Four Na2S2O4-reduced Na-vermiculites, each with some trioctahedral mica interstratified, were oxidized with H2O2 at pH 6·5 and again reduced with Na2S2O4 in suspensions at pH 7·5–8·0. The layer charge (CEC + K+), measured at pH 6·50, did not change significantly when octahedral Fe was oxidized (7–92 mmole 100g−1) or reduced (6–71 mmole 100 g−1). Electroneutrality was maintained within the octahedral sheet when Fe was oxidized or reduced. When Fe(II) was oxidized, electroneutrality was maintained by deprotonation of octahedral OH− groups,
(a)${\left\{ {{{\left[ {Fe\left( {II} \right)} \right]}_2}M{g_4}{O_4}{{\left( {OH} \right)}_4}} \right\}^{ \pm 0}}\leftrightarrows{\left\{ {{{\left[ {Fe\left( {III} \right)} \right]}_2}M{g_4}{O_4}{{\left( {OH} \right)}_2}O_2^*} \right\}^ {\pm 0}} + 2{e^ - } + 2{H^ + }$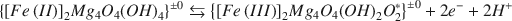
and by ejection of (dissolution of structural) octahedral metallic cations,
(b)${\left\{ {{{\left[ {Fe\left( {II} \right)} \right]}_5}Mg{O_4}{{\left( {OH} \right)}_4}} \right\}^{ \pm 0}} \rightarrow {\left\{ {{{\left[ {Fe\left( {III} \right)} \right]}_4}{O_4}{{\left( {OH} \right)}_4}} \right\}^{ \pm 0}} + 5{e^ - } + F{e^{3 + }} + M{g^{2 + }}.$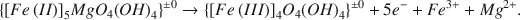
When Fe(III) was reduced, electroneutrality was maintained by reprotonation of the deprotonated sites (O*, equation a). Reaction (b) was not reversible. Thus, reversibility of the reaction, Fe(II) ⇄ Fe(III) within the octahedral sheet decreased with increasing amount of ejected metallic cations. The amount of Fe(III) and Mg2+ ejected per Fe(II) oxidized was related to the degree of vermiculitization, being greatest with Na-degraded biotite [0·03 Fe3+ and 0·11 Mg2+ per Fe(II) oxidized] and lowest (nearly zero) with South African vermiculite. The number of deprotonated (O*) and reversible sites increased from 0·69 per Fe(II) oxidized with the K-depleted biotite to approximately 1·0 with South African vermiculite. The weathering increment was small since, of the total amount of Fe + Mg, less than 1·3 per cent was ejected from any of the four vermiculitic materials. When biotite was K-depleted, about 20 m-equiv of layer charge per 100g (300°C basis) was lost, while 51 mmole of Fe(II) per 100g was oxidized in the presence of Na2S2O4 and 82 mmoles in its absence in the aqueous suspensions. Since sequential reduction-oxidation-reduction treatments of K-depleted biotite and mica-containing vermiculites did not cause significant changes in layer charge (r2 = 0·04), the layer charge changes were concluded to be entirely independent of the oxidation or reduction of Fe in these minerals.