and 3).
Figure 3 gives an overview of the MR estimates results, and Supplementary Tables 2 and 3 give a thorough presentation of the outcomes of the sensitivity analysis and a leave-one-out analysis. Additionally, we used scatter plots (Figures 4 and 5) to graphically show the discrete and cumulative impacts computed using various approaches on the outcome datasets derived from the exposure datasets. In addition, forest plots were produced and are shown in Figures 6 and 7. Among them, the scatterplot visualizes to us how well the various models fit the relationship between dysmenorrhea and epilepsy. The figure shows that the contribution of each data to the overall result is basically similar. The IVW model, which has a larger slope than the other models, was more positive in reaching the result that dysmenorrhea has a causal relationship with epilepsy, which is the same as the previous results (Figures 4 and 5).
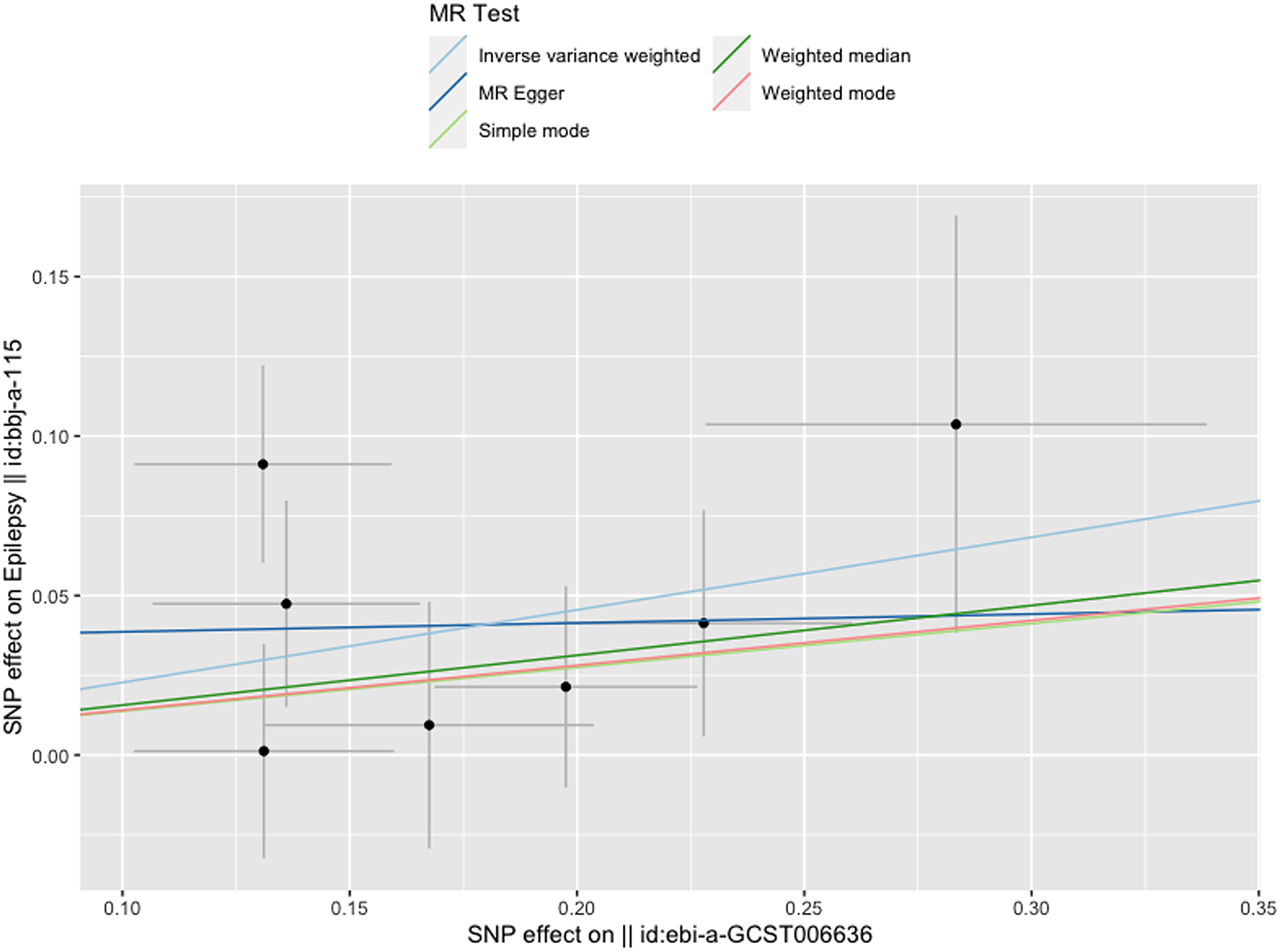
Figure 4. Scatterplot of dysmenorrhea on epilepsy.
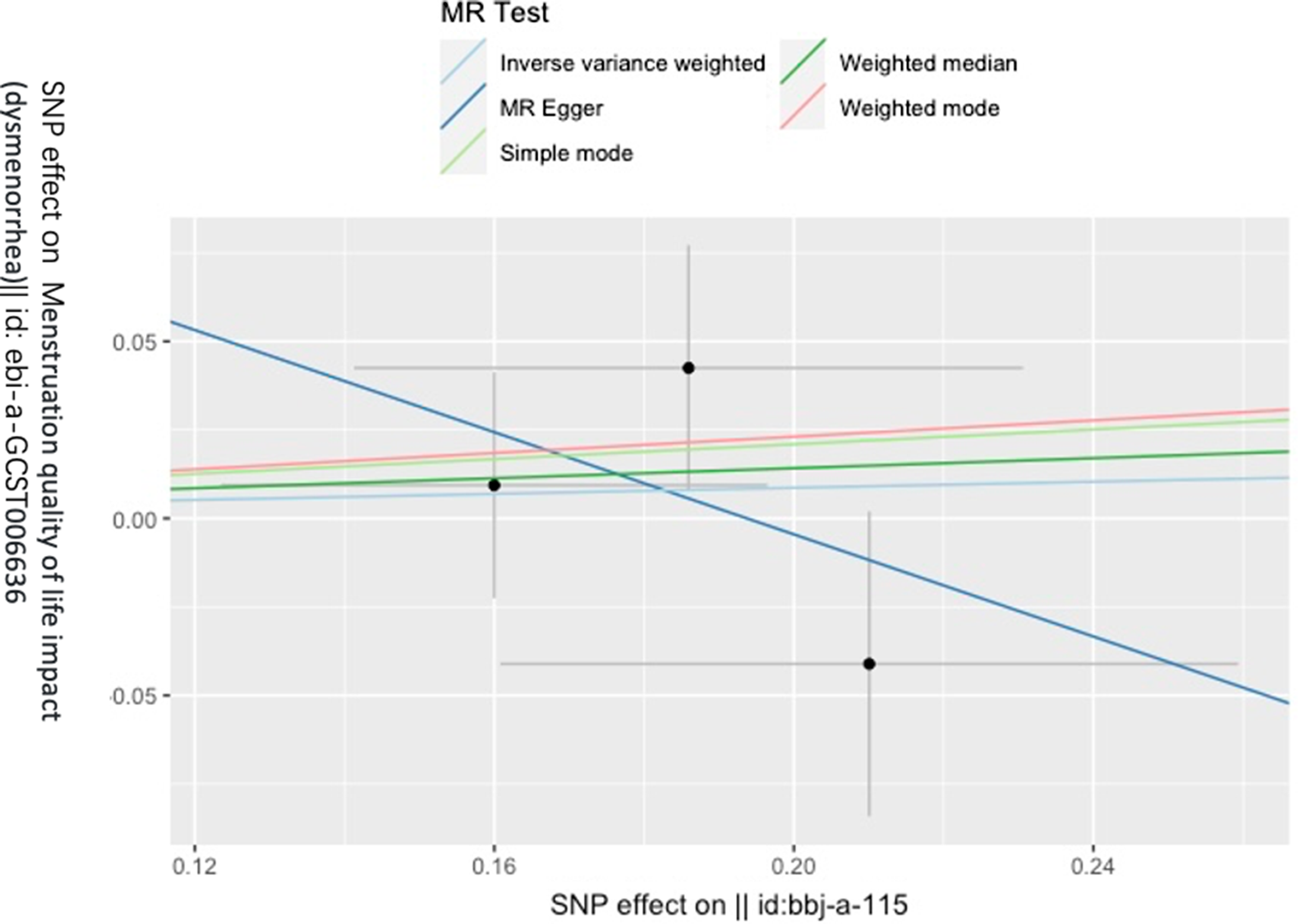
Figure 5. Scatterplot of epilepsy on dysmenorrhea.
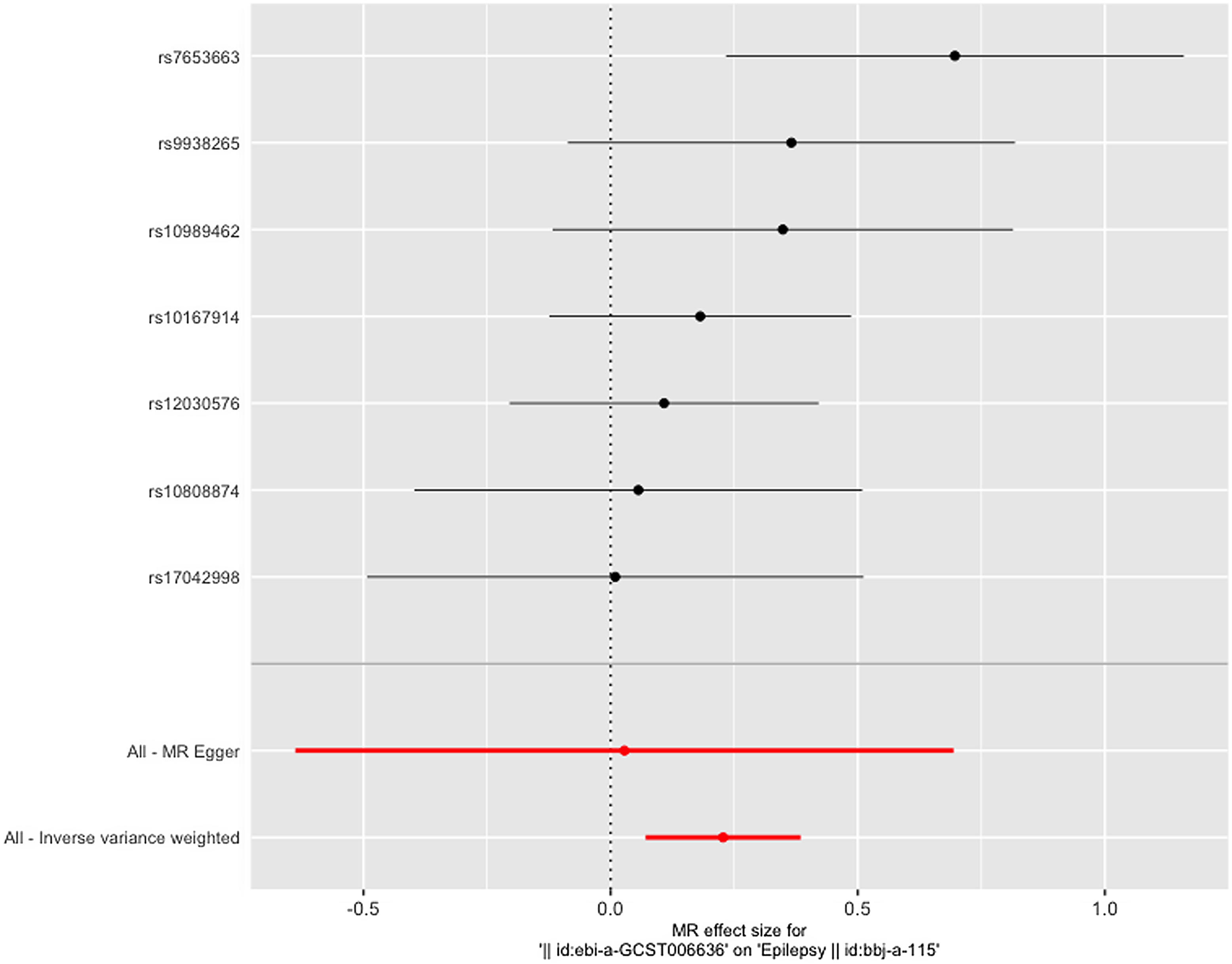
Figure 6. Leave-one-out figure of dysmenorrhea on epilepsy.
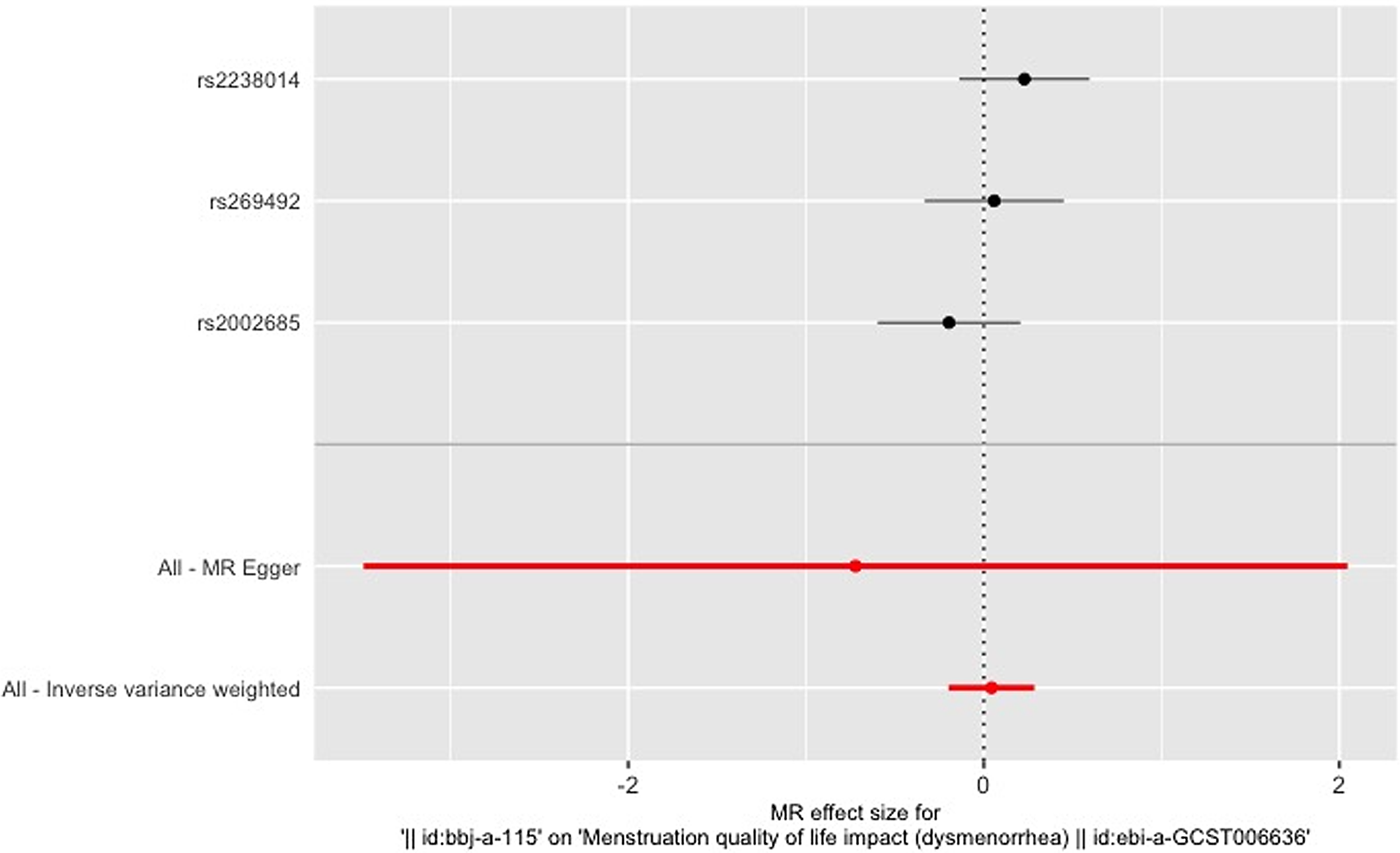
Figure 7. Leave-one-out figure of epilepsy on dysmenorrhea.
Discussion
This is the first study based on a bidirectional MR between dysmenorrhea and epilepsy in an East Asian population. Our MR results suggest that the occurrence of dysmenorrhea is causally related to epilepsy, whereas epilepsy has no causal effect on dysmenorrhea. This observation is supported by consistent results from the IVW model and weighted median analysis. The disease datasets used in this study were obtained from different databases with low sample overlap, increasing the reliability of our conclusions.
It is worth mentioning that the forest plot shows that the effect of only one SNP is significantly different from zero, and the rest of the SNPs do not show a significant effect, but the total IVW results show a significant effect; excluding this SNP still retains a significant effect, and we believe that such a result proves that looking at the results of a single SNP in isolation alone can be biased, and that when all the instrumental variables that have been rigorously removed from the chain of disequilibrium are treated as a whole analysis, the results are more comprehensive and objective and informative.
It is very difficult and beyond the scope of this study to fully understand the impact of the interaction between genetic and environmental factors on the development of these diseases, but we still try to make an analytical study of the mechanisms that generate their causality. First, central sensitization may play an important role in the causal relationship between dysmenorrhea and epilepsy. The most common form of dysmenorrhea is primary dysmenorrhea, whose widely accepted mechanism of occurrence is the uterine overproduction of prostaglandins (PGs; Josimovich, Reference Josimovich2013). PGs mediate myocardial and vasoconstriction, sensitization of painful nerve fibers, and ultimately pain (Iacovides et al., Reference Iacovides, Avidon and Baker2015). Whereas nociceptive hypersensitivity (increased pain sensitivity) and anomalous pain are the two main features of central sensitization (Sandkühler, Reference Sandkühler2009; Seminowicz et al., Reference Seminowicz, Laferriere, Millecamps, Yu, Coderre and Bushnell2009; Woolf, Reference Woolf2011). It is noteworthy that other pain conditions that occur concurrently with PDM, such as low back pain, irritable bowel syndrome and fibromyalgia, usually manifest as central sensitization (Arendt-Nielsen et al., Reference Arendt-Nielsen, Morlion, Perrot, Dahan, Dickenson, Kress, Wells, Bouhassira and Drewes2018; Liu et al., Reference Liu, Li, Hong, Huo, Chang, Wang, Huang, Li and Zhang2023). The path mechanism of epilepsy can be explained by neuronal hyperexcitability (Winawer et al., Reference Winawer, Connors and Investigators2013; Zarcone & Corbetta, Reference Zarcone and Corbetta2017). As-Sanie et al. (Reference As-Sanie, Kim, Schmidt-Wilcke, Sundgren, Clauw, Napadow and Harris2016) demonstrated that increased concentrations of glutamine and glutamate within the anterior insula, as well as increased neuronal connectivity from the anterior insula to the medial prefrontal cortex, were positively correlated with pain intensity. Based on the above, dysmenorrhea leading to enhanced central sensitization and thus epilepsy is a possible causal pathway. Second, dysmenorrhea and epilepsy may be mediated by inflammation. Dysmenorrhea due to endometriosis is associated with inflammatory stimuli (Vercellini et al., Reference Vercellini, Viganò, Somigliana and Fedele2014). Some studies have shown that inflammation-related cytokines are associated with dysmenorrhea (Ahn et al., Reference Ahn, Singh and Tayade2017; Kiyama, Reference Kiyama2020). At the same time, inflammatory molecules such as IL-1β, TNF-α, IL-6, and HMGB1 affect neuronal function by inducing rapid post-translational changes in glutamate receptor subunit composition and/or phosphorylation. Together, these microenvironmental changes lead to overexcitation of neuronal networks and lower seizure thresholds (Chen et al., Reference Chen, Lai, Huang, Wu and Huang2022; Vezzani et al., Reference Vezzani, Fujinami, White, Preux, Blümcke, Sander and Löscher2016). Third, the gut microbiota may play a role in the causal relationship between dysmenorrhea and epilepsy. Some studies have shown gut microbiota-immune system interactions with dysmenorrhea due to endometriosis (García-Peñarrubia et al., Reference García-Peñarrubia, Ruiz-Alcaraz, Martínez-Esparza, Marín and Machado-Linde2020). Experimental evidence linking gut ecological dysregulation to immune dysregulation by Laschke and Menger (Reference Laschke and Menger2016) suggests a hypothesized role for the gut microbiota in the pathogenesis of endometriosis. This hypothesis is supported by the findings of Bailey and Coe (Reference Bailey and Coe2002) regarding the correlation between endometriosis and alterations in the gut microbiota of rhesus monkeys. A growing number of recent studies have demonstrated the relevance of gut microbes to epilepsy (Dahlin & Prast-Nielsen, Reference Dahlin and Prast-Nielsen2019; Olson et al., Reference Olson, Vuong, Yano, Liang, Nusbaum and Hsiao2018; Socała et al., Reference Socała, Doboszewska, Szopa, Serefko, Włodarczyk, Zielińska, Poleszak, Fichna and Wlaź2021; Sorboni et al., Reference Sorboni, Moghaddam, Jafarzadeh-Esfehani and Soleimanpour2022; Yue et al., Reference Yue, Cai, Xiao, Zhan and Zeng2022). The microbiota has been shown to synthesize and respond to many neurotransmitters, including 22-hydroxytryptamine and GABA (H.-X. Wang & Wang, Reference Wang and Wang2016). Ecological disorders in which they are reduced can cause epileptic manifestations (Amlerova et al., Reference Amlerova, Šroubek, Angelucci and Hort2021; Casillas-Espinosa et al., Reference Casillas-Espinosa, Powell and O’Brien2012; Scheffer et al., Reference Scheffer, Berkovic, Capovilla, Connolly, French, Guilhoto, Hirsch, Jain, Mathern, Moshé, Nordli, Perucca, Tomson, Wiebe, Zhang and Zuberi2017).
The generalizability and extent of our findings may be impacted by several constraints, notwithstanding the significant insights garnered from our investigation. First, our research is restricted to people from East Asia, so it is not yet clear if it applies to populations elsewhere. Second, a more thorough approach that involves subgroup analysis of dysmenorrhea may offer more insightful and nuanced findings given the intrinsic variability of patients with dysmenorrhea. Subtypes of the illness may be more closely connected with epilepsy. Third, due to the limitations of the database, the power calculation of the selected IVs could be further enhanced.
Conclusions
By employing a bidirectional two-sample MR method in this investigation, we achieved major strides toward comprehending the intricate link between dysmenorrhea and epilepsy. Our results support the effects of dysmenorrhea on epilepsy susceptibility and development by both extending and analyzing prior research on the processes underlying both conditions. However, no link between epilepsy and dysmenorrhea was shown to be causal. These findings have significant implications for patient counseling and raise the possibility of curing dysmenorrhea as a therapeutic aspect for managing epilepsy.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/thg.2023.48.
Acknowledgments
We appreciated all the genetics consortiums for making the GWAS summary data publicly available.
Financial support
None.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics statement
This study used publicly available de-identified data from participant studies that were approved by an ethical standards committee concerning human experimentation. No separate ethical approval was required in this study.










