Stroke is the major cause of morbidity and mortality( Reference Sacco, Kasner and Broderick 1 ). Ischaemic stroke and haemorrhagic stroke are the two main types of stroke, with ischaemic stroke being responsible for 80–90 % of all stroke cases( Reference Larsson 2 ). Diet may play an important role in the aetiology of stroke by affecting various risk factors of stroke, such as hypertension, inflammation, platelet aggregation and endothelial function( Reference Larsson 2 ). Although total fat intake has not been related to stroke risk, specific fatty acids may have a role( Reference Larsson 2 ). PUFA, which exist as n-3 and n-6 PUFA, have been associated with lower risk of CHD( Reference Mozaffarian and Wu 3 – Reference Wu, Lemaitre and King 6 ), but their role in the aetiology of stroke is less clear. In prospective studies, consumption of long-chain n-3 PUFA has been associated with modestly lower risk of stroke( Reference Chowdhury, Stevens and Gorman 7 ), but only a few studies have investigated the association with the intermediate-chain length n-3 PUFA α-linolenic acid (ALA) and the results have been mixed( Reference Larsson, Virtamo and Wolk 8 – Reference He, Rimm and Merchant 10 ). The n-6 PUFA have not generally been associated with stroke risk( Reference He, Merchant and Rimm 11 – Reference Yaemsiri, Sen and Tinker 14 ).
Assessment of dietary intakes by subjective methods such as FFQ is subject to misclassification, which creates random error, and therefore can attenuate the associations between dietary factors and risk of diseases. Circulating fatty acids provide an objective measure of exposure, and are thus less subject to random error. However, previous studies that have used circulating PUFA as exposure have not produced consistent findings( Reference Wu, Lemaitre and King 6 – Reference Larsson, Virtamo and Wolk 8 , Reference Yaemsiri, Sen and Tinker 14 – Reference Sakai, Kakutani and Tokuda 21 ). Therefore, the aim of our study was to investigate the associations between serum n-3 and n-6 PUFA and risk of stroke in men.
Fish is a major source of not only long-chain n-3 PUFA but also methylmercury, which is found especially in large and old predatory fish. We have previously shown in this study population that high exposure to Hg was associated with higher risk of CVD and with attenuation of the inverse association of long-chain n-3 PUFA with the risk( Reference Virtanen, Voutilainen and Rissanen 22 , Reference Virtanen, Laukkanen and Mursu 23 ). Therefore, we also investigated whether Hg exposure is associated with risk of stroke and whether Hg could modify the association between long-chain n-3 PUFA and risk of stroke. Previous studies in populations with lower exposure to Hg have not found an association between Hg exposure and risk of stroke( Reference Wennberg, Bergdahl and Stegmayr 24 – Reference Mozaffarian, Shi and Morris 26 ). Furthermore, unlike previous studies, we assessed Hg exposure by measuring the hair Hg concentration, which is considered as the best marker for long-term Hg exposure( Reference Roman, Walsh and Coull 27 ).
Methods
Study design and population
Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD) is a population based, randomly selected sample of men from Eastern Finland. It has been designed to explore the associations between risk factors and risk of CVD, atherosclerosis, stroke and other chronic diseases( Reference Salonen 28 ). The baseline examinations were performed in 1984–1989( Reference Salonen 28 ). A total of 2682 men who were 42, 48, 54 or 60 years old at baseline (82·9 % of those eligible) were recruited. This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Research Ethics Committee of the University of Kuopio. Written informed consent was obtained from all subjects/patients. Subjects with history of CVD or stroke (n 709) or with missing data on serum PUFA (n 131) or hair Hg (n 14) were excluded, leaving a total of 1828 men.
Serum fatty acid measurements
Serum esterified fatty acid and NEFA were specified in one GC run without pre-separation as described( Reference Laaksonen, Lakka and Lakka 29 ). Serum fatty acids were extracted using chloroform–methanol. The chloroform phase was evaporated and treated with sodium methoxide, which methylated esterified fatty acids. Quantification was carried out with reference standards purchased from NU-Chek Prep Inc. Each analyte had an individual reference standard, and the internal standard was eicosane. Fatty acids were chromatographed in an NB-351 capillary column (HNU-Nordion) using a Hewlett-Packard 5890 Series II GC (Hewlett-Packard Company, since 1999 Agilent Technologies Inc.) with a flame ionisation detector. Results were obtained in micromoles per litre and presented as proportions of total serum fatty acids. The CV% for repeated measurements of fatty acids was 8·7 % for linoleic acid (LA, 18 : 2n-6), 11·6 % for γ-linolenic acid (GLA, 18 : 3n-6), 9·9 % for arachidonic acid (AA, 20 : 4n-6), 8·6 % for ALA (18 : 3n-3), 10·4 % for EPA (20 : 5n-3), 12·7 % for docosapentaenoic acid (DPA, 22 : 5n-3) and 13·3 % for DHA (22 : 6n-3).
Other measurements
The subjects gave their fasting blood samples between 08.00 and 10.00 hours at baseline examinations in 1984–1989. They were instructed to abstain from ingesting alcohol for 3 d and from smoking and eating for 12 h before providing samples. Detailed descriptions of the determination of blood glucose( Reference Salonen 28 ), assessment of medical history and medications( Reference Salonen 28 ), family history of diseases( Reference Salonen 28 ), smoking( Reference Salonen 28 ) and alcohol consumption( Reference Salonen 28 ) have been published. Diabetes was defined as self-reported diabetes mellitus or fasting blood glucose of 6·7 mmol/l or more. Education was assessed in years by using a self-administrated questionnaire. Physical activity was assessed using the KIHD 12-Month Leisure-Time Physical Activity Questionnaire( Reference Lakka, Venalainen and Rauramaa 30 ). Serum C-reactive protein (CRP) was measured using an immunometric assay (Immulite High Sensitivity CRP Assay; DPC). BMI was computed as weight (kg):square of height (m2). Dietary intake of foods and nutrients was assessed at the time of blood sampling using 4-d food recordings( Reference Voutilainen, Rissanen and Virtanen 31 ). Hg in hair was determined by flow injection analysis-cold vapour atomic absorption spectrometry and amalgamation, as described previously( Reference Salonen, Seppanen and Nyyssonen 32 ).
Ascertainment of follow-up events
Incident strokes between 1984 and 1992 were observed through the FINMONICA (Finnish Monitoring Trends and Determinants in Cardiovascular Diseases) stroke register( Reference Kurl, Laukkanen and Rauramaa 33 ). Information regarding the stroke incident between 1993 and 2012 was collected through computerised linkage to the national hospital discharge registry. The diagnosis of stroke was based on sudden onset of clinical signs or focal or global disturbance of cerebral function lasting 24 h (except in the case of sudden death or if interrupted by surgical intervention) with no apparent cause other than a vascular origin. Each suspected stroke (International Classification of Diseases (ICD)-9 codes 430–439 and ICD-10 codes I60–I68 and G45–G46) was classified into the following: (1) a definite stroke, (2) no stroke or (3) an unclassifiable event. The FINMONICA stroke register data were annually re-checked with the data obtained from the computerised national hospital discharge and death registers. Definite strokes and unclassifiable events were included in the group of any stroke. Each definite stroke was classified into (1) an ischaemic stroke (ICD-9 codes 433–434; ICD-10 code I63) or (2) a haemorrhagic stroke (ICD-9 codes 430–431; ICD-10 codes I60–I61). If the subject had multiple non-fatal strokes during follow-up, the first stroke was considered as the end point. Computed tomography (CT) was performed in 90 % of the patients by 1993, and CT, MRI and autopsy reached 100 % by 1997. Every resident of Finland has a unique personal identifier that is used in registers. There were no losses to follow-up.
Statistical analysis
Subjects were divided into quartiles according to the mean serum n-3 PUFA, serum n-6 PUFA and the Hg content in hair. The univariate relationships between serum PUFA and hair Hg and baseline characteristics were assessed by means and linear regression analysis (for continuous variables) or χ 2 tests (for categorical variables). Associations between serum PUFA, hair Hg and stroke risk were analysed using Cox regression models. Three different models were used. The first model was adjusted for age and examination year. The second model further included potential confounders such as BMI, smoking status, physical activity and alcohol intake. Model 3 further included possible effect modifiers such as systolic blood pressure, diabetes, serum HDL- and LDL-cholesterol and TAG, and serum CRP. Cohort mean was used to replace missing values in covariates (<0·5 %). Tests of linear trend were conducted by assigning median values for each category of exposure variable and treating those as a single continuous variable. Statistical significance of the interactions on a multiplicative scale was assessed by likelihood ratio tests using a cross-product term. All P-values were two-tailed (α=0·05). Data were analysed using SPSS 21.0 for Windows (IBM Corp.).
Results
At baseline, higher serum concentrations of both n-3 and n-6 PUFA were associated with generally more favourable health and lifestyle characteristics, such as higher leisure-time physical activity, education and serum HDL-cholesterol concentrations and lower systolic blood pressure and serum TAG and less smoking, whereas higher hair Hg content was associated with generally less favourable characteristics such as lower education and leisure-time physical activity and higher serum LDL-cholesterol concentration and smoking (Table 1).
Table 1 Baseline characteristics according to quartiles of serum n-3 PUFA, n-6 PUFA and hair mercury concentrations (Mean values and standard deviations; percentages)

During the average follow-up of 21·2 years (minimum–maximum 0·3–28·8 years), 202 men (11·1 %) experienced a stroke. Of all strokes, 153 were ischaemic and fifty-one were haemorrhagic strokes. After adjustment for age and examination year (model 1 in Tables 2–4), the only statistically significant association was observed between serum ALA and risk of haemorrhagic stroke (hazard ratio (HR) in the highest v. the lowest quartile 0·33, 95 % CI 0·13, 0·86, P trend=0·03). However, further multivariate adjustments attenuated the association (models 2 and 3). No statistically significant associations were found with the other n-3 or n-6 PUFA (Tables 2–4, online Supplementary Tables S1 and S2).
Table 2 Risk of incident total stroke in quartiles (Q) of serum n-3 and n-6 PUFA (Hazard ratios (HR) and 95 % confidence intervals)

DPA, docosapentaenoic acid; ALA, α-linolenic acid; LA, linoleic acid; AA, arachidonic acid.
* Model 1: adjusted for age and examination year.
† Model 2: adjusted for model 1 plus BMI, smoking status, physical activity, alcohol intake.
‡ Model 3: adjusted for model 2 plus systolic blood pressure, diabetes, HDL-cholesterol, LDL-cholesterol, serum TAG, C-reactive protein.
Table 3 Risk of incident ischaemic stroke in quartiles (Q) of serum n-3 and n-6 PUFA (Hazard ratios (HR) and 95 % confidence intervals)

DPA, docosapentaenoic acid; ALA, α-linolenic acid; LA, linoleic acid; AA, arachidonic acid.
* Model 1: adjusted for age and examination year.
† Model 2: adjusted for model 1 plus BMI, smoking status, physical activity, alcohol intake.
‡ Model 3: adjusted for model 2 plus systolic blood pressure, diabetes, HDL-cholesterol, LDL-cholesterol, serum TAG, C-reactive protein.
Table 4 Risk of incident haemorrhagic stroke in quartiles of serum n-3 and n-6 PUFA (Hazard ratios (HR) and 95 % confidence intervals)

DPA, docosapentaenoic acid; ALA, α-linolenic acid; LA, linoleic acid; AA, arachidonic acid.
* Model 1: adjusted for age and examination year.
† Model 2: adjusted for model 1 plus BMI, smoking status, physical activity, alcohol intake.
‡ Model 3: adjusted for model 2 plus systolic blood pressure, diabetes, HDL-cholesterol, LDL-cholesterol, serum TAG, C-reactive protein.
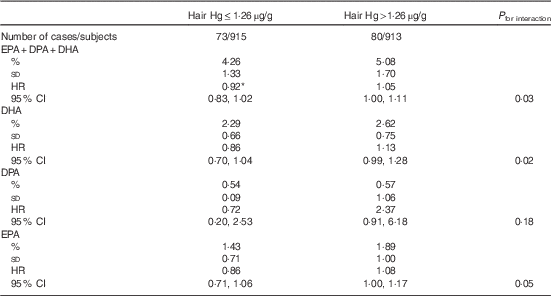
The mean hair Hg concentration was 1·90 (sd 1·95) µg/g. Higher hair Hg concentration was not associated with the risk of total stroke, ischaemic stroke or haemorrhagic stroke (Table 5). Hair Hg did not modify the associations between long-chain n-3 PUFA and risk of total stroke or haemorrhagic stroke (online Supplementary Tables S3 and S4). However, among those with hair Hg content above the median (1·26 µg/g), each 0·5 %-unit higher serum total long-chain n-3 PUFA concentration was associated with 5 % (HR 1·05; 95 % CI 1·00, 1·11) higher risk of ischaemic stroke and among those with hair Hg below the median with 8 % lower risk (HR 0·92; 95 % CI 0·83, 1·02; P interaction=0·03; Table 6). The results were generally similar for the individual long-chain n-3 PUFA EPA, DPA and DHA (Table 6).
Table 5 Risk of incident total stroke, ischaemic stroke and haemorrhagic stroke in quartiles of hair mercury (Hazard ratios (HR) and 95 % confidence intervals)

* Model 1: adjusted for age and examination year.
† Model 2: adjusted for model 1 plus BMI, smoking status, physical activity, alcohol intake.
‡ Model 3: adjusted for model 2 plus systolic blood pressure, diabetes, HDL-cholesterol, LDL-cholesterol, serum TAG, C-reactive protein.
Table 6 Ischaemic stroke associated with each 0·5 %-unit increase in serum long-chain n-3 PUFA, stratified by the median hair mercury content (Percentages and standard deviations; hazard ratios (HR) and 95 % confidence intervals)
Discussion
In this prospective population-based cohort study among middle-aged and older men, serum n-3 or n-6 PUFA or hair Hg were not associated with the risk of stroke. However, among those with hair Hg content above the median, higher serum long-chain n-3 PUFA concentration was associated with an increased risk of ischaemic stroke.
Previously, in this study population, higher serum long-chain n-3 PUFA concentration has been inversely associated with risk factors for stroke, such as high blood pressure( Reference Virtanen, Nyantika and Kauhanen 34 ), CRP( Reference Reinders, Virtanen and Brouwer 35 ) and atrial fibrillation( Reference Virtanen, Mursu and Voutilainen 36 ), and with lower risk of sudden cardiac death( Reference Reinders, Virtanen and Brouwer 35 ), CHD( Reference Mozaffarian, Lemaitre and King 37 ) and CVD( Reference Virtanen, Voutilainen and Rissanen 22 ). Despite the inverse associations with the risk factors, in the present study, higher serum long-chain n-3 PUFA concentration was not associated with lower risk of stroke. This is consistent with the findings from a meta-analysis, which found an inverse association between fish or long-chain n-3 PUFA intakes and risk of stroke, whereas no association was found with circulating long-chain n-3 PUFA( Reference Chowdhury, Stevens and Gorman 7 ). The reasons for this inconsistency are somewhat unclear, because circulating long-chain n-3 PUFA are an established biomarker for intake of fatty acids( Reference Hodson, Skeaff and Fielding 38 ). Results from randomised trials with fish oil in patients with established CVD are also inconsistent( Reference Larsson, Virtamo and Wolk 39 , Reference Rizos, Ntzani and Bika 40 ).
The plant-based n-3 PUFA ALA has been associated with modestly lower risk of CVD( Reference Pan, Chen and Chowdhury 4 ); however, except for the inverse association with circulating ALA concentration found in a small nested case–control study( Reference Simon, Fong and Bernert 15 ), associations with stroke risk have generally not been observed with either dietary or circulating ALA( Reference Larsson, Virtamo and Wolk 8 – Reference He, Rimm and Merchant 10 , Reference Wiberg, Sundstrom and Arnlov 17 – Reference Fretts, Mozaffarian and Siscovick 20 ), supporting our findings.
Although there is good evidence that higher intake or circulating levels of n-6 PUFA, especially LA, are associated with lower risk of CHD( Reference Farvid, Ding and Pan 5 , Reference Wu, Lemaitre and King 6 ), the associations with the risk of stroke are less clear. Studies examining the associations with dietary intake of n-6 PUFA have generally not found an association with stroke risk( Reference He, Merchant and Rimm 11 – Reference Yaemsiri, Sen and Tinker 14 ), whereas circulating LA concentrations have in some studies been associated with a lower risk( Reference Iso, Sato and Umemura 16 , Reference Wiberg, Sundstrom and Arnlov 17 , Reference Yamagishi, Folsom and Steffen 19 ). However, as in our cohort, this has not been observed in all studies( Reference Wu, Lemaitre and King 6 , Reference Simon, Fong and Bernert 15 , Reference De Goede, Verschuren and Boer 18 ). Our results on the lack of association with AA, GLA and dihomo-γ-linolenic acid are consistent with other findings from other observational studies( Reference Wu, Lemaitre and King 6 , Reference Yaemsiri, Sen and Tinker 14 , Reference Wiberg, Sundstrom and Arnlov 17 , Reference Yamagishi, Folsom and Steffen 19 , Reference Sakai, Kakutani and Tokuda 21 ).
In the KIHD cohort, high hair Hg content was associated with a higher risk of CVD and with attenuation of the inverse association between n-3 PUFA and these events( Reference Virtanen, Voutilainen and Rissanen 22 , Reference Virtanen, Laukkanen and Mursu 23 ). In the present analyses, such attenuation was observed with the risk of ischaemic stroke, but not with total strokes or haemorrhagic strokes. In fact, among those with high hair Hg content, serum long-chain n-3 PUFA were associated with increased risk of ischaemic stroke. This was unexpected, and also the possible mechanism for this is unclear, because hair Hg was not associated with a higher risk of ischaemic stroke. Whether the increased risk is due to true biological effect of the long-chain n-3 PUFA on the aetiology of ischaemic stroke among those with higher exposure to Hg or whether it occurred by chance is not known. However, our results, together with the observations from other cohorts( Reference Wennberg, Bergdahl and Stegmayr 24 – Reference Mozaffarian, Shi and Morris 26 ), suggest that Hg exposure has little impact on the risk of stroke.
The strengths of the present study include the prospective and population-based design, extensive database on potential confounders and mediators and the use of serum n-3 and n-6 PUFA and hair Hg as measures of exposure. Serum fatty acids and hair Hg are both established biomarkers for exposure( Reference Roman, Walsh and Coull 27 , Reference Hodson, Skeaff and Fielding 38 ). Unlike dietary assessment methods, these biomarkers are objective measures, and therefore less subject to misclassification, which would reduce the associations towards the null. The potential weakness is that the study included only middle-aged and older Caucasian men, and thus the results may not be generalisable to other populations or to women. In theory, because we evaluated several associations, it is possible that the significant associations may have occurred due to type I error. Furthermore, the number of haemorrhagic strokes was low, and therefore the findings regarding the associations between PUFA and haemorrhagic stroke risk should be interpreted cautiously.
In summary, our findings suggest that higher circulating n-3 and n-6 PUFA or hair Hg are not associated with risk of incident stroke in middle-aged and older men.
Acknowledgements
The study was supported by the University of Eastern Finland. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
The authors’ contributions were as follows: R. D., S. K., T.-P. T. and J. K. V. contributed to the conception and design of the study; S. K. and T.-P. T. acquired data; R. D. and J. K. V. analysed the data and interpreted the results; R. D. drafted the manuscript; and all authors critically revised the paper and approved the final version.
The authors declare that there are no conflicts of interest.
Supplementary Material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/10.1017/S0007114516000982











